Abstract
The lymphatic circulation mediates drainage of fluid and cells from the periphery through lymph nodes, facilitating immune detection of lymph-borne foreign antigens. The 10.1.1 monoclonal antibody recognizes a lymphatic endothelial antigen, here purified by antibody affinity chromatography. SDS-PAGE and mass spectrometry identified mCLCA1 as the 10.1.1 antigen, a 90 kD cell surface protein expressed in lymphatic endothelium and stromal cells of spleen and thymus. The 10.1.1 antibody affinity chromatography also purified LFA-1, an integrin that mediates leukocyte adhesion to endothelium. This mCLCA1-LFA-1 interaction has functional consequences, as lymphocyte adhesion to lymphatic endothelium was blocked by 10.1.1 antibody bound to endothelium, or by LFA-1 antibody bound to lymphocytes. Lymphocyte adhesion was increased by cytokine treatment of lymphatic endothelium, in association with increased expression of ICAM-1, an endothelial surface protein that is also a ligand for LFA-1. By contrast, mCLCA1 expression and the relative contribution of mCLCA1 to lymphocyte adhesion were unaffected by cytokine activation, demonstrating that mCLCA1 and ICAM-1 interactions with LFA-1 are differentially regulated. mCLCA1 also bound to the LFA-1-related Mac-1 integrin that is preferentially expressed on leukocytes. mCLCA1-mediated adhesion of Mac-1- or LFA-1-expressing leukocytes to lymphatic vessels and lymph node lymphatic sinuses provides a new target for investigation of lymphatic involvement in leukocyte adhesion and trafficking during the immune response.
INTRODUCTION
The lymphatic system has a central role in immune surveillance and in the adaptive immune response (1, 2). Lymph drains from the periphery through lymphatic vessels into LNs, to present antigens to lymphocytes that enter LNs via HEVs (3, 4)). Lymphatic vessels also transport immune cells including mast cells, neutrophils, or dendritic cells from the periphery to draining LNs as a rapid response to infection (5–7). Lymphocytes and other leukocytes also increase their adhesion and transmigration through the HEVs, and accumulate within LNs to mount an adaptive immune response to infection. Similar adhesion mechanisms could mediate leukocyte trafficking through the lymphatic system and through vascular HEVs (1). However, little is known yet about the involvement of lymphatic endothelium in regulation of leukocyte trafficking through the lymphatic system in homeostasis, or during immune responses (1). The development of antibodies against lymphatic endothelial markers such as LYVE-1 and Prox-1 (8, 9) now allows detailed investigation of the contributions of the lymphatic system to immune functions.
The entry of lymphocytes into LNs from the bloodstream via HEVs involves an adhesion and transmigration pathway that has been extensively characterized, and which serves as the model of endothelial/immune system cross-talk (reviewed by (10–12)). For example, naïve lymphocytes entering peripheral LNs from the bloodstream express L-selectin that recognizes sialyl Lewis X modifications on CD34 or GlyCAM-1 glycoproteins expressed on the HEV, resulting in lymphocyte slowing and rolling along the HEV. Lymphocyte LFA-1 then binds to endothelial ICAM-1 to arrest rolling and promote leukocyte adhesion to the HEV. LFA-1 binding activity is increased by inducers such as CCL21 chemokine or PMA treatment of lymphocytes, while ICAM-1 on HEVs is induced by cytokines such as TNFα, to strongly increase the affinity of this adhesive interaction (10, 13). This regulated process is critical for cytokine activation of the immune response, to accelerate leukocyte entry into LNs. LFA-1 is expressed on B and T lymphocytes, macrophages, and neutrophils (14). Leukocyte arrest on HEVs can also be promoted by α4β1 integrin adhesion to endothelial VCAM-1. This mechanism is particularly important for leukocyte entry into the bone marrow, although it makes some contribution to lymphocyte trafficking via LN HEVs (15).
Upon arrest from rolling, lymphocytes transmigrate through HEV endothelium to enter the LN parenchyma, by trans-cellular or para-cellular mechanisms involving a number of proteins including ICAM-1, JAM-A, and CD99 (11, 12). Leukocytes then migrate to the B or T cell regions, where they encounter soluble antigen or antigen-presenting cells (4, 16). In the absence of immune stimulation, lymphocytes transit through lymphatic sinus endothelium to exit LNs via the lymphatic sinuses within a day (17). Lymphatic endothelium expresses ICAM-1 and VCAM-1 (18, 19), so that lymphatic sinuses of the LN could also potentially mediate adhesion and/or transmigration of immune cells by the same LFA-1- and α4 β1-dependent mechanisms used by HEVs. A recent study found that ICAM-1 and VCAM-1 mRNA are up-regulated 8- and 214-fold after TNFα, treatment of primary murine lymphatic endothelium, respectively, suggesting that lymphatic endothelium can regulate leukocyte trafficking during the immune response (20).
LFA-1 is primarily expressed in T and B cells, so that it is the major ligand for ICAM-1-mediated lymphocyte adhesion to endothelium. Mac-1 is an LFA-1-related integrin that is preferentially expressed on leukocytes including granulocytes, dendritic cells, and macrophages, which also binds to ICAM-1 to promote adhesion and migration of leukocytes through blood vessels (10). Thus far, studies of lymphatic endothelial ICAM-1 interaction with Mac-1 suggest a key role in macrophage adhesion and migration (21). ICAM-1- and Mac-1-mediated adhesion to lymphatic endothelium also can suppress dendritic cell maturation (22). Much remains to be learned about the involvement of these and other adhesion molecules in leukocyte trafficking through the lymphatic system during the immune response.
Our interest in leukocyte/lymphatic endothelial adhesion interactions arose from our studies of the 10.1.1 monoclonal antibody, which recognizes an antigen expressed on the surface of lymphatic endothelium (23). The 10.1.1 antibody was generated by immunizing hamsters with murine thymic stromal cells, to obtain an antibody recognizing specific cell types in lymphoid organs (24). The 10.1.1 antibody recognizes lymphatic vessels in the skin and colon, intestinal lacteals, and lymphatic sinuses of lymph nodes, suggesting that it is widely expressed on lymphatic endothelium (23). The expression of 10.1.1 on lymphatic vessels and LN lymphatic sinuses overlaps with that of other lymphatic endothelial markers Prox-1, podoplanin, and LYVE-1, making it a useful marker of lymphatic endothelium (23, 25). In the thymus, the 10.1.1 antigen is expressed on stromal medullary epithelium, and it is concentrated at sites of thymocyte contact, suggesting that this antigen functions in adhesion (24). In this study, affinity chromatography was used to purify the antigen recognized by the 10.1.1 antibody, as a first step to identify and characterize its function in the lymphatic system and in lymphoid organs. The 10.1.1 antigen was discovered to be a lymphatic endothelial surface ligand for LFA-1 and Mac-1, that is a major mediator of lymphocyte adhesion to lymphatic endothelium.
MATERIALS AND METHODS
Mice and Antibodies
C57BL/6J wild type mice (Jackson Laboratories) were housed in sterile micro-isolator rooms under specific pathogen-free conditions. Experimental methods involving animals were approved by Fred Hutchinson Cancer Research Center Animal Care and Use Committee.
Syrian hamster antibodies used were 10.1.1 (24) and hamster IgG (Jackson Immunoresearch). Rat antibodies used were CD18 (clone YTS213.1, Upstate), CD18 (clone M18/2), CD11a (M17/4), CD11b (MI/70) and F4/80 (BM3; BD Pharmingen), MECA-32 (SP2/0; Developmental Studies Hybridoma Bank), LYVE-1 (ALY7; eBioscience), and rat IgG (Sigma) were used. Secondary goat antibodies were anti-Syrian Hamster HRP (Jackson ImmunoResearch), anti-Rat HRP (SouthernBiotech), anti-hamster IgG Alexa 568, and anti-rat IgG Alexa 568.
Purification of 10.1.1 antigen
Lysate was prepared from 14 C57Bl/6J mouse spleens by Dounce homogenization (B pestle) in 20 ml Lysis Buffer (1% Triton X-100, 50 mM Tris pH 7.4, 0.3 M NaCl, 0.5 mM EDTA, 10 mM iodoacetamide, and Complete Protease Inhibitor (Boehringer-Mannheim), incubation on ice 30 min, and centrifugation at 4000 × g for 10 min. The supernatant was brought to 1% sodium deoxycholate, and centrifuged at 43,000 × g for 40 min. Nonspecific Syrian hamster IgG (Jackson Immunoresearch) and Protein G Sepharose-purified 10.1.1 antibody were linked to CNBr-activated Sepharose 4B (Sigma) for antibody affinity chromatography (26). Spleen lysates (100 mg protein) were first passed through 0.5 ml hamster-IgG-Sepharose in wash buffer (0.14 M NaCl, 0.5% Triton X-100, 0.5% deoxycholate, 0.01 M Tris pH 8.0), bound to 0.5 ml 10.1.1 IgG-Sepharose, washed with stringent buffer (0.5 M NaCl, 0.5% Nonidet P-40, and 0.05 M Tris HCl (pH 8.0)), and antigen was then eluted with 2% SDS. Fractions were subjected to SDS PAGE on 7% Tris-acetate NUPAGE gels (Invitrogen), followed by Coomassie Blue staining.
Protein identification by in-gel digestion and mass spectrometry
Gel bands were excised, denatured with 60% methanol, reduced with 10 mM dithiothreitol at 60 °C for 1 h, and alkylated with 50 mM iodoacetamide at room temperature in the dark for 30 min. Methanol concentration was reduced to 20% by adding ammonium bicarbonate (50 mM), and proteins were digested with trypsin (Promega) at 37 °C for 6 h at a protein-to-enzyme ratio of 50:1 (w/w), dried in a SpeedVac, and resuspended in 50 mM NH4HCO3. Peptides (12 pmol) were injected for LC-MS/MS analysis in a nano-LC system interfaced with a linear ion trap mass spectrometer (Thermo Electron, San Jose, CA). Instrument settings included spray voltage of 1.5 kV, ion transfer tube temperature 200 °C, and collision gas pressure 1.3 Torr. Voltages across capillary and quadrupole lenses were tuned for optimal signal intensity using the +2 charge state ion of angiotensin I (m/z 649). For each LC-MS/MS analysis, a MS survey scan (m/z 400–1600) was followed by three data- dependent MS/MS scans. Tandem mass spectrometry spectra were searched against the National Cancer Institute human sequence database using Comet. Database search results were validated using PeptideProphet cutoff >0.95, and peptides were then assigned a protein identification using ProteinProphet.
Tissue culture and transfection
293, MS1, and EL-4 cells (ATCC) were cultured as recommended. SV-LEC cells were grown in DME plus 10% fetal bovine serum at 37°C (27). 293 cells were transfected with mCLCA1-, mCLCA2- (28), mAST- (murine angiostatin; (29)) or parental- pcDNA plasmids by calcium phosphate treatment (30), and harvested after 48 h.
Immunoprecipitation and immunoblotting
For immunoprecipitation, lysates were prepared in Lysis Buffer (Figures 2, 4A, and 4B). For mixed lysate immunoprecipitations, cells were lysed in low stringency buffer (1% NP-40, 0.05M TRIS pH 8.0, Complete™ protease inhibitor cocktail, 10 mM iodoacetimide), and protein concentration measured by Bradford assay before mixing 800 μg of EL-4 or bone marrow lysates with 800 μg of 293 cell lysate. Lysates were precleared with Protein-G beads (Amersham) 4x, bound to 25 μl antibody-coated protein G beads at 4°C 1.5 h, washed 4x in buffer (0.5% NP-40, 0.3 M NaCl, 0.0005 mM EDTA, 0.05 M Tris (pH 7.4)) and resuspended in non-reducing SDS sample buffer (0.25M Tris pH 6.8, 50% Glycerol, 10% w/v SDS, 0.005% w/v Bromophenol Blue). For immunoblotting, cells or tissues were prepared for SDS PAGE by lysing in non-reducing SDS sample buffer, before electrophoresis on 7% SDS-PAGE gels, and immunoblotting.
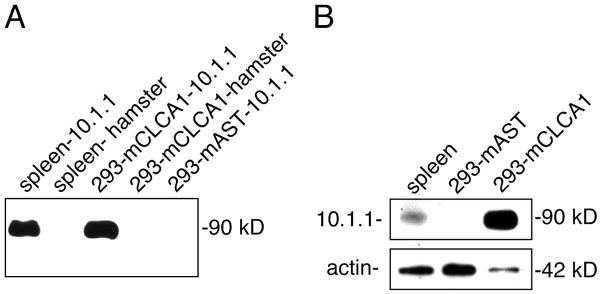
Figure 2. The 10.1.1 antibody recognizes mCLCA1.
A). Immunoprecipitation of spleen or transfected 293 cell lysates with 10.1.1 or hamster antibodies, followed by SDS-PAGE and immunoblotting with 10.1.1 antibody. The 90 kD species is detected after 10.1.1 antibody immunoprecipitation of lysates from spleen, and a slightly smaller protein is detected in immunoprecipitates from 293 cells transfected with mCLCA1, but not from control mAST-transfected 293 cell lysates. Hamster IgG does not immunoprecipitate these proteins. B). SDS-PAGE and immunoblotting with 10.1.1 antibody identifies a 90 kD protein in spleen lysates and a slightly smaller protein in 293 cells transfected with pcDNA-mCLCA1 plasmid (293-mCLCA1), but not with pcDNA-mAST plasmid (293-mAST). Blots were re-probed with antibody to detect 42 kD actin as a loading control.
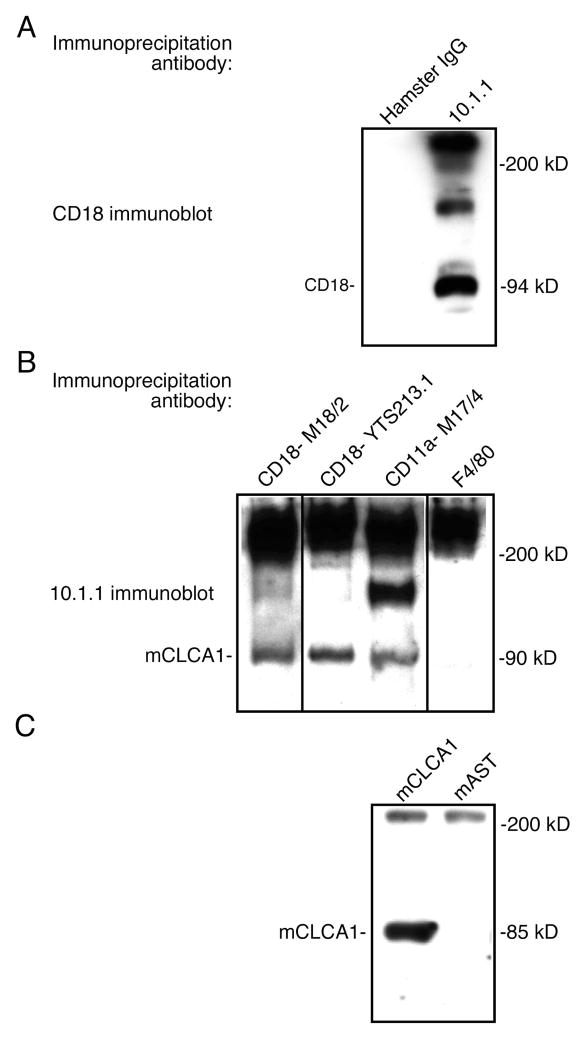
Figure 4. mCLCA1 binds to lymphocyte LFA-1.
A). Spleen lysates were immunoprecipitated with hamster IgG or 10.1.1 antibody, resolved by SDS-PAGE, and immunoblotted with CD18 antibody. The 10.1.1 antibody immunoprecipitates the 94 kD CD18 protein. B). Spleen lysates were immunoprecipitated with CD18, CD11a, or F4/80 rat IgG2a,κ antibodies, and western blotted with 10.1.1 antibody. LFA-1 CD18 and CD11a subunit antibodies immunoprecipitate the 90 kD 10.1.1 antigen, while unrelated F4/80 antibody does not. C). EL-4 thymic lymphoma lysates expressing LFA-1 were mixed with lysates from 293 cells transfected with pcDNA-mCLCA1 or pcDNA-mAST, immunoprecipitated with CD18 antibody, and immunoblotted with 10.1.1 antibody. mCLCA1 is immunoprecipitated from these mixed lysates by CD18 antibody.
Immunostaining
293 cells were plated on glass coverslips in 6 well plates (Corning) at 4×104 cells/well for 48 h. Cryosections were prepared using tissues frozen in Tissue-Tek OCT compound (Sakura). Samples were fixed in −20°C acetone 10 min, air dried 15 min, and fixed in phosphate-buffered formalin for 10 min, followed by immunostaining and mounting in Prolong Gold (Invitrogen). For transmission electron microscopy, mice were perfused with 0.1 M cacodylate buffer (pH 7.4), containing 4% paraformaldehyde and 1 mM CaCl2. Floating tissue sections (50–100 μm thick) were sequentially reacted with primary and secondary antibodies, then reacted to demonstrate peroxidase activity and embedded for transmission electron microscopy. Sections were viewed and photographed with a Philips 201 electron microscope. Details of the immuoperoxidase procedures are described elsewhere (24, 31).
RT-PCR
RNA was purified using TRIZOL (Invitrogen), reverse transcribed, and PCR-amplified using 0.3 mM forward and reverse primers (mCLCA-1 forward primer: GTGGACCAGCCTTTCTACATGTCTAG, mCLCA-1 reverse primer: TGTGACACACAGTTGCCTCTCTCA, mCLCA-2 forward primer: GGACCGGCCTTTCTACATTTCTAG, mCLCA-2 reverse primer: TCGTGGACCACCTTCTTGCCTGTG) (32). PCR amplification was performed at 95°C 5 min, followed by 32 cycles of 95°C, 64°C and 72°C for 30 sec each.
Lymphocyte adhesion assay
SV-LEC cells (10,000 cells/well) or MS1 cells (15,000 cells/well) were plated on 96 well plates. Cells were untreated or pretreated with murine TNFα (10 ng/ml, eBioscience) and IL-1β (5pg/ml, Peprotech) for 24 hours. Lymphocytes were dissociated from spleens of C57Bl/6J mice with a plunger, and passed through a 200 μm nylon filter, followed by red blood cell lysis and removal of B lymphocytes and macrophages by passage through a nylon wool column, resulting in >80% pure T cells (33). The cells were labeled with BCECF fluorescent dye (Molecular Probes) and were sometimes activated with 10ng/ml PMA (Sigma) 30 min at 37°C. Lymphocytes or endothelium were sometimes pre-treated with antibodies 30 min, before the 30 min adhesion assay using 130,000 lymphocytes per well (34). Plates were rinsed 5x, and read using a Fluoroskan Ascent fluorometer (Thermo Labsystems). Percent bound lymphocytes were determined by comparison to serially diluted input lymphocytes.
RESULTS
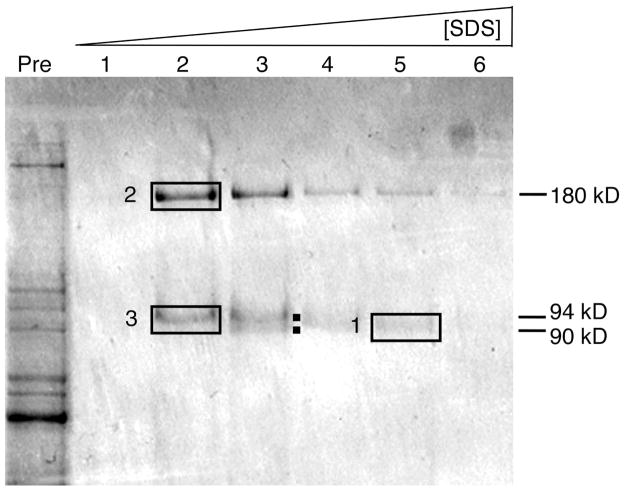
The antigen recognized by the 10.1.1 antibody is expressed on the surface of lymphatic endothelial cells. In order to identify and characterize the function of this protein, we used 10.1.1 antibody affinity chromatography to purify the corresponding antigen from spleen, where it is expressed at high levels. Mouse spleen lysates were passed through a nonspecific hamster IgG-agarose column, bound to a 10.1.1 antibody-agarose column, and eluted with SDS. SDS-PAGE purification of the eluted fractions consistently identified several protein bands (Fig. 1), which were subjected to in-gel trypsin digestion and mass spectrometric analysis. The strongly bound protein band #1 at 90 kD in fraction 5 (Fig. 1) included two distinct proteins, mCLCA1, a 90 kD glycoprotein, and LFA-1β (CD18), a 94 kD glycoprotein, both detected by multiple high confidence peptide identifications (Table 1). The 94 kD LFA-1β protein is preferentially eluted in early fractions, while the 90 kD mCLCA1 protein required higher SDS concentrations for elution (Fig. 1). Sequencing of the 180 kD band #2 in fraction 2, identified LFA-1α (CD11a), the major dimerization partner of LFA-1β (26). Thus 10.1.1 antibody affinity chromatography co-purified mCLCA1 and the LFA-1 heterodimer.
Figure 1. 10.1.1 antibody affinity purification of the antigen.
SDS PAGE analysis of input spleen lysate (“Pre”) and fractions #1–6 eluted with SDS from 10.1.1 antibody-sepharose column. Gel bands 1 to 3 were subjected to mass spectrometric identification. Protein bands of 90 and 94 kD both elute in fraction #3 (dots).
Table I.
Summary of mass spectrometry peptide identification from SDS PAGE-purified gel bands 1 to 3, depicted in Figure 1. The number of unique peptides identified and percent coverage of the full-length protein are given.
| Peptide Identification
| ||||
|---|---|---|---|---|
| Gel Band Number
|
Molecular Weight (kD)
|
Identification
|
Number of Unique Peptides
|
Coverage (percent)
|
| 1 | 90 | mCLCA1 | 40 | 38 |
| 1 | 94 | LFA-1β | 32 | 44 |
| 2 | 180 | LFA-1α | 3 | 3 |
| 3 | 94 | LFA-1β | 2 | 2 |
The mCLCA1 gene encodes the 10.1.1 antigen
The 10.1.1 antibody affinity chromatography purified several candidates to encode the lymphatic endothelial antigen. The 10.1.1 antigen and mCLCA1 are both glycosylated membrane proteins migrating at about 90 kD (24, 28), so that mCLCA1 was the first candidate we examined. Here we present several lines of evidence that lead us to conclude that mCLCA1 is the 10.1.1 antigen. mCLCA1 is a member of the CLCA gene family, which encodes six membrane-associated or integral membrane proteins in mice (reviewed by (35, 36)). Most CLCA gene family members are thought to function in calcium-dependent chloride ion transport, perhaps by interacting with chloride channel proteins. Initial characterization suggested that mCLCA1 is also involved in tumor cell survival and metastasis (37, 38). Otherwise little is known yet about the functions of mCLCA1 or other CLCA gene family members.
Immunoprecipitation was used to test whether the 10.1.1 antibody recognizes the mCLCA1 gene product. The 10.1.1 antibody immunoprecipitates a 90 kD protein from spleen lysates, while hamster IgG does not, as revealed by immunoblotting with 10.1.1 antibody (Fig. 2A). The 10.1.1 antibody also immunoprecipitated the slightly smaller ~85 kD mCLCA protein exogenously expressed from 293 cells, while hamster IgG did not (Fig. 2A). This size discrepancy is likely due to glycosylation, as N-glycosidase digestion of the spleen lysate reduces the molecular weight of 10.1.1 antigen by about 5 kD ((24) and data not shown). As an additional negative control, pcDNA-mAST-transfected cells producing the unrelated murine angiostatin protein did not immunoprecipitate any protein (29). These findings that the 10.1.1 antibody specifically recognizes mCLCA1 were confirmed by direct immunoblotting experiments. The 10.1.1 antibody detects a protein of about 90 kD in spleen, and a smaller 85 kD protein in mCLCA1-transfected 293 cells, but not in pcDNA-mAST-transfected cells (Fig. 2B). These studies demonstrate that the 10.1.1 antibody specifically recognizes the mCLCA1 gene product.
The mCLCA1 gene was recently duplicated in rodents to generate the closely related mCLCA2 gene (39). While these genes are 95% identical in amino acid sequence, mCLCA2 shows a distinct pattern of tissue expression in the thymus and involuting mammary gland (32, 39). We tested whether the 10.1.1 antibody recognizes these closely related proteins by immunofluorescent staining of 293 cells transfected with mCLCA1, mCLCA2, or control pcDNA expression plasmids. The 10.1.1 antibody recognizes both mCLCA1 and mCLCA2 on the cell surface, and also overexpressed antigen trapped in cytoplasmic vesicles surrounding DAPI-stained nuclei (Fig. 3A). The cell surface expression of mCLCA1 and mCLCA2 resembles that of the 10.1.1 antigen on lymphatic endothelium (23).
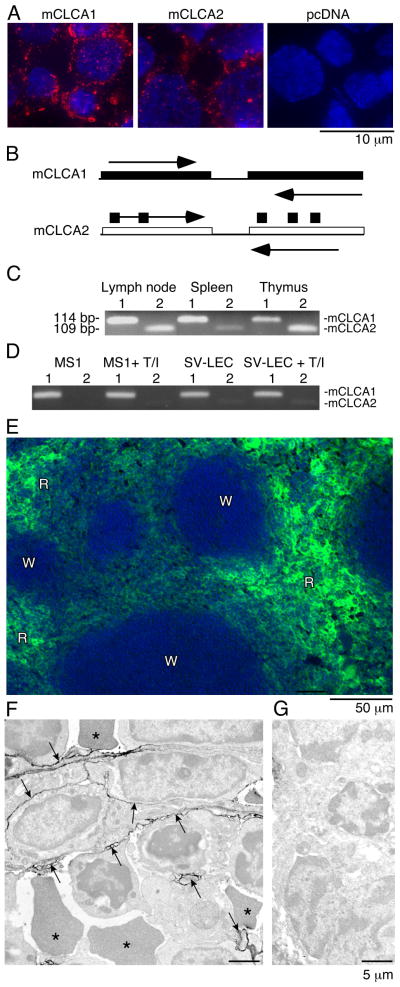
Figure 3. mCLCA1 is expressed in lymphatic endothelium and in lymphoid organs.
A). 10.1.1 antibody (red) stains cell membrane and cytoplasmic vesicles of 293 cells transfected with pcDNA-mCLCA1 or pcDNA-mCLCA2 expression constructs, with blue DAPI nuclear counterstaining. 293 cells transfected with pcDNA plasmid do not stain. B). PCR primers containing divergent sequences were used to specifically amplify the mCLCA1 or mCLCA2 cDNAs. C). RT-PCR of 114 bp mCLCA1 (1) or 109 bp mCLCA2 (2) identifies mCLCA1 and mCLCA2 mRNA expression in lymphoid organs. D). RT-PCR of mCLCA1 and mCLCA2 in MS1 and SV-LEC cells demonstrates that mCLCA1 is the major species in endothelial cells. TNFα/IL1β treatment for 48 h (T/I) does not alter mCLCA1 mRNA abundance. E). Spleen cryosections were immunostained for 10.1.1 antigen, revealing expression throughout the red pulp (R), but not the white pulp (W). F). Immunoperoxidase electron microscopy (Philips 201) of splenic red pulp immunostained with 10.1.1 antibody. The 10.1.1 antibody reactivity, evidenced by electron-dense peroxidase reaction product, is associated with stromal cell processes outlined by arrows, interspersed amongst leukocytes and red blood cells (*). G). No labeling is evident by electron microscopy when 10.1.1 antibody was replaced with normal hamster IgG.
The expression of mCLCA1 and mCLCA2 mRNA was compared in different lymphoid organs and cell types by RT-PCR using gene-specific primers (Fig. 3B). mCLCA1 mRNA is the major species expressed in LN and spleen, while mCLCA2 is preferentially expressed in thymus (Fig. 3C), in agreement with previous mRNA expression surveys (32). mCLCA1 is the major species in the SV-LEC cell line (Fig. 3D), which is a lymphatic endothelial isolate expressing the lymphatic markers Prox-1 and LYVE-1 (27). The mCLCA1 mRNA is also the major species expressed in MS1 endothelial cells (Fig. 3D), which were isolated from SV40 T antigen-transformed pancreatic islets (40). Taken together, these findings are consistent with the mCLCA1 protein encoding the 10.1.1 antigen of lymphatic vessels and LN lymphatic sinuses (23). In the thymus, mCLCA1 and mCLCA2 are likely restricted to stromal medullary epithelium, based on previous 10.1.1 antibody immunostaining of these cells (24).
The spleen selectively expresses mCLCA1, with very little mCLCA2 expression (Fig. 3C). We performed immunostaining with 10.1.1 antibody to identify the cells expressing mCLCA1. The red pulp of the spleen immunostained with 10.1.1 antibody, while white pulp was negative (Fig. 3E). The major cellular components of the red pulp are venous sinusoids, blood cells, and specialized stromal cells. Transmission electron microscopy demonstrated that the major 10.1.1 antibody-positive cells are stromal cells with irregular processes (Fig. 3F), distributed amongst leukocytes and red blood cells (R). This electron-dense reaction product was due to specific 10.1.1 antibody binding, as no labeling was observed when hamster IgG was used for immunostaining (Fig. 3G). Thus mCLCA1 expression is a feature of specialized stromal cells of the splenic red pulp, and of lymphatic endothelium and thymic stromal epithelium.
Lymphatic endothelial mCLCA1 binds lymphocyte LFA-1
Both subunits of LFA-1 (CD11a and CD18) co-purified with mCLCA1 by 10.1.1 antibody affinity chromatography (Table 1). Lymphocyte LFA-1 binds to ICAM-1 expressed on HEVs, to promote lymphocyte adhesion and transmigration into LNs. Our finding that mCLCA1 binds to LFA-1 suggests that this interaction could be involved in lymphocyte adhesion to lymphatic endothelium. The 105 kD ICAM-1 protein (24) was not purified by 10.1.1 antibody affinity chromatography (Fig. 1), indicating that the mCLCA1-LFA-1 interaction is distinct from ICAM-1-LFA-1 interaction. Immunoprecipitation and immunoblotting were used to test whether mCLCA1 specifically binds to the CD11a and CD18 subunits of LFA-1 in vitro. First, the 10.1.1 antibody was used to immunoprecipitate spleen lysates, to test whether this co-purifies the CD18 subunit of LFA-1, as detected by SDS PAGE and immunoblotting with CD18 antibody. The 94 kD CD18 subunit was specifically immunoprecipitated by 10.1.1 antibody but not by hamster IgG (Fig. 4A), supporting the idea that CD18 and mCLCA1 have a strong affinity for each other. Second, immunoblotting of immunoprecipitates prepared using CD18 antibodies or a CD11a antibody isolated the 90 kD 10.1.1 antigen, while immunoprecipitation with an isotype control IgG2a,κ antibody (F4/80) did not (Fig. 4B). These findings confirm that the CD11a and CD18 LFA-1 subunits both bind to the 10.1.1 antigen under stringent conditions, no matter which antibody was used for the initial immunoprecipitation.
Finally, the mCLCA1 protein was directly tested for its ability to bind to LFA-1. mCLCA1 or control angiostatin were expressed from pcDNA plasmids in 293 cells, and EL-4 murine thymic lymphoma cells were used as a source of LFA-1 (26). Lysates were mixed, immunoprecipitated with CD18 antibody, and immunoblotted with 10.1.1 antibody. The CD18 antibody immunoprecipitated the 85 kD mCLCA1 gene product from mCLCA1-transfected 293 cells (Fig. 4C), but not from mAST-transfected cells, demonstrating that mCLCA1 is the 85–90 kD protein that specifically binds to LFA-1.
mCLCA1 mediates lymphocyte adhesion to lymphatic endothelium
Our finding that mCLCA1 strongly binds to LFA-1 in vitro suggests that this interaction could contribute to lymphatic endothelial adhesion to lymphocytes in vivo. Functional assays were developed to test whether mCLCA1 is involved in LFA-1-mediated binding of lymphocytes to lymphatic endothelium. Purified splenic lymphocytes were labeled with fluorescent BCECF dye, bound to endothelial cells under static conditions, and washed extensively before fluorimeter measurement of bound cells (34). The lymphocyte preparations were >80% T cells, with the remainder B cells and macrophages (33). SV-LEC and MS1 endothelial cells were used for these assays, as they both express mCLCA1 (Fig. 3D).
Two treatments were tested to optimize lymphocyte binding to lymphatic endothelium. First, PMA treatment activates LFA-1 to increase lymphocyte adhesion to vascular endothelium (13). PMA treatment of lymphocytes increased their binding to SV-LEC lymphatic endothelial cells by 50%, and to MS1 endothelial cells by 322% (Fig. 5A). Lymphocytes were therefore PMA-treated in subsequent experiments, to maximize LFA-1 adhesion activity. Second, cytokines such as TNFα and IL1β increase the affinity of HEV endothelium for lymphocytes, by increasing the expression of adhesive proteins including the LFA-1 binding partner ICAM-1 (19, 20). TNFα/IL1β pre-treatment of SV-LEC cells for 48 h increased their lymphocyte binding activity by 48% (hamster IgG samples; Fig. 5B) and increased MS1 endothelial cell binding by 92% (data not shown). These findings demonstrate that treatments known to enhance lymphocyte adhesion to vascular endothelium exert similar effects on lymphatic endothelial cells.
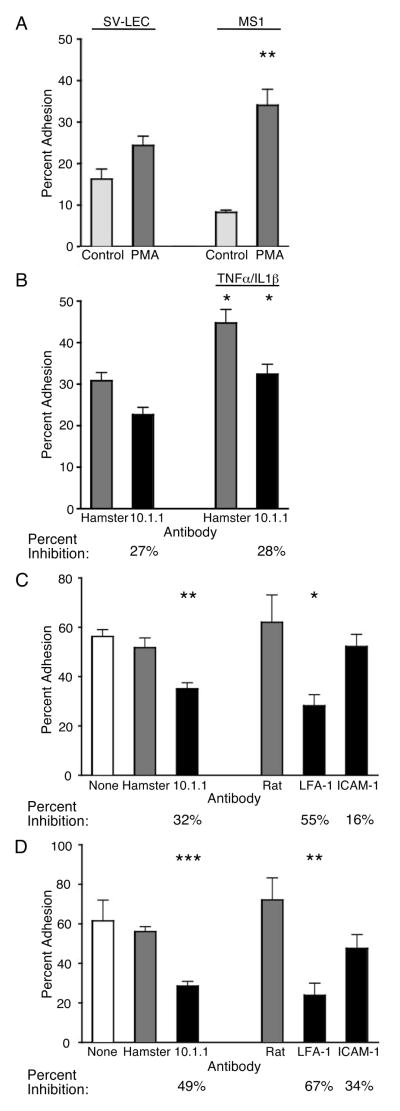
Figure 5. mCLCA1 contributes to lymphatic endothelial cell adhesion to lymphocytes.
A). PMA activation increases binding of BCECF-labeled splenic lymphocytes to SV-LEC or MS1 endothelial cells in static adhesion assays. B). TNFα/IL1β treatment of SV-LEC cells increases PMA-treated lymphocyte adhesion. The 10.1.1 antibody inhibits lymphocyte adhesion to control or TNFα/IL1β-treated endothelium to the same extent, relative to hamster IgG-treated samples. C). 10.1.1 but not hamster IgG blocked PMA-treated lymphocyte adhesion to TNFα/IL1β-treated SV-LEC. LFA-1 antibody blocked lymphocyte adhesion to SV-LEC cells, while ICAM-1 antibody or nonspecific rat IgG did not significantly block binding. D). 10.1.1 antibody but not hamster IgG blocked lymphocyte adhesion to MS1 cells. LFA-1 antibody blocked lymphocyte adhesion to MS1 cells, while ICAM-1 antibody or nonspecific rat IgG did not significantly block binding. Two-tailed unpaired t test analysis identified assays showing statistically significant inhibition (* = p < 0.02, ** = p < 0.005, *** = p < 0.0001). Standard errors are shown.
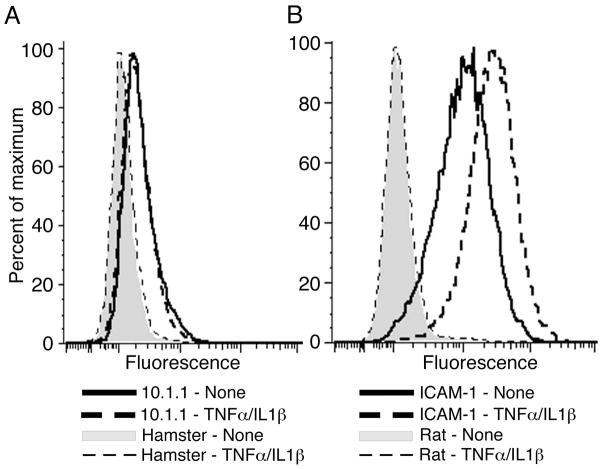
The involvement of mCLCA1 in lymphocyte adhesion to lymphatic endothelium was assessed by incubating endothelial cells with 20 μg of 10.1.1 or control hamster antibody before lymphocyte addition. This antibody concentration was selected as titration experiments indicated that this concentration gave maximal inhibition of lymphocyte binding (data not shown). The 10.1.1 antibody blocked 27% of PMA-treated lymphocyte adhesion to control SV-LEC cells (Fig. 5B). 10.1.1 antibody inhibition of adhesion remained at 28% when SV-LEC were activated by TNFα/IL1β treatment (Fig. 5B), indicating that mCLCA1 adhesion activity is constitutive, while some other molecule such as ICAM-1 mediates cytokine-induced lymphocyte binding activity. In fact, RT-PCR demonstrated no change in mCLCA1 mRNA levels in SV-LEC or MS1 cells after cytokine treatment (Fig. 3D). 10.1.1 immunostaining and flow cytometry confirmed that mCLCA1 surface expression on SV-LEC cells was not affected by cytokine treatment (Fig. 6A). In contrast, surface ICAM-1 expression was strongly induced by TNFα/IL1β treatment (Fig. 6B), in agreement with previous findings that cytokine treatment induces ICAM-1 and increases lymphatic endothelial adhesiveness (18, 19). Taken together, these findings demonstrate that mCLCA1 contributes to lymphatic endothelial adhesion in a constitutive manner, which is distinct from cytokine-induced LFA-1-ICAM-1 interactions.
Figure 6. Cytokines activate lymphatic endothelial ICAM-1 but not mCLCA1.
A). mCLCA1 expressed on the surface of SV-LEC cells is detected by 10.1.1 antibody immunostaining and flow cytometry, and it is not increased by TNFα//IL1β treatment. B). ICAM-1 surface expression is induced by TNFα//IL1β treatment of SV-LEC cells.
The contributions of LFA-1, ICAM-1, and 10.1.1 to lymphocyte adhesion were compared in antibody blocking experiments, using monoclonal antibodies with demonstrated neutralizing activity in this assay (34, 41). TNFα/IL1β treatment of the SV-LEC cells was used to maximize ICAM-1 binding activity. The LFA-1 (CD18) antibody showed the strongest effect to block 55% of lymphocyte adhesion to SV-LEC (Fig. 5C), in agreement with previous reports that LFA-1 is a major mediator of lymphocyte adhesion to endothelium (42). The 10.1.1 antibody significantly blocked 32% of the binding in these assays, while neutralizing ICAM-1 antibody (43) reduced binding by only 16% (Fig. 5C). This ICAM-1 antibody preparation is biologically active however, as it efficiently bound to SV-LEC cells (Fig. 6B). These findings demonstrate that mCLCA1 significantly contributes to lymphocyte adhesion activity of SV-LEC cells.
Antibody inhibition experiments using TNFα/IL1β--treated MS1 endothelial cells also demonstrated that mCLCA1 is a major contributor to lymphocyte adhesion. LFA-1 antibody treatment significantly blocked 67% of the adhesion, while 10.1.1 antibody treatment significantly decreased lymphocyte binding to MS1 cells by 49% (Fig. 5D). Again, the ICAM-1 antibody showed a smaller effect to decrease binding by only 34% (Fig 5D). Thus mCLCA1-LFA-1 adhesion predominates over ICAM-1-mediated adhesion in both endothelial cell lines, even under conditions where ICAM-1 adhesion activity is stimulated by cytokine treatment.
mCLCA1 binds to the LFA-1-related leukocyte integrin Mac-1
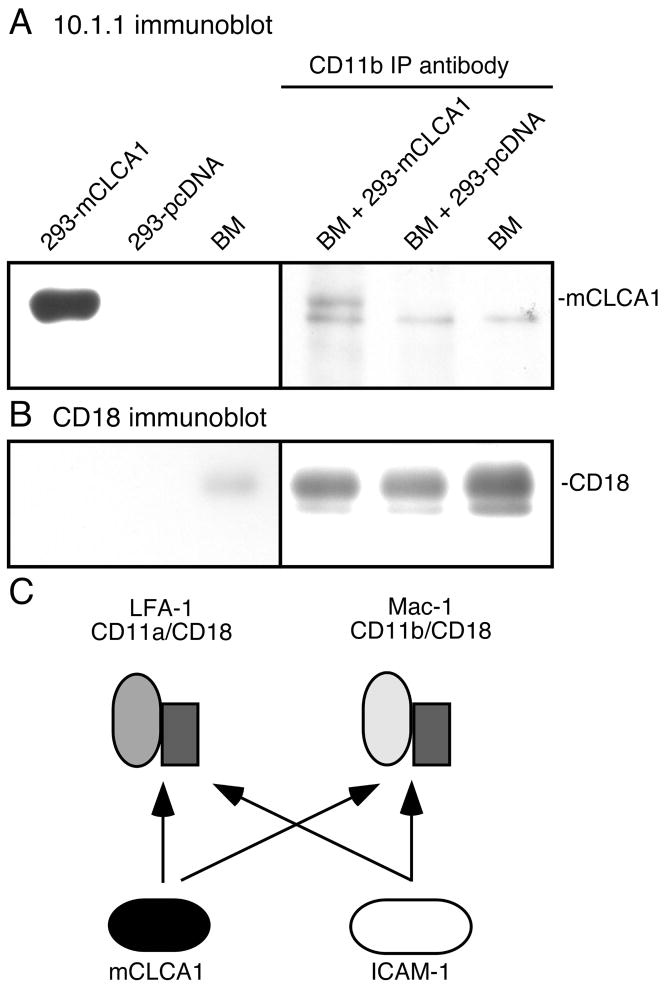
ICAM-1 binds not only to LFA-1, but also to the related leukocyte integrin Mac-1 (44). mCLCA1 could therefore potentially also bind to Mac-1. Mac-1 was not detected in our antibody affinity purification (Fig. 1), however the spleen contains much more LFA-1 than Mac-1 (26). We therefore used bone marrow as an enriched source of Mac-1-expressing leukocytes, to test whether mCLCA1 also binds to Mac-1. Bone marrow (BM) lysates were incubated with lysates of mCLCA1- or control pcDNA-transfected 293 cells, and immunoprecipitated with Mac-1 CD11b antibody. 10.1.1 antibody immunoblotting detects the mCLCA1 protein in CD11b immunoprecipitates including bone marrow plus mCLCA1-transfected 293 cells (Fig. 7A). Immunoprecipitates of lysates of bone marrow with 293 cells transfected with pcDNA detected a nonspecific band but not the larger mCLCA1 species (Fig. 7A). As a loading control, similar yields of the Mac-1 subunit of CD18 were detected in each immunoprecipitate (Fig. 7B). These findings demonstrate that mCLCA1 specifically binds to Mac-1, so that it could potentially regulate the interaction of Mac-1-expressing leukocytes with lymphatic endothelium. The ability of mCLCA1 to bind both Mac-1 and LFA-1 is a feature shared with ICAM-1, suggesting that lymphatic endothelial mCLCA1 and ICAM-1 could potentially compete for binding to leukocytes expressing LFA-1 or to Mac-1, as depicted in Fig. 7C.
Figure 7. mCLCA1 binds to leukocyte Mac-1.
A). Immunoblotting with 10.1.1 antibody identifies mCLCA1 in lysates from 293 cells transfected with mCLCA1 but not in bone marrow (BM) lysates, shown in the left panel. In the right panel, bone marrow lysates expressing Mac-1 were mixed with lysates from 293 cells transfected with mCLCA1 or pcDNA, immunoprecipitated with CD11b antibody, and immunoblotted with 10.1.1 antibody. mCLCA1 is immunoprecipitated from BM and 293-mCLCA lysates by the CD11b antibody, but not from BM and 293-pcDNA lysates. B). Stripping and re-blotting with CD18 antibody demonstrate similar protein recovery in immunoprecipitates. C). mCLCA1 and ICAM-1 of lymphatic endothelium can compete for binding to LFA-1 and Mac-1.
DISCUSSION
Our purification and characterization of the 10.1.1 antigen identified the 90 kD glycosylated surface protein mCLCA1. mCLCA1 is expressed at high levels in lymphatic endothelium and in specialized stromal cells of lymphoid organs, matching the tissue-restricted expression of the 10.1.1 antigen. mCLCA1 may contribute to multiple cellular functions in addition to its LFA-1 and Mac-1 binding activity. Previous studies demonstrated that mCLCA1 binds to β4 integrin in vitro (38), and that it can promote hematogenous metastasis (37). In addition, mCLCA1 influences calcium-dependent chloride channel conductance by an unknown mechanism (36). Lymphatic endothelial cells show enriched expression of a variety of ion transporters relative to vascular endothelium, and lymphatic vessels are active in transendothelial fluid and vesicle transport (45), so that mCLCA1 could potentially contribute to these specialized lymphatic endothelial functions. Some or all of these diverse activities could contribute to mCLCA1 functions in lymphatic vessels and lymphoid organs.
The 10.1.1 antibody affinity chromatography identified a strong interaction of mCLCA1 with the leukocyte surface molecule LFA-1. This finding was initially surprising, as ICAM-1 is assumed to be the major binding partner of LFA-1 on lymphatic endothelium, as it is on vascular endothelium (1). Our adhesion assays using neutralizing antibodies demonstrated that LFA-1 is the major determinant of lymphocyte adhesion to lymphatic endothelium, in agreement with previous studies of vascular endothelium (46, 47). Interestingly, the 10.1.1 antibody blocked lymphocyte adhesion more effectively than the ICAM-1 antibody in both SV-LEC and MS1 cells, suggesting that mCLCA1 is the major LFA-1 binding partner in lymphatic endothelium.
mCLCA1 is expressed on lymphatic vessels, where it could function in LFA-1-dependent leukocyte adhesion during trafficking from the periphery, and on LN lymphatic sinuses, where it could contribute to leukocyte adhesion within LNs. The 10.1.1 antibody affinity chromatography co-purified LFA-1 but not ICAM-1, suggesting that the mCLCA1-LFA-1 interaction does not permit ICAM-1 binding. In this case, lymphatic endothelial mCLCA1 and ICAM-1 could compete for LFA-1 binding activity, as depicted in Fig. 7C. ICAM-1 is strongly induced by TNFα/IL1β treatment of lymphatic endothelium of lymphatic endothelium, while mCLCA1 is not affected by these cytokines, so that the relative contributions of ICAM-1 and mCLCA1 to leukocyte adhesion to lymphatic endothelium could vary during an immune response. However, our adhesion assays demonstrated a major role of mCLCA1 in LFA-1-mediated adhesion even after ICAM-1 expression was activated by cytokine treatment, suggesting that mCLCA1 is a major mediator of leukocyte adhesion to lymphatic endothelium during inflammation.
The 10.1.1 antibody recognizes native and denatured mCLCA1, and also exhibits neutralizing activity, making it a useful reagent for identification and characterization of murine lymphatic endothelium, including lymphatic vessels and LN lymphatic sinuses (23). Importantly, mCLCA1 is expressed on the cell surface, facilitating flow cytometric purification of lymphatic endothelial cells with the 10.1.1 antibody (48). Only a few markers of lymphatic endothelium have been developed thus far, and no single marker identified thus far is specific to lymphatic endothelium. Prox-1 is a transcription factor that is expressed in several organs in addition to lymphatic vessels (49). Podoplanin is a membrane protein expressed on a variety of cell types including lymphatic endothelium (50), while LYVE-1 is a membrane protein expressed in lymphatic and tumor vascular endothelium and macrophages, that is down-regulated by cytokines (20, 51). Thus far, mCLCA1 appears to be constitutively expressed in LNs or in lymphatic endothelium (23). mCLCA1-specific reagents should therefore be useful to identify lymphatic endothelium alone or in combination with these other lymphatic markers in normal or inflamed organs.
The mCLCA1 and mCLCA2 genes resulted from a recent gene duplication event in rodents, so that these closely related proteins likely have similar functions (39). However, mCLCA1 and mCLCA2 show very different tissue expression patterns (32). mCLCA2 is preferentially expressed in the thymus, presumably in 10.1.1 antibody-positive thymic stromal epithelium that was used as the antigen for 10.1.1 hybridoma production (24), and also in the involuting mammary gland (32). mCLCA2 could potentially also function in LFA-1 or Mac-1 binding and leukocyte adhesion in these tissues, as it is so similar to mCLCA1 in sequence (95% amino acid identity; (39)). Interestingly, the thymic medullary epithelial 10.1.1 antigen (predominantly mCLCA2) is induced by IL-1β treatment (24), while we find that lymphatic endothelial mCLCA1 is not IL-1β-responsive, suggesting complexity in regulation of the expression of these genes. The 10.1.1 antigen is selectively expressed on thymic stromal epithelium at sites of thymocyte binding (24), which could involve LFA-1-mCLCA2 adhesion. The human gene CLCA family member most closely related to mCLCA1/2 at the amino acid sequence level is hCLCA3 (69% identical), a cell surface protein of unknown function (36). Further studies will be required to determine whether hCLCA3 performs similar lymphatic endothelial adhesion functions in humans.
The mCLCA1 and ICAM-1 proteins share no regions of amino acid sequence similarity, even though they both show a strong affinity for LFA-1 and Mac-1. Distinct immunoglobulin-like domains of ICAM-1 mediate binding to LFA-1 and Mac-1 (44), neither or which are found in the mCLCA1 protein. Distinct domains of mCLCA1 could mediate binding to LFA-1 and Mac-1, or the same mCLCA1 domain could bind to the CD18 subunit shared by the LFA-1 and Mac-1 heterodimers. Biochemical investigations will be required to identify the sites of mCLCA1 interaction with LFA-1 and Mac-1, for comparison with those of ICAM-1. The ability of mCLCA1 and ICAM-1 to independently bind to LFA-1 or to Mac-1 increases the complexity of regulation of leukocyte adhesion to lymphatic endothelium.
Our finding that mCLCA1 binds to Mac-1 suggests that mCLCA1 contributes to adhesion of Mac-1-expressing granulocytes, dendritic cells, natural killer cells, or macrophages to lymphatic endothelium. Mac-1 and ICAM-1 interaction is important for adhesion and migration of lymphocytes within blood vessels (10), so that mCLCA1 could serve similar functions to regulate trafficking in the lymphatic system. The expression of mCLCA1 in stromal cells of the splenic red pulp could also potentially mediate adhesion of resident granulocytes or other leukocytes abundant in red pulp. Mac-1 mediates peritoneal macrophage efflux to lymphatics during resolution of inflammation (21), which could also potentially involve mCLCA1-Mac-1 interaction. Thus Mac-1-mediated adhesion to mCLCA1 could potentially impact a variety of leukocyte interactions with lymphatic endothelium, thymic stroma, or splenic red pulp stroma.
The exact role of mCLCA1 interaction with LFA-1 and Mac-1 in regulation of lymphocyte and leukocyte adhesion and trafficking in vivo remains to be defined. Immunotherapeutic drugs blocking LFA-1 or ICAM-1 activity can inhibit abnormal lymphocyte activation and pathology in chronic inflammatory or autoimmune diseases including rheumatoid arthritis, psoriasis, and multiple sclerosis (11, 52). Our discovery that LFA-1- mCLCA1 interaction is distinct from the LFA-1-ICAM-1 interaction makes it a new target for therapeutic manipulation of pathological immune responses. Similarly, the mCLCA1-Mac-1 interaction provides a new target to block undesirable leukocyte adhesion and trafficking. Further investigation of the functional significance of lymphatic endothelial or lymphoid organ stromal mCLCA1 adhesion to Mac-1 or LFA-1-expressing leukocytes will also provide insight to the trafficking of immune cells through the lymphatic circulation and lymphoid organs.
Acknowledgments
We thank Maria Harrell, Brian Iritani, Paul Neiman, Karen Spratt, and Elizabeth Wayner for their advice, and Yihai Cao for generously providing CMV-mAST plasmid.
Abbreviations used in this paper
- LN
lymph node
- HEV
high endothelial venule
- BCECF
2′,7′-Bis- (2-Carboxyethyl)-5- (And-6)- carboxyfluorescein
References
- 1.Johnson LA, Jackson DG. Cell traffic and the lymphatic endothelium. Ann N Y Acad Sci. 2008;1131:119–133. doi: 10.1196/annals.1413.011. [DOI] [PubMed] [Google Scholar]
- 2.Swartz MA, Hubbell JA, Reddy ST. Lymphatic drainage function and its immunological implications: From dendritic cell homing to vaccine design. Semin Immunol. 2008;20:147–156. doi: 10.1016/j.smim.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, Fiette L, Saeland S, Gicquel B, Winter N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–1850. doi: 10.1182/blood-2005-03-1281. [DOI] [PubMed] [Google Scholar]
- 6.McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection.[see comment] Nature Immunology. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 7.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 8.Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem. 2001;276:19420–19430. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- 9.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 10.Carlos T, Harlan J. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2102. [PubMed] [Google Scholar]
- 11.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 12.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–196. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 13.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 14.Kurzinger K, Reynolds T, Germain RN, Davignon D, Martz E, Springer TA. A novel lymphocyte function-associated antigen (LFA-1): cellular distribution, quantitative expression, and structure. J Immunol. 1981;127:596–602. [PubMed] [Google Scholar]
- 15.Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen MJ, Hamann A, Hogg N. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J Exp Med. 1999;189:1467–1478. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajenoff M, Glaichenhaus N, Germain RN. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J Immunol. 2008;181:3947–3954. doi: 10.4049/jimmunol.181.6.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sprent J. Lymphocyte migration in vivo assessed with radioisotopes. In: Herzenberg L, Weir DM, editors. The Lymphoid System. Blackwell Science Inc; Cambridge MA: 1996. [Google Scholar]
- 18.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawa Y, Sugimoto Y, Ueki T, Ishikawa H, Sato A, Nagato T, Yoshida S. Effects of TNF-alpha on leukocyte adhesion molecule expressions in cultured human lymphatic endothelium. J Histochem Cytochem. 2007;55:721–733. doi: 10.1369/jhc.6A7171.2007. [DOI] [PubMed] [Google Scholar]
- 20.Johnson LA, Prevo R, Clasper S, Jackson DG. Inflammation-induced uptake and degradation of the lymphatic endothelial hyaluronan receptor LYVE-1. J Biol Chem. 2007;282:33671–33680. doi: 10.1074/jbc.M702889200. [DOI] [PubMed] [Google Scholar]
- 21.Cao C, Lawrence DA, Strickland DK, Zhang L. A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood. 2005;106:3234–3241. doi: 10.1182/blood-2005-03-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, Skobe M. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol. 2009;183:1767–1779. doi: 10.4049/jimmunol.0802167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruddell A, Mezquita P, Brandvold KA, Farr A, Iritani BM. B lymphocyte-specific c-Myc expression stimulates early and functional expansion of the vasculature and lymphatics during lymphomagenesis. American Journal of Pathology. 2003;163:2233–2245. doi: 10.1016/S0002-9440(10)63581-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farr A, Nelson A, Hosier S, Kim A. A novel cytokine-responsive cell surface glycoprotein defines a subset of medullary thymic epithelium in situ. J Immunology. 1993;150:1160–1171. [PubMed] [Google Scholar]
- 25.Ruddell A, Kelly-Spratt KS, Furuya M, Parghi SS, Kemp CJ. p19/Arf and p53 suppress sentinel lymph node lymphangiogenesis and carcinoma metastasis. Oncogene. 2008;27:3145–3155. doi: 10.1038/sj.onc.1210973. [DOI] [PubMed] [Google Scholar]
- 26.Kurzinger K, Springer TA. Purification and structural characterization of LFA-1, a lymphocyte function-associated antigen, and Mac-1, a related macrophage differentiation antigen associated with the type three complement receptor. J Biol Chem. 1982;257:12412–12418. [PubMed] [Google Scholar]
- 27.Ando T, Jordan JTP, Wang Y, Jennings MH, Houghton J, Alexander JS. Isolation and characterization of a novel mouse lymphatic endothelial cell line: SV-LEC. Lymphat Res Biol. 2005;3:105–115. doi: 10.1089/lrb.2005.3.105. [DOI] [PubMed] [Google Scholar]
- 28.Gandhi R, Elble RC, Gruber AD, Schreur KD, Ji HL, Fuller CM, Pauli BU. Molecular and functional characterization of a calcium-sensitive chloride channel from mouse lung. J Biol Chem. 1998;273:32096–32101. doi: 10.1074/jbc.273.48.32096. [DOI] [PubMed] [Google Scholar]
- 29.Cao Y, O’Reilly MS, Marshall B, Flynn E, Ji RW, Folkman J. Expression of angiostatin cDNA in a murine fibrosarcoma suppresses primary tumor growth and produces long-term dormancy of metastases. Journal of Clinical Investigation. 1998;101:1055–1063. doi: 10.1172/JCI1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller AD, Miller DG, Garcia JV, Lynch CM. Use of retroviral vectors for gene transfer and expression. Methods in Enzymology. 1993;217:580–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- 31.Farr AG, Nakane PK. Immunohistochemistry with enzyme labeled antibodies: a brief review. J Immunol Methods. 1981;47:129–144. doi: 10.1016/0022-1759(81)90114-9. [DOI] [PubMed] [Google Scholar]
- 32.Leverkoehne I, Horstmeier BA, von Samson-Himmelstjerna G, Scholte BJ, Gruber AD. Real-time RT-PCR quantitation of mCLCA1 and mCLCA2 reveals differentially regulated expression in pre- and postnatal murine tissues. Histochem Cell Biol. 2002;118:11–17. doi: 10.1007/s00418-002-0420-4. [DOI] [PubMed] [Google Scholar]
- 33.Hathcock K. Current Protocols in Immunology. John Wiley and Sons, Inc; 2007. T cell enrichment by nonadherance to nylon; pp. 3.2.1–3.2.4. [DOI] [PubMed] [Google Scholar]
- 34.Mobley JL, Shimizu Y. Current Protocols in Immunology. John Wiley and Sons, Inc; 2003. Measurement of cellular adhesion under static conditions; pp. 7.28.21–27.28.22. [DOI] [PubMed] [Google Scholar]
- 35.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 36.Loewen ME, Forsyth GW. Structure and function of CLCA proteins. Physiol Rev. 2005;85:1061–1092. doi: 10.1152/physrev.00016.2004. [DOI] [PubMed] [Google Scholar]
- 37.Abdel-Ghany M, Cheng HC, Elble RC, Lin H, DiBiasio J, Pauli BU. The interacting binding domains of the beta(4) integrin and calcium-activated chloride channels (CLCAs) in metastasis. J Biol Chem. 2003;278:49406–49416. doi: 10.1074/jbc.M309086200. [DOI] [PubMed] [Google Scholar]
- 38.Abdel-Ghany M, Cheng HC, Elble RC, Pauli BU. Focal adhesion kinase activated by beta(4) integrin ligation to mCLCA1 mediates early metastatic growth. J Biol Chem. 2002;277:34391–34400. doi: 10.1074/jbc.M205307200. [DOI] [PubMed] [Google Scholar]
- 39.Lee D, HSKYKJCKBM CC. Induction of mouse Ca2+-sensitive chloride channel 2 gene during involution of mammary gland. BBRC. 1999;264:933–937. doi: 10.1006/bbrc.1999.1583. [DOI] [PubMed] [Google Scholar]
- 40.Arbiser J, Moses M, Fernandez C, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, Byers H, Folkman J. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Madrid F, Simon P, Thompson S, Springer TA. Mapping of antigenic and functional epitopes on the alpha- and beta-subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983;158:586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 43.Dang LH, Rock KL. Stimulation of B lymphocytes through surface Ig receptors induces LFA-1 and ICAM-1-dependent adhesion. J Immunol. 1991;146:3273–3279. [PubMed] [Google Scholar]
- 44.Diamond MS, Staunton DE, Marlin SD, Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991;65:961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- 45.Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci U S A. 2002;99:16069–16074. doi: 10.1073/pnas.242401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamann A, Jablonski-Westrich D, Duijvestijn A, Butcher EC, Baisch H, Harder R, Thiele HG. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J Immunol. 1988;140:693–699. [PubMed] [Google Scholar]
- 47.Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med. 1998;187:205–216. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, Farr AG, Tung KS, Engelhard VH. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 207:681–688. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO Journal. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–921. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D’Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. 2005;115(2):247–57. 2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giblin PA, Lemieux RM. LFA-1 as a key regulator of immune function: approaches toward the development of LFA-1-based therapeutics. Curr Pharm Des. 2006;12:2771–2795. doi: 10.2174/138161206777947731. [DOI] [PubMed] [Google Scholar]