Abstract
The amyloid β-protein (Aβ) is subject to proteolytic degradation by a diverse array of peptidases and proteinases, known collectively as Aβ-degrading proteases (AβDPs). A growing number of AβDPs have been identified, which, under physiological and/or pathophysiological conditions, contribute significantly to the determination of endogenous cerebral Aβ levels. Despite more than a decade of investigation, the complete set of AβDPs remains to be established, and our understanding of even well-established AβDPs is incomplete. Nevertheless, the study of known AβDPs has contributed importantly to our understanding of the molecular pathogenesis of Alzheimer disease (AD) and has inspired the development of several novel therapeutic approaches to the regulation of cerebral Aβ levels. In this article, we discuss the general features of Aβ degradation and introduce the best-characterized AβDPs, focusing on their diverse properties and the numerous conceptual insights that have emerged from the study of each.
Cerebral amyloid β-protein (Aβ) levels—and the pathogenesis of Alzheimer disease—are determined in part by the activities of Aβ-degrading proteases (AβDPs) such as neprilysin and insulin-degrading enzyme.
Amyloid β-protein (Aβ) is a normal product of cellular metabolism (Haass et al. 1993) derived from the amyloid precursor protein (APP) by the successive action of the β- and γ-secretases (see Haass et al. 2011). As is true for any other peptide, the production of Aβ is normally counterbalanced by its elimination via any of several processes operating in parallel, including proteolytic degradation, cell-mediated clearance, passive and active transport, and the aggregation and deposition of Aβ into insoluble aggregates. Although the relative importance of these different pathways remains to be established, a growing body of evidence suggests that proteolytic degradation is a particularly significant determinant of cerebral Aβ levels and, by extension, Alzheimer disease (AD) pathogenesis.
It has long been hypothesized that sporadic forms of AD may be attributable to defective clearance of Aβ (Selkoe 2001; Tanzi et al. 2004). Nevertheless, despite the obvious appeal of this simple idea, it had remained little more than a theoretic possibility. Recently, using newly developed techniques for quantifying the rates of Aβ production and clearance within the cerebrospinal fluid (CSF) in humans (Bateman et al. 2006), it was confirmed that sporadic AD patients do indeed exhibit significant defects in the clearance of CSF Aβ (Mawuenyega et al. 2010). Although these experiments cannot distinguish precisely which clearance mechanisms are impaired in these patients, these findings—together with the evidence reviewed in this article—lend strong support to the idea that defective Aβ degradation may be operative in AD.
Widespread interest in Aβ degradation did not take hold until the turn of the 21st century. A key turning point in the field came with the first study that was explicitly designed to examine Aβ degradation in the living animal (Iwata et al. 2000). In addition to identifying neprilysin (NEP) as one of the principal Aβ-degrading proteases (AβDPs), this study highlighted the pathophysiological significance of Aβ degradation to AD pathogenesis generally, thereby igniting interest in this previously underappreciated aspect of Aβ metabolism. A growing list of AβDPs have been identified which, by virtue of their diverse features, contribute in unique ways to the overall economy of brain Aβ. In this article, we provide an overview of the general features of Aβ degradation followed by a brief description of the some of the best characterized AβDPs and their diverse properties. We conclude with a discussion of the feasibility of developing therapies targeting Aβ proteolysis.
GENERAL FEATURES
Aβ Levels Are Potently Regulated by Proteolytic Degradation
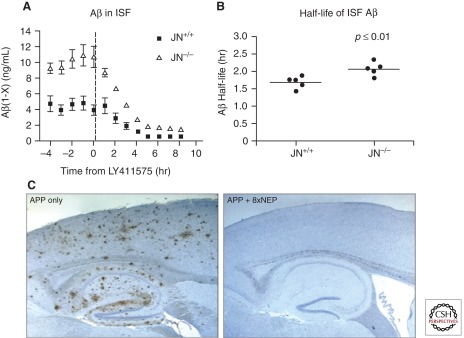
Aβ is degraded by a large set of proteases with diverse characteristics (Table 1). Abundant evidence shows that AβDPs, both collectively and in many cases individually, contribute substantially to the determination of cerebral Aβ levels (Eckman and Eckman 2005; Leissring 2008; Leissring and Saido 2007; Turner and Nalivaeva 2007). In an illustrative study, the half-life of Aβ in brain interstitial fluid (ISF) was quantified in APP transgenic mice lacking or expressing NEP (Fig. 1A; Farris et al. 2007). This was accomplished by using in vivo microdialysis to quantify interstitial Aβ levels as a function of time before and after pharmacologic blockade of Aβ production (Farris et al. 2007). Genetic deletion of NEP resulted in a doubling of steady-state Aβ levels and, notably, a significant increase in the half-life of ISF Aβ (Fig. 1B). Conversely, transgenic overexpression of NEP in neurons by eightfold in an APP mouse model lowered Aβ levels by around 90% and, notably, prevented the development of any amyloid plaques or downstream cytopathology when examined up to 14 months of age (Fig. 1C; Leissring et al. 2003). These and many other findings strongly suggest that AβDPs occupy an “upstream” position within the amyloid cascade that may be surpassed only by the proteases involved in Aβ production itself.
Table 1.
Proteases implicated in the degradation of Aβ
| Type | Protease | Max. relative brain Aβ levels in KOa | Aβ substratesb | |||
|---|---|---|---|---|---|---|
| Aβ40 | Aβ42 | Oligos | Fibrils | Subcellular localizationc | ||
| Metallo | NEP | 2.0 | 2.0 | Synth | No | Ex, ER, G |
| NEP2 | 1.3 | 1.6 | Ex, ER, G | |||
| hMMEL | Ex, ER, G | |||||
| ECE1 | 1.3d | 1.3d | Ex, ER, G, Endo | |||
| ECE2 | 1.3 | 1.3 | Ex, ER, G, Endo | |||
| ACE | N.S. | N.S. | Ex, ER, G | |||
| MMP2 | 1.2 | 1.3 | Yes | Ex, ER, G | ||
| MMP9 | N.S. | 1.3 | Yes | Ex, ER, G | ||
| MMP14/MT1-MMP | Yes | Ex, ER, G | ||||
| CD147/EMMPRIN | Ex, ER, G, Endo | |||||
| IDE | 1.6 | 1.4 | No | No | Ex, ER, Endo, Lyso, Mito | |
| Serine | Plasmin | N.S. | N.S. | Natural | Yes | Ex, ER, G |
| Acylpeptide hydrolase | Natural | Ex, Cyto | ||||
| Myelin basic protein | Yes | Ex, ER, G | ||||
| Aspartyl | Cathepsin D | N.S. | 3.0 | Yes | Endo, Lyso | |
| BACE1 | 0.0 | 0.0 | Endo, Lyso | |||
| BACE2 | N.S. | N.S. | No | Endo, Lyso | ||
| Cysteine | Cathepsin B | N.S. | N.S. | Yes | Ex, Endo, Lyso | |
| Threonine | Proteasome | Cyto | ||||
| Other | Catalytic antibodies | – | ||||
aData reflect the maximum published values for endogenous cerebral Aβ levels in mice lacking both copies of individual AβDPs, expressed relative to wild-type controls. KO, knockout; N.S., no significant difference.
bAggregated forms of Aβ known to be degraded by individual AβDPs. Synth, synthetic Aβ oligomers; Natural, naturally secreted Aβ oligomers.
cEx, extracellular space; ER, endoplasmic reticulum; Endo, endosomes; Lyso, lysosomes; Mito, mitochondria; Cyto, cytosol.
dEffect induced by deletion of one copy of ECE1.
Figure 1.
Aβ degradation is a potent determinant of brain Aβ levels and amyloid pathology. (A) Effects of genetic deletion of NEP on Aβ levels in the interstitial fluid (ISF) of the J9 line of APP transgenic mice monitored by in vivo microdialysis before and after blockade of Aβ production with the γ-secretase inhibitor, LY411575. Note that steady-state levels of Aβ are approximately doubled in J9 mice lacking NEP (JN−/−). (Panel A is adapted from Farris et al. 2007; reprinted, with permission, from the authors.) (B) The half-life of ISF Aβ determined from the data in (A). Note that deletion of NEP results in a statistically significant (P < 0.01) 23% increase in the half-life of ISF Aβ, from 1.7 to 2.1 hours. (C) Transgenic overexpression of NEP by eightfold results in the complete prevention of amyloid plaque formation in the J20 line of APP transgenic mice up to 14 months of age. (Panel B is adapted from Leissring et al. 2003; reprinted, with permission, from the author.)
Net Aβ Levels Reflect the Balance between Rates of Production and Clearance
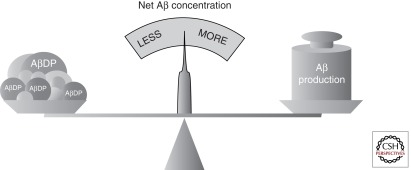
Aβ is generated and eliminated continuously, and the absolute concentration of Aβ, within a given compartment and at a given instant, is determined jointly by these opposing forces. An instructive analogy is that of a balance (Fig. 2), wherein the absolute rate of Aβ production, represented by a weight on one arm, is counterbalanced by the overall rate of Aβ clearance, represented by a large collection of diverse counterweights on the other arm.
Figure 2.
The balance analogy illustrates the relationship between AβDPs and net Aβ levels. By analogy with a balance, net Aβ concentrations (represented by the position of the pointer on the scale) are determined by the rate of Aβ production (represented by a single weight on one arm) relative to the overall rate of Aβ clearance (represented by a collection of counterweights on the other). Aβ clearance is performed by a collection of AβDPs (dark gray counterweights) working jointly with each other and with other eliminative processes (light gray counterweights). See text for additional details.
The balance analogy serves to illustrate several general features of Aβ degradation:
-
Net Aβ levels are determined by the relative, rather than absolute, rates of Aβ production and elimination.
Net Aβ concentrations can be elevated either by an increase in Aβ production or by a decrease in the overall rate of its elimination, and the converse is also true. However, no change in net Aβ levels will occur if these opposing forces vary in indirect proportion to one another—only if one changes with respect to the other.
-
AβDPs work cooperatively with each other and with other catabolic processes to eliminate Aβ.
The catabolism of Aβ is mediated not only by multiple AβDPs but also by a diverse array of eliminative processes, including diffusion, passive and active transport, protein–protein interactions, aggregation, and deposition. These processes all operate simultaneously, in complex combinations that vary regionally and by subcellular compartment.
-
Net Aβ levels are determined by the sum total of all catabolic processes.
Despite the complexity of Aβ catabolism, assuming production to be constant, the parameter most relevant to the determination of Aβ levels is the overall rate of Aβ catabolism, determined by the totality of all contributing processes. As a consequence, AβDPs and other Aβ-eliminating processes are functionally interchangeable, at least with respect to their influence determining net concentrations of Aβ.
-
Proteolytic degradation of Aβ normally operates at or near its functional capacity.
In mice, genetic deletion of any one of several, markedly different AβDPs can result in significant elevations in endogenous cerebral Aβ (Table 1). These increases in net Aβ levels occur in a gene dosage-dependent manner, and simultaneous deletion of two different AβDPs has also been shown to produce roughly additive effects. Taken together, these findings show that multiple AβDPs exist, each of which is rate limiting in the determination of cerebral Aβ concentrations. More significantly, these findings suggest that there is little or no reserve capacity in the overall catabolism of cerebral Aβ.
-
The mechanistic relationship between Aβ and AβDPs is bidirectional.
Not only do AβDPs regulate Aβ via proteolytic degradation, but Aβ itself can also disrupt the function of AβDPs, either directly, via competitive inhibition, or indirectly, via a wide range of secondary processes triggered by Aβ accumulation, such as oxidative damage. Conversely, aggregated Aβ can also stimulate the production or activation of certain AβDPs. In these and other ways, AβDPs and Aβ interact bidirectionally.
Aβ Production and Degradation Are Asymmetric
The balance analogy, although illustrative of the mutual interdependence of Aβ production and degradation, fails to completely capture several fundamental asymmetries between the two processes. Collectively, these asymmetries offer important insights into the contribution of AβDPs to the normal regulation of cerebral Aβ levels and, by extension, the pathogenesis and potential treatment of AD.
Few Sources versus Many Diverse Sinks
Perhaps the most fundamental asymmetry is the difference in sheer complexity between Aβ production and degradation. Full-length Aβ peptides are produced by just two proteases, which act within a comparatively limited subset of subcellular compartments, primarily within neuronal cells. Aβ degradation, in contrast, is mediated by a considerably larger number of proteases, each with unique Aβ avidities, pH optima and, perhaps most critically, different regional, cellular, and subcellular localizations.
AβDPs Define Different Pools of Aβ
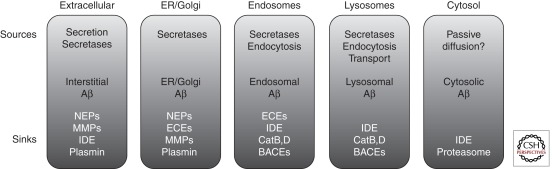
Proteolytic degradation of Aβ is the terminal event that defines the lifespan of a substantial portion of all Aβ peptides produced. By determining the temporal lifetime of individual Aβ molecules, proteolytic degradation also indirectly determines the spatial extent to which each molecule can be transported away from its site of production. As illustrated in Figure 3, AβDPs thus help to define specific pools of Aβ, the temporal and spatial extent of which is defined jointly by production and degradation. In light of the substantial variety in the regional and subcellular localization of many AβDPs (Table 1), it is evident that many different pools of Aβ exist, each contributing differently to overall Aβ levels and, potentially, to AD pathogenesis (Fig. 3). As such, functionally or spatially distinct AβDPs represent experimental probes for establishing the relative importance of individual pools of Aβ, which might then be more selectively targeted for therapeutic benefit.
Figure 3.
AβDPs regulate and help define distinct pools of Aβ. Aβ can be conceptualized as existing in distinct pools localized to different subcellular compartments (rounded rectangles). Each pool is characterized by different “sources” of Aβ (e.g., secretases, secretion) and different combinations of AβDPs. Because AβDPs vary considerably in terms of their subcellular localization, pH optima and other properties, Aβ within different subcellular compartments is regulated by diverse combinations of AβDPs.
Aβ Degradation Is Catalytic and Irreversible
Proteolytic degradation is catalytic and irreversible, meaning that a single AβDP molecule can effect the permanent elimination of a large number of Aβ molecules, while itself remaining unchanged. Although it is true that Aβ production is also mediated by catalytic processes that can be rate limiting, in practice, Aβ production appears to be substrate limited. This can be seen from the fact that increases in APP expression—for instance, in Down’s syndrome or in APP transgenic mice—result in roughly proportional increases in net Aβ production, both in the brain and in the periphery. Because small changes in the activities of multiple AβDPs can result in large changes in net Aβ levels, the catalytic nature of AβDPs suggests they are important both for the etiology and the potential treatment of AD.
Aβ Degradation Is Prone to a Range of Environmental and Age-Associated Insults
The pathogenesis of AD is known to be influenced by a range of environmental insults, whereas aging itself is known to be characterized by the accrual of oxidative damage (Zhu et al. 2007), as well as a general decrease in the expression of many proteins (Lu et al. 2004). AβDPs, in turn, are known to be vulnerable to a range of potentially damaging exogenous influences, including pharmacological inhibition, environmental insults, and age-related oxidative damage (Wang et al. 2003; Caccamo et al. 2005; Shinall et al. 2005; Neant-Fery et al. 2008). Given that age is the principle risk factor for AD, these considerations suggest that defective clearance of Aβ is likely to be operative not only in sporadic forms of AD, as was recently confirmed experimentally (Mawuenyega et al. 2010), but even in those cases attributable to increased production of Aβ due to genetic disturbances.
Aβ Degradation Can Take Place Distal to Sites of Production
The study of AβDPs has confirmed other evidence suggesting that Aβ exists in a dynamic equilibrium between various compartments, such as the secretory pathway, the endolysosomal system, the interstitial space, CSF, and even compartments outside the brain such as the circulatory system. Because these compartments are interconnected, either through physical contiguity or through active and passive Aβ transport, the degradation of Aβ in one compartment can result in the lowering of Aβ in the others. As a consequence, AβDPs can regulate net Aβ levels at sites distal to its production. This principle has an important therapeutic corollary. Whereas therapies aimed at blocking Aβ production must necessarily act locally, within Aβ-producing cells, therapies aimed at increasing Aβ catabolism are capable of exerting their effect in multiple compartments, including compartments outside the blood–brain barrier. In a striking demonstration of this principle, overexpression of NEP exclusively in the periphery (in skeletal muscle) was recently shown to lower steady-state Aβ levels and amyloid plaque deposition in brain (Liu et al. 2009).
SPECIFIC Aβ-DEGRADING PROTEASES
A large number of AβDPs have been identified to date (Table 1), but the state of our knowledge about each varies considerably. AβDPs can be classified by enzymological type (e.g., metalloproteases, cysteine proteases, etc.), by the assembly state of the Aβ substrates they hydrolyze (e.g., peptidases, oligopeptidases, or fibrillases), and by their subcellular localization (Table 1). There is a further, functional distinction between endogenous regulators, which regulate brain Aβ levels under physiological conditions, and pathogenic regulators, which are operative under pathological conditions, and these categories need not be mutually exclusive. In principle, a third functional category of AβDPs might be termed therapeutic regulators, which, it is important to emphasize, do not necessarily need to belong to either of the former categories to be effective.
In the following subsections, we briefly introduce the best characterized AβDPs, together with catalytic antibodies and endogenous protease inhibitors, focusing on the distinguishing features of each and the principles that have been learned from their study. Experimental evidence strongly suggests that additional AβDPs remain to be identified. For instance, simultaneous inhibition of multiple zinc-metalloproteases by i.c.v. infusion of the broad-spectrum metalloprotease inhibitor, phosphoramidon, resulted in a remarkable >fivefold increase in endogenous cerebral Aβ levels (Eckman et al. 2006). The magnitude of this increase is far greater than that seen by genetic deletion of any single AβDP (Table 1) or even from simultaneous deletion of multiple AβDPs (Eckman et al. 2006). In a similar finding, i.c.v. infusion of thiorphan in mice lacking both NEP and a related protease NEP2, nevertheless resulted in large increases in cerebral Aβ (Hafez et al. 2011). These and other findings strongly suggest that additional AβDPs remain to be identified that normally participate in Aβ catabolism and/or that might be used therapeutically.
Zinc-Metalloproteases
Neprilysin
The most extensively investigated and best characterized AβDP is NEP, a member of the M13 clan of zinc-metalloproteases (Howell et al. 1995; Hersh and Rodgers 2008). NEP was once termed “enkephalinase” because enkephalin is one of its best substrates in vitro (Turner 1998). However, enkephalin levels in the cerebral cortex were unchanged in NEP knockout (KO) mice (Saria et al. 1997; Iwata and Saido, unpubl. data), suggesting that NEP alone does not determine the steady-state levels of enkephalin in vivo. This is probably because there exist redundant catabolic mechanism(s) that involve exopeptidase(s), other endopeptidase(s), or both. In contrast, levels of both Aβ40 and Aβ42 are twofold higher in NEP KO mice than the levels in wild-type controls (Table 1; Iwata et al. 2001), suggesting that NEP is an important endogenous regulator of Aβ.
NEP was first identified as an important AβDP in an experimental paradigm in which the degradation of radiolabeled Aβ42 injected into rat hippocampus was monitored in the presence or absence of different protease inhibitors (Iwata et al. 2000; Saido and Iwata 2006). NEP is a type II membrane-associated peptidase, the active site of which faces the lumenal or extracellular side of membranes (Roques et al. 1993; Turner 2004; Turner et al. 2001), a topology that is ideally suited for the degradation of largely extracytoplasmic peptides such as Aβ. NEP is almost exclusively expressed in neurons, not in glia, and the peptidase, after synthesis in the soma, is axonally transported to presynaptic terminals (Fukami et al. 2002), presumably in a manner similar to that in which APP is transported. Therefore, presynaptic terminals and nearby intracellular (lumenal) locations are likely to be the sites of Aβ degradation by NEP (Iwata et al. 2004). Importantly, the levels of Aβ inversely correlate with the gene dosage of NEP and thus with its enzymatic activity. These observations suggest that even partial loss of NEP expression/activity can cause the elevation of Aβ levels and could therefore induce amyloidosis on a long-term basis, in a similar manner to familial AD-causing gene mutations. The results also suggest that the rate constant for the intraparenchymal degradation of Aβ by NEP could account for as much as 50% of the total clearance activity (Saito et al., 2003).
Several insights have emerged from the study of NEP in APP transgenic mice. As discussed above, genetic deletion leads to an approximate doubling of steady-state levels of cerebral Aβ while accelerating amyloid deposition (see Fig. 1A). Qualitative pathological differences have emerged, as well. For example, deletion of NEP in the J9 line of transgenic mice led to the emergence of cerebral amyloid angiopathy that was not present in mice expressing two functional copies of NEP (Farris et al. 2007).
The therapeutic value of overexpressing NEP has also been investigated in APP transgenic mouse models. For example, as mentioned above, a cross between the J20 line of APP transgenic mice and a transgenic mouse that expresses eightfold higher levels of NEP (8xNEP) resulted in up to a 90% reduction in steady-state Aβ levels and the complete prevention of amyloid plaque formation and associated cytopathology when examined at up to 14 months of age (Fig. 1C; Leissring et al. 2003). NEP has been reported to degrade Aβ oligomers that impair neuronal plasticity and cognitive function in APP-Tg mice (Huang et al. 2006), although a different study saw no decrease in oligomers (Meilandt et al. 2009) (discussed below). As another potential therapeutic benefit, neuropeptide Y fragments generated by NEP-catalyzed proteolysis have been shown to be neuroprotective (Rose et al. 2009). Although these and other findings illustrate the potential benefits of therapeutic overexpression of NEP, there may also be risks. For example, the 8xNEP transgenic line has been shown to alternatively prevent or promote premature lethality in a strain-dependent manner (Leissring et al. 2003; Meilandt et al. 2009).
Like all known AβDPs, NEP degrades monomeric Aβ. Interestingly, some of the pathogenic APP mutations that reside within the Aβ sequence render Aβ monomers more resistant to NEP-catalyzed proteolysis (Tsubuki et al. 2003; Betts et al. 2008). It is less clear whether NEP can directly degrade oligomeric Aβ species. In vitro, NEP was reported to degrade oligomeric forms of synthetic Aβ (Kanemitsu et al. 2003), but it was incapable of degrading naturally secreted Aβ oligomers isolated from cultured cells (Leissring et al. 2003), suggesting that differences in the Aβ oligomer preparation might matter. Two findings in APP transgenic mice raise additional questions. On the one hand, deletion of NEP in 2 different mouse models was found to increase the concentration of Aβ oligomers (Huang et al. 2006; Farris et al. 2007). On the other hand, a cross between the 8xNEP line and the J20 line of APP transgenic mice resulted in dramatic decreases in monomeric Aβ levels and prevented all plaque formation (as reported previously by Leissring et al. 2003), yet oligomeric Aβ levels were unchanged (Meilandt et al. 2009). Moreover, in the latter study, NEP overexpression failed to reverse the learning and memory deficits present in the J20 line (Meilandt et al. 2009). Because different promoters were used, the extent to which the NEP and APP transgenes were coexpressed in the same population of neurons is not clear. Nevertheless, whether coexpressed appreciably or not, this result implies that NEP might not be capable of clearing at least some naturally produced Aβ oligomers.
NEP-Like Peptidases
Several close homologs of NEP are also implicated as candidate AβDPs (Table 1; Shirotani et al. 2001). For example, genetic ablation of NEP2 produces net increases in cerebral Aβ levels that are additive with those produced by deletion of NEP (Hafez et al. 2011). Another phosphoramidon-sensitive NEP homolog, human membrane metalloendopeptidase-like protein (hMMEL), was recently found to degrade Aβ in cultured cells (Huang et al. 2008). Although the exact contribution of each is still under investigation, it seems likely that the collective action of these and other NEP-like peptidases contribute significantly to the determination of cerebral Aβ levels.
Endothelin-Converting Enzymes
Two additional members of the M13 family of zinc metalloproteases, endothelin-converting enzymes 1 and 2 (ECE1, ECE2), are also known to be endogenous regulators of Aβ (Table 1; Eckman et al. 2001, 2003). In contrast to NEP and NEP-like peptidases, which are most active at neutral pH, ECEs have an acidic pH optimum and are therefore active primarily within acidic subcellular compartments (Table 1). As a consequence, ECEs primarily degrade Aβ at intracellular sites (Eckman et al. 2003). This point is important, because, together with other evidence (Leissring 2008), it serves to show that the vast majority of Aβ degradation likely occurs before the secretion of the monomer into the extracellular space.
Angiotensin-Converting Enzyme
Another important vasopeptidase implicated in the degradation of Aβ is angiotensin-converting enzyme (ACE) (Carvalho et al. 1997; Hu et al. 2001). Because pharmaceutical ACE inhibitors are widely used to treat hypertension, the question of whether ACE is an endogenous regulator of Aβ is a critical one. At present, the balance of the evidence suggests that it is not. Oral administration of the widely used ACE inhibitor, captopril, to APP transgenic mice resulted in no significant elevation in cerebral Aβ levels (Hemming et al. 2007b). Moreover, genetic deletion of ACE failed to produce any significant elevation in steady-state levels of endogenous Aβ (Table 1; Eckman et al. 2006). Nevertheless, because there is also genetic evidence that variants in the Ace gene are associated with the risk for late-onset AD (Bertram et al. 2007), it will be important to gain further clarity about the exact role of ACE in the degradation of Aβ under physiological and pathophysiological conditions.
Matrix-Metalloproteinases
Matrix-metalloproteinases (MMPs) represent another important group of AβDPs that can be distinguished, in part, by their ability to degrade both monomeric and fibrillar forms of Aβ (Table 1; Yan et al. 2006a). Multiple MMPs have been implicated in the degradation of Aβ, including MMP2 (Roher et al. 1994), MMP9 (Yan et al. 2006a) and MMP14 (a.k.a. MT1-MMP) (Liao and Van Nostrand 2010) but only a subset have been investigated in vivo. Relative to other AβDPs, MMPs are comparatively weak endogenous regulators of Aβ. For example, deletion of MMP2 or MMP9 in mice resulted in modest but statically significant increases in endogenous cortical and hippocampal Aβ (Yin et al. 2006) (Table 1). However, some special properties of MMPs suggest they are likely to be of considerably greater importance in a pathological context. First, MMPs normally exist as latent pro-enzymes that can be proteolytically processed to become fully active (Van Wart and Birkedal-Hansen 1990). Interestingly, extracellular matrix metalloproteinase inducer (EMMPRIN; CD147), one of the proteases responsible for activating MMPs by this mechanism, was found to lower Aβ levels in cultured cells by inducing multiple MMPs (Vetrivel et al. 2008). Second, basal expression of MMPs is low but can be stimulated by pathological insults, including Aβ itself (Deb and Gottschall 1996). Consistent with these features, in APP transgenic mice, MMPs were found to be up-regulated in astrocytes adjacent to amyloid deposits (Yin et al. 2006). Moreover, in the same mice, i.c.v. infusion of the broad-spectrum MMP inhibitor, GM6001, resulted in significant increases (∼50%) in both the steady-state levels and the half-life of ISF Aβ (Yin et al. 2006).
Insulin-Degrading Enzyme
Insulin-degrading enzyme (IDE) is another well-established AβDP that has been extensively investigated for its role in Aβ degradation using a wide array of experimental approaches, ranging from enzymological analyses to human molecular genetics (Hersh 2006). Although IDE is a zinc-metalloprotease, it belongs to a separate superfamily with distinct evolutionary origins, referred to as “inverzincins” because they feature a zinc-binding motif (HxxEH) that is inverted with respect to the canonical one (HExxH) present in most known zinc-metalloproteases (Becker and Roth 1992). The crystal structure of IDE is unusual, resembling a clam shell, with a large internal chamber formed from two bowl-shaped halves connected by a flexible linker (Shen et al. 2006). Because oligomeric and fibrillar forms of Aβ are too large to fit completely into its internal chamber, IDE is strictly a peptidase, i.e., it exclusively degrades monomeric Aβ.
Although functionally similar to vasopeptidases (e.g., ACE) in showing a preference for monomeric Aβ, IDE differs substantially in terms of its subcellular localization. It is well established that IDE is most abundant in the cytosol (Falkevall et al. 2006) and also present within mitochondria (Leissring et al. 2004; Farris et al. 2005), but there is less certainty about its presence in other subcellular compartments (Leissring et al. 2004), with various studies reporting its presence in peroxisomes (Kuo et al. 1994), endosomes (Hamel et al. 1991), the endoplasmic reticulum (Carpenter et al. 2010), and lysosomes (MA Leissring, unpubl.). Like most other AβDPs, IDE is also present in the extracellular space (Table 1), both in secreted (Qiu et al. 1998) and cell-associated (Vekrellis et al. 2000) forms. IDE lacks a canonical signal peptide sequence (Leissring et al. 2004) and it is exported independent of the classical secretory pathway (Zhao et al. 2009). The precise nature of the underlying secretion mechanism remains obscure, but accruing evidence suggests that it is mediated at least partly by exosomes (Bulloj et al. 2010; Tamboli et al. 2010).
Abundant evidence suggests that IDE is the major AβDP secreted into the medium of cultured cells (Qiu et al. 1998). For example, in cultured primary neurons, genetic deletion of IDE resulted in >90% decrease in the initial degradation rate of physiological levels of exogenous Aβ monomers (Farris et al. 2003), and similar results are seen with a wide variety of different cultured cells (MA Leissring, unpubl.). However, in vivo, genetic deletion of IDE resulted in elevations in cerebral Aβ levels which, although comparable to those induced by many AβDPs, are smaller than might be expected from results in cultured cells (Table 1; Farris et al. 2003). Two factors may contribute to this interesting disparity. First, although IDE is present in CSF (Qiu et al. 1998), it is likely that IDE accumulates in the medium of cultured cells to a greater extent than it does in extracellular fluids in vivo. Second, IDE KO mice suffer from chronic hyperinsulinemia (Farris et al. 2003; Abdul-Hay et al. 2011), which triggers age-dependent compensatory adaptations, including severe insulin and glucose intolerance (Abdul-Hay et al. 2011). The secondary consequences of IDE ablation thus obscure the impact of this important AβDP on brain Aβ levels. New pharmacologic inhibitors of IDE (Leissring et al. 2010) should make it possible to circumvent these compensatory changes and determine the direct contribution of IDE to cerebral brain Aβ levels.
Serine Proteases
Plasmin
Three functionally related serine proteases have been linked directly and indirectly to Aβ degradation: plasmin and urokinase-type and tissue-type plasminogen activators (uPA and tPA, respectively). Of these, only plasmin has been shown to directly degrade Aβ; like MMPs, it can degrade both monomeric and fibrillar forms (Table 1; Van Nostrand and Porter 1999; Tucker et al. 2000). tPA and uPA, however, are responsible for converting the inactive zymogen of plasmin (plasminogen) into its active form. The latter process is normally inhibited by the endogenous inhibitor, plasminogen activator inhibitor1 (PAI1; Myohanen and Vaheri 2004), and it is of great interest that pharmaceuticals which disrupt PAI1 have been developed that effectively lower brain Aβ in APP transgenic mice (Jacobsen et al. 2008). tPA is an excellent example of a pathologic regulator of Aβ, because it is stimulated by fibrillar proteins including Aβ (Van Nostrand and Porter 1999). uPA is of interest because of evidence linking variability around the gene for uPA (PLAU) to late-onset AD (Serretti et al. 2007).
Acylpeptide Hydrolase
A second serine protease implicated in the degradation of Aβ is acylpeptide hydrolase (APH), a predominantly cytosolic enzyme that catalyzes the hydrolysis of amino-terminally acetylated amino acids from small peptides (Table 1; Yamin et al. 2007). Intriguingly, APH has been reported to show a preference for degrading naturally secreted Aβ dimers and trimers (Yamin et al. 2009).
Myelin Basic Protein
In rather remarkable discovery, myelin basic protein (MBP), which is known to possesses endogenous serine protease activity, was recently identified as a bona fide AβDP (Liao et al. 2009). As is true for plasmin and APH, MBP can degrade both monomeric and fibrillar forms of Aβ (Table 1; Liao et al. 2009).
Cysteine Proteases
Cathepsin B
Cysteine proteases were initially implicated in Aβ degradation by in vivo pharmacological studies (Frautschy et al. 1998). However, only one cysteine protease, cathepsin B (CatB), has so far been specifically implicated in the degradation of Aβ in vivo (Mueller-Steiner et al. 2006). Interestingly, CatB is predominantly present within the endolysosomal protein degradation pathway (Mort and Buttle 1997), which is known to degrade Aβ and which is compromised in AD (Glabe 2001). CatB is secreted by exocytosis in certain pathological conditions (Mort and Buttle 1997) and has also been found to be present within extracellular amyloid plaques in AD (Mueller-Steiner et al. 2006). However, it is unclear whether CatB is operative in these compartments, because it exhibits optimal activity at pH 5–6 (Koga et al. 1991). Unlike most other known AβDPs, CatB is an endoprotease (Mort and Buttle 1997), but it is unusual for also having dipeptidyl carboxypeptidase activity (Mueller-Steiner et al. 2006).
Aspartyl Proteases
Cathepsin D
A second lysosomal protease implicated in Aβ degradation is the aspartyl protease, cathepsin D (CatD) (Leissring and Saido 2007). This role for CatD was initially discovered from analysis of brain homogenates, where it was shown to be the principal protease responsible for Aβ degradation at acidic pH (Hamazaki 1996; McDermott and Gibson 1996). Confirming its physiological relevance, CatD KO mice were recently found to have significant elevations in steady-state endogenous brain Aβ (Leissring et al. 2009). Consistent with its high activity in brain homogenates, deletion of CatD resulted in cerebral brain Aβ42 levels threefold higher than those in wild-type littermates, the largest increase observed in any AβDP KO mouse model (Table 1). Intriguingly, Aβ40 levels were unaffected in these mice, resulting in increases in the Aβ42/40 ratio that are comparable to those induced by presenilin mutations (Leissring et al. 2009). Consistent with an effect on the critical Aβ42/40 ratio, deletion of CatD, unlike that of any other known AβDP, accelerates the onset of plaque formation. In the TgCRND8 APP transgenic mice, which normally develop amyloid plaques beginning at 3 months of age, deletion of CatD elicits plaque formation by just 3 weeks of age (MA Leissring, unpubl.). The differential increase in Aβ42 seen in the CatD KO mice has an intriguing mechanistic basis. Unlike CatB, CatD does not convert Aβ42 to Aβ40. Rather, CatD degrades Aβ42 and Aβ40 in a highly differential manner, with the affinity for Aβ42 and Aβ40 CatD being in the low nanomolar and low micromolar range, respectively, a factor that may drive preferential degradation of Aβ42 at low concentrations. At the same time, the turnover rate of Aβ42 is very slow, around 100-fold lower than that of Aβ40. Quite interestingly, the strong affinity of Aβ42 together with its slow turnover rate render Aβ42 a potent competitive inhibitor of CatD, even at relatively low (midnanomolar) concentrations (Leissring et al. 2009). Together with accumulating human molecular genetic evidence linking CatD to late-onset AD (Bertram et al. 2007), these findings suggest that CatD is a physiological and pathological regulator of Aβ, and they further suggest that CatD might be a downstream target of Aβ42 itself.
BACE1
Ironically, the major protease implicated in β-secretase activity, β-site APP cleaving enzyme 1 (BACE1; a.k.a. memapsin 2), is also capable of directly degrading Aβ (Fluhrer et al. 2003). Given BACE1’s key role in Aβ production, the physiological relevance of this finding is difficult to assess but may explain the finding that transgenic overexpression of very high levels of BACE1 paradoxically resulted in reduced Aβ deposition in vivo (Lee et al. 2005).
BACE2
BACE2, a close homolog of BACE1, also avidly degrades Aβ in vitro, exhibiting a catalytic efficiency that is around 50-fold greater than BACE1 (Abdul-Hay and Leissring 2011), higher in fact than the published values for any other known AβDP. Nevertheless, BACE2 KO mice show no net elevation in endogenous cerebral Aβ levels (Table 1; MA Leissring and SO Abdul-Hay, unpubl.). This is likely because BACE2 is expressed in astrocytes and other glia but not in neurons, which carry out the majority of Aβ production (Dominguez et al. 2005). Although these results suggest that BACE2 is not a physiologic regulator of Aβ, BACE2 might play some role in a pathological context because adult astrocytes are known to avidly degrade Aβ (Wyss-Coray et al. 2003).
The Proteasome
Aβ is also degraded by the proteasome (a.k.a., multicatalytic proteinase) by as-yet undetermined catalytic subunits (Lopez Salon et al. 2003). The proteasome is localized to the cytosol (Table 1) and, given that Aβ is produced in lumenal compartments, might therefore be assumed to play no physiologic role in Aβ degradation. However, some experimental evidence suggests that Aβ42 can diffuse passively from the lumen of the ER into the cytosol, where it is degraded jointly by the proteasome and IDE (Fig. 3; Schmitz et al. 2004). These and other findings—including evidence that Aβ accumulates within other intracellular organelles such as mitochondria (Yan et al. 2006b)—suggest that ill-defined pools of Aβ may exist that are degraded by certain AβDPs.
Aβ-Degrading Catalytic Antibodies
As is true for the secretases involved in Aβ production, the therapeutic targeting of AβDPs is complicated by the fact that each degrades multiple substrates besides Aβ. Catalytic antibodies have been suggested as an alternative that, by virtue of their higher specificity for particular antigenic targets, might improve the selectivity for Aβ. A surprisingly large number of Aβ-degrading immunoglobulins (Igs) and Ig-fragments have been discovered or engineered (Taguchi et al. 2008a). Although the catalytic efficiencies of most Aβ-degrading antibodies is currently orders of magnitude slower than AβDPs, the technology exists to engineer existing antibodies or select new ones with improved properties (Taguchi et al. 2008b). Interestingly, Aβ-degrading antibodies are present in the sera of normal subjects and, notably, are increased in AD patients (Paul et al. 2010). Such antibodies can be harvested and may have therapeutic potential (Taguchi et al. 2008a). The exciting potential of catalytic Aβ-degrading antibodies makes this a topic worthy of continued investigation.
ENDOGENOUS INHIBITORS OF Aβ DEGRADATION
Several endogenous protease inhibitors have also been implicated in the regulation of Aβ degradation. Certainly the most interesting example is the nonneuronal isoform of APP itself (APP751), which was in fact identified initially as a serine protease inhibitor (protease nexin II; Van Nostrand and Cunningham 1987; Van Nostrand et al. 1989) due to the presence of a Kunitz-type serine protease inhibitor (KPI) domain present in the longer APP isoforms (Ponte et al. 1988). The KPI domain inhibits Aβ degradation in cell culture by as-yet undetermined serine proteases (Naidu et al. 1995) and, intriguingly, transgenic mice overexpressing KPI-containing APP isoforms were found to have more severe amyloid pathology than mice expressing equivalent levels of APP lacking this domain (Higgins et al. 1993). It was later discovered that a second inhibitor domain exists within all isoforms of APP (Miyazaki et al. 1993), which has been mapped (to residues 579–601 of APP770; Higashi and Miyazaki 2003) and shown to potently and selectively inhibit MMP2 (Higashi and Miyazaki 2008). Another serine protease inhibitor, alpha-1 antichymotrypsin, which was identified as a constituent of amyloid plaques (Abraham et al. 1988), has also been shown to inhibit the degradation of Aβ in vitro and in vivo (Abraham et al. 2000). The cysteine protease inhibitor cystatin C, which has been genetically linked to late-onset AD (Bertram et al. 2007), also regulates Aβ degradation by inhibiting CatB (Sun et al. 2008), although other mechanisms may also contribute to cystatin C’s overall effect on amyloid plaque formation (Gauthier et al. 2011). Finally, as noted already, pharmacologic inhibitors of PAI1, which normally blocks that conversion of plasminogen to plasmin by tPA and uPA, have been shown to attenuate amyloid deposition in APP transgenic mice (Jacobsen et al. 2008).
THERAPEUTIC APPROACHES BASED ON Aβ DEGRADATION
One strategy for the treatment of chronically elevated Aβ levels in AD would be gene therapy using an AβDP. The introduction of NEP into the brains of APP transgenic mice using viral vectors has been shown to attenuate Aβ pathology, leading to improved cognitive function (Marr et al. 2003; Iwata et al. 2004; El-Amouri et al. 2008; Spencer et al. 2008). Although gene therapy for the treatment of Parkinson’s disease in humans has already gained substantial momentum (Feng and Maguire-Zeiss 2010), its application to AD has not been as prominent, presumably due to the difference in the size and extent of the affected brain regions. However, in the very early stage of disease development, introduction of the NEP gene into the entorhinal cortex, which leads to expression of NEP in the hippocampus (Iwata et al. 2004), might generate a useful therapeutic effect. On the other hand, a significant reduction in cerebral Aβ levels and plaque formation has been achieved by expression of NEP in transplanted astrocytes (Hemming et al. 2007a), suggesting that neurons do not necessarily need to be directly infected with AβDPs to be effective. Substantial advances in gene therapy technology are anticipated in the coming years. IDE, ECEs, MMPs, BACE2, or other AβDPs could also be used in a similar manner to NEP, whereas the plasmin system should be more cautiously considered due to potential adverse side effects caused by hemorrhages (Murray et al. 2011).
AβDPs can be targeted by pharmacological therapies as well. Compared to the approach of inhibiting Aβ production, the notion that drugs could be developed which chronically stimulate Aβ degradation would seem to be impractical. However, because many AβDPs are regulated at least in part by endogenous inhibitors, it is feasible to enhance Aβ degradation via drugs that disrupt protease–inhibitor interactions. Indeed, this approach has been pursued preclinically, as illustrated by the development of PAI1 inhibitors that effectively promote Aβ degradation by plasmin (Jacobsen et al. 2008). Pharmacologic enhancement of the expression of AβDPs is another potential strategy. For example, neuronal NEP activity has been shown to be controlled by a neuropeptide, somatostatin (Saito et al. 2005), likely involving the phosphorylation status of the cytoplasmic domain of NEP (Kakiya R, Saito T, and Saido T, unpubl.). It should be feasible to develop synthetic small-molecule agonists that stimulate NEP by activating somatostatin receptors, such as the type four receptor, which is present exclusively in brain. Finally, for certain AβDPs, it may be feasible to develop compounds that directly activate proteolytic degradation. Consistent with this, compound screening has identified drug-like molecules that increase Aβ degradation by IDE several-fold (Cabrol et al. 2009).
CONCLUSIONS
Perhaps the most fundamental question yet to be answered is why Aβ is deposited in sporadic AD, which accounts for >99% of AD cases. It should be noted that the number of sporadic AD patients will grow as the average life expectancy increases, whereas the number of early-onset familial AD patients should remain proportional to the total population. The hypothesis that Aβ accumulation results at least in part from an age-dependent decline of Aβ degradation provides a plausible mechanism that may account for a substantial portion of AD cases. Virtually all humans accumulate Aβ in the brain as they age (Funato et al. 1998; Morishima-Kawashima et al. 2000), suggesting that Aβ deposition may be an unavoidable consequence of aging which may in turn place fundamental limits on the health of the brain. Because the conversion of “normal aging” to AD via mild cognitive impairment appears to be a continuous process caused primarily by the gradual acceleration of Aβ accumulation, we may ultimately be able to implement pre-symptomatic interventions which include Aβ-reducing strategies utilizing degradation and clearance mechanisms to maintain lower Aβ levels during later life (Saito et al. 2003).
ACKNOWLEDGMENTS
This work is supported by grants from the American Health Assistance Foundation and the CART Fund (to M.A.L.).
Footnotes
Editors: Dennis J. Selkoe, Eckhard Mandelkow, and David M. Holtzman
Additional Perspectives on The Biology of Alzheimer Disease available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Abdul-Hay SO, Leissring MA 2011. Functional cDNA screening identifies BACE-2 as a principal beta-amyloid-degrading protease. In Alzheimer’s Association International Conference on Alzheimer’s Disease, Proposal No. 17630. AAIC, Paris [Google Scholar]
- Abdul-Hay SO, Kang D, McBride M, Li L, Zhao J, Leissring MA 2011. Deletion of insulin-degrading enzyme elicits antipodal, age-dependent effects on glucose and insulin tolerance. PLoS One 6: e20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham CR, Selkoe DJ, Potter H 1988. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer’s disease. Cell 52: 487–501 [DOI] [PubMed] [Google Scholar]
- Abraham CR, McGraw WT, Slot F, Yamin R 2000. Alpha 1-antichymotrypsin inhibits A beta degradation in vitro and in vivo. Ann NY Acad Sci 920: 245–248 [PubMed] [Google Scholar]
- Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM 2006. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med 12: 856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AB, Roth RA 1992. An unusual active site identified in a family of zinc metalloendopeptidases. Proc Natl Acad Sci 89: 3835–3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE 2007. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat Genet 39: 17–23 [DOI] [PubMed] [Google Scholar]
- Betts V, Leissring MA, Dolios G, Wang R, Selkoe DJ, Walsh DM 2008. Aggregation catabolism of disease-associated intra-Abeta mutations: Reduced proteolysis of Abeta A21G by neprilysin. Neurobiol Dis 31: 442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulloj A, Leal MC, Xu H, Castano EM, Morelli L 2010. Insulin-degrading enzyme sorting in exosomes: A secretory pathway for a key brain amyloid-beta degrading protease. J Alzheimers Dis 19: 79–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrol C, Huzarska MA, Dinolfo C, Rodriguez MC, Reinstatler L, Ni J, Yeh L-A, Cuny GD, Stein RL, Selkoe DJ, et al. 2009. Small-molecule activators of insulin-degrading enzyme discovered through high-throughput compound screening. PLoS One 4: e5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Oddo S, Sugarman MC, Akbari Y, LaFerla FM 2005. Age- and region-dependent alterations in Abeta-degrading enzymes: Implications for Abeta-induced disorders. Neurobiol Aging 26: 645–654 [DOI] [PubMed] [Google Scholar]
- Carpenter JE, Jackson W, de Souza GA, Haarr L, Grose C 2010. Insulin-degrading enzyme binds to the nonglycosylated precursor of varicella-zoster virus gE protein found in the endoplasmic reticulum. J Virol 84: 847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho KM, Franca MS, Camarao GC, Ruchon AF 1997. A new brain metalloendopeptidase which degrades the Alzheimer beta-amyloid 1–40 peptide producing soluble fragments without neurotoxic effects. Braz J Med Biol Res 30: 1153–1156 [DOI] [PubMed] [Google Scholar]
- Deb S, Gottschall PE 1996. Increased production of matrix metalloproteinases in enriched astrocyte and mixed hippocampal cultures treated with beta-amyloid peptides. J Neurochem 66: 1641–1647 [DOI] [PubMed] [Google Scholar]
- Dominguez D, Tournoy J, Hartmann D, Huth T, Cryns K, Deforce S, Serneels L, Camacho IE, Marjaux E, Craessaerts K, et al. 2005. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem 280: 30797–30806 [DOI] [PubMed] [Google Scholar]
- Eckman EA, Eckman CB 2005. Abeta-degrading enzymes: Modulators of Alzheimer’s disease pathogenesis and targets for therapeutic intervention. Biochem Soc Trans 33: 1101–1105 [DOI] [PubMed] [Google Scholar]
- Eckman EA, Reed DK, Eckman CB 2001. Degradation of the Alzheimer’s amyloid beta peptide by endothelin-converting enzyme. J Biol Chem 276: 24540–24548 [DOI] [PubMed] [Google Scholar]
- Eckman EA, Watson M, Marlow L, Sambamurti K, Eckman CB 2003. Alzheimer’s disease beta-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J Biol Chem 278: 2081–2084 [DOI] [PubMed] [Google Scholar]
- Eckman EA, Adams SK, Troendle FJ, Stodola BA, Kahn MA, Fauq AH, Xiao HD, Bernstein KE, Eckman CB 2006. Regulation of steady-state beta-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J Biol Chem 281: 30471–30478 [DOI] [PubMed] [Google Scholar]
- El-Amouri SS, Zhu H, Yu J, Marr R, Verma IM, Kindy MS 2008. Neprilysin: An enzyme candidate to slow the progression of Alzheimer’s disease. Am J Pathol 172: 1342–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkevall A, Alikhani N, Bhushan S, Pavlov PF, Busch K, Johnson KA, Eneqvist T, Tjernberg L, Ankarcrona M, Glaser E 2006. Degradation of the amyloid beta-protein by the novel mitochondrial peptidasome, PreP. J Biol Chem 281: 29096–29104 [DOI] [PubMed] [Google Scholar]
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S 2003. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci 100: 4162–4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris W, Leissring MA, Hemming ML, Chang AY, Selkoe DJ 2005. Alternative splicing of human insulin-degrading enzyme yields a novel isoform with a decreased ability to degrade insulin and amyloid beta-protein. Biochemistry 44: 6513–6525 [DOI] [PubMed] [Google Scholar]
- Farris W, Schutz SG, Cirrito JR, Shankar GM, Sun X, George A, Leissring MA, Walsh DM, Qiu WQ, Holtzman DM, et al. 2007. Loss of neprilysin function promotes amyloid plaque formation and causes cerebral amyloid angiopathy. Am J Pathol 171: 241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng LR, Maguire-Zeiss KA 2010. Gene therapy in Parkinson’s disease: Rationale and current status. CNS Drugs 24: 177–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhrer R, Multhaup G, Schlicksupp A, Okochi M, Takeda M, Lammich S, Willem M, Westmeyer G, Bode W, Walter J, et al. 2003. Identification of a beta-secretase activity, which truncates amyloid beta-peptide after its presenilin-dependent generation. J Biol Chem 278: 5531–5538 [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Horn DL, Sigel JJ, Harris-White ME, Mendoza JJ, Yang F, Saido TC, Cole GM 1998. Protease inhibitor coinfusion with amyloid beta-protein results in enhanced deposition and toxicity in rat brain. J Neurosci 18: 8311–8321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami S, Watanabe K, Iwata N, Haraoka J, Lu B, Gerard NP, Gerard C, Fraser P, Westaway D, St George-Hyslop P, et al. 2002. Abeta-degrading endopeptidase, neprilysin, in mouse brain: Synaptic and axonal localization inversely correlating with Abeta pathology. Neurosci Res 43: 39–56 [DOI] [PubMed] [Google Scholar]
- Funato H, Yoshimura M, Kusui K, Tamaoka A, Ishikawa K, Ohkoshi N, Namekata K, Okeda R, Ihara Y 1998. Quantitation of amyloid beta-protein (A beta) in the cortex during aging and in Alzheimer’s disease. Am J Pathol 152: 1633–1640 [PMC free article] [PubMed] [Google Scholar]
- Gauthier S, Kaur G, Mi W, Tizon B, Levy E 2011. Protective mechanisms by cystatin C in neurodegenerative diseases. Front Biosci (Schol Ed) 3: 541–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe C 2001. Intracellular mechanisms of amyloid accumulation and pathogenesis in Alzheimer’s disease. J Mol Neurosci 17: 137–145 [DOI] [PubMed] [Google Scholar]
- Haass C, Hung AY, Schlossmacher MG, Oltersdorf T, Teplow DB, Selkoe DJ 1993. Normal cellular processing of the beta-amyloid precursor protein results in the secretion of the amyloid beta peptide and related molecules. Ann NY Acad Sci 695: 109–116 [DOI] [PubMed] [Google Scholar]
- *.Haass C, Kaether C, Sisodia S, Thinakaran G 2011. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 10.1101/cshperspect.a006270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez D, Huang JY, Huynh AM, Valtierra S, Rockenstein E, Bruno AM, Lu B, DesGroseillers L, Masliah E, Marr RA 2011. Neprilysin-2 is an important beta-amyloid degrading enzyme. Am J Pathol 178: 306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazaki H 1996. Cathepsin D is involved in the clearance of Alzheimer’s beta-amyloid protein. FEBS Lett 396: 139–142 [DOI] [PubMed] [Google Scholar]
- Hamel FG, Mahoney MJ, Duckworth WC 1991. Degradation of intraendosomal insulin by insulin-degrading enzyme without acidification. Diabetes 40: 436–443 [DOI] [PubMed] [Google Scholar]
- Hemming ML, Patterson M, Reske-Nielsen C, Lin L, Isacson O, Selkoe DJ 2007a. Reducing amyloid plaque burden via ex vivo gene delivery of an Aβ-degrading protease: A novel therapeutic approach to Alzheimer disease. PLoS Med 4: e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming ML, Selkoe DJ, Farris W 2007b. Effects of prolonged angiotensin-converting enzyme inhibitor treatment on amyloid beta-protein metabolism in mouse models of Alzheimer disease. Neurobiol Dis 26: 273–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh LB 2006. The insulysin (insulin degrading enzyme) enigma. Cell Mol Life Sci 63: 2432–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh LB, Rodgers DW 2008. Neprilysin and amyloid beta peptide degradation. Curr Alzheimer Res 5: 225–231 [DOI] [PubMed] [Google Scholar]
- Higashi S, Miyazaki K 2003. Identification of a region of beta-amyloid precursor protein essential for its gelatinase A inhibitory activity. J Biol Chem 278: 14020–14028 [DOI] [PubMed] [Google Scholar]
- Higashi S, Miyazaki K 2008. Identification of amino acid residues of the matrix metalloproteinase-2 essential for its selective inhibition by beta-amyloid precursor protein-derived inhibitor. J Biol Chem 283: 10068–10078 [DOI] [PubMed] [Google Scholar]
- Higgins LS, Catalano R, Quon D, Cordell B 1993. Transgenic mice expressing human β-APP751, but not mice expressing β-APP695, display early Alzheimer’s disease-like histopathology. Ann NY Acad Sci 695: 224–227 [DOI] [PubMed] [Google Scholar]
- Howell S, Nalbantoglu J, Crine P 1995. Neutral endopeptidase can hydrolyze β-amyloid(1–40) but shows no effect on β-amyloid precursor protein metabolism. Peptides 16: 647–652 [DOI] [PubMed] [Google Scholar]
- Hu J, Igarashi A, Kamata M, Nakagawa H 2001. Angiotensin-converting enzyme degrades Alzheimer amyloid β-peptide (Aβ); retards Aβ aggregation, deposition, fibril formation; and inhibits cytotoxicity. J Biol Chem 276: 47863–47868 [DOI] [PubMed] [Google Scholar]
- Huang SM, Mouri A, Kokubo H, Nakajima R, Suemoto T, Higuchi M, Staufenbiel M, Noda Y, Yamaguchi H, Nabeshima T, et al. 2006. Neprilysin-sensitive synapse-associated amyloid-β peptide oligomers impair neuronal plasticity and cognitive function. J Biol Chem 281: 17941–17951 [DOI] [PubMed] [Google Scholar]
- Huang JY, Bruno AM, Patel CA, Huynh AM, Philibert KD, Glucksman MJ, Marr RA 2008. Human membrane metallo-endopeptidase-like protein degrades both beta-amyloid 42 and beta-amyloid 40. Neuroscience 155: 258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, et al. 2000. Identification of the major Abeta1–42-degrading catabolic pathway in brain parenchyma: Suppression leads to biochemical and pathological deposition. Nat Med 6: 143–150 [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC 2001. Metabolic regulation of brain Abeta by neprilysin. Science 292: 1550–1552 [DOI] [PubMed] [Google Scholar]
- Iwata N, Mizukami H, Shirotani K, Takaki Y, Muramatsu S, Lu B, Gerard NP, Gerard C, Ozawa K, Saido TC 2004. Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-beta peptide in mouse brain. J Neurosci 24: 991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JS, Comery TA, Martone RL, Elokdah H, Crandall DL, Oganesian A, Aschmies S, Kirksey Y, Gonzales C, Xu J, et al. 2008. Enhanced clearance of Abeta in brain by sustaining the plasmin proteolysis cascade. Proc Natl Acad Sci 105: 8754–8759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemitsu H, Tomiyama T, Mori H 2003. Human neprilysin is capable of degrading amyloid beta peptide not only in the monomeric form but also the pathological oligomeric form. Neurosci Lett 350: 113–116 [DOI] [PubMed] [Google Scholar]
- Koga H, Yamada H, Nishimura Y, Kato K, Imoto T 1991. Multiple proteolytic action of rat liver cathepsin B: Specificities and pH-dependences of the endo- and exopeptidase activities. J Biochem 110: 179–188 [DOI] [PubMed] [Google Scholar]
- Kuo WL, Gehm BD, Rosner MR, Li W, Keller G 1994. Inducible expression and cellular localization of insulin-degrading enzyme in a stably transfected cell line. J Biol Chem 269: 22599–22606 [PubMed] [Google Scholar]
- Lee EB, Zhang B, Liu K, Greenbaum EA, Doms RW, Trojanowski JQ, Lee VM 2005. BACE overexpression alters the subcellular processing of APP and inhibits Abeta deposition in vivo. J Cell Biol 168: 291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring MA 2008. The AβCs of Aβ-cleaving proteases. J Biol Chem 283: 29645–29649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring MA, Saido TC 2007. Aβ degradation. In Alzheimer’s disease: Advances in genetics, molecular and cellular biology (ed. Sisodia S, Tanzi R), pp. 157–178 Springer, New York [Google Scholar]
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ 2003. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40: 1087–1093 [DOI] [PubMed] [Google Scholar]
- Leissring MA, Farris W, Wu X, Christodoulou DC, Haigis MC, Guarente L, Selkoe DJ 2004. Alternative translation initiation generates a novel isoform of insulin-degrading enzyme targeted to mitochondria. Biochem J 383: 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring MA, Reinstatler L, Sahara T, Roman R, Sevlever D, Saftig P, Levites Y, Golde TE, Burgess JD, Ertekin-Taner N, et al. 2009. Cathepsin D selectively degrades Aβ42 and tau: Implications for Alzheimer disease pathogenesis. In Society for Neuroscience, Program No. 139108 Society for Neuroscience, Chicago [Google Scholar]
- Leissring MA, Malito E, Hedouin S, Reinstatler L, Sahara T, Abdul-Hay SO, Choudhry S, Maharvi GM, Fauq AH, Huzarska M, May PS, Choi S, et al. 2010. Designed inhibitors of insulin-degrading enzyme regulate the catabolism and activity of insulin. PLoS One 5: e10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao MC, Van Nostrand WE 2010. Degradation of soluble and fibrillar amyloid β-protein by matrix metalloproteinase (MT1-MMP) in vitro. Biochemistry 49: 1127–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao MC, Ahmed M, Smith SO, Van Nostrand WE 2009. Degradation of amyloid β protein by purified myelin basic protein. J Biol Chem 284: 28917–28925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Studzinski C, Beckett T, Guan H, Hersh MA, Murphy MP, Klein R, Hersh LB 2009. Expression of neprilysin in skeletal muscle reduces amyloid burden in a transgenic mouse model of Alzheimer disease. Mol Ther 17: 1381–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Salon M, Pasquini L, Besio Moreno M, Pasquini JM, Soto E 2003. Relationship between beta-amyloid degradation and the 26S proteasome in neural cells. Exp Neurol 180: 131–143 [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA 2004. Gene regulation and DNA damage in the ageing human brain. Nature 429: 883–891 [DOI] [PubMed] [Google Scholar]
- Marr RA, Rockenstein E, Mukherjee A, Kindy MS, Hersh LB, Gage FH, Verma IM, Masliah E 2003. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci 23: 1992–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ 2010. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JR, Gibson AM 1996. Degradation of Alzheimer’s beta-amyloid protein by human cathepsin D. Neuroreport 7: 2163–2166 [DOI] [PubMed] [Google Scholar]
- Meilandt WJ, Cisse M, Ho K, Wu T, Esposito LA, Scearce-Levie K, Cheng IH, Yu GQ, Mucke L 2009. Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J Neurosci 29: 1977–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Hasegawa M, Funahashi K, Umeda M 1993. A metalloproteinase inhibitor domain in Alzheimer amyloid protein precursor. Nature 362: 839–841 [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Oshima N, Ogata H, Yamaguchi H, Yoshimura M, Sugihara S, Ihara Y 2000. Effect of apolipoprotein E allele ε4 on the initial phase of amyloid β-protein accumulation in the human brain. Am J Pathol 157: 2093–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort JS, Buttle DJ 1997. Cathepsin B. Int J Biochem Cell Biol 29: 715–720 [DOI] [PubMed] [Google Scholar]
- Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, Yu G, Esposito L, Mucke L, et al. 2006. Antiamyloidogenic and neuroprotective functions of cathepsin B: Implications for Alzheimer’s disease. Neuron 51: 703–714 [DOI] [PubMed] [Google Scholar]
- Murray IV, Proza JF, Sohrabji F, Lawler JM 2011. Vascular metabolic dysfunction in Alzheimer’s disease: A review. Exp Biol Med 236: 772–782 [DOI] [PubMed] [Google Scholar]
- Myohanen H, Vaheri A 2004. Regulation and interactions in the activation of cell-associated plasminogen. Cell Mol Life Sci 61: 2840–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu A, Quon D, Cordell B 1995. Beta-amyloid peptide produced in vitro is degraded by proteinases released by cultured cells. J Biol Chem 270: 1369–1374 [DOI] [PubMed] [Google Scholar]
- Neant-Fery M, Garcia-Ordonez RD, Logan TP, Selkoe DJ, Li L, Reinstatler L, Leissring MA 2008. Molecular basis for the thiol sensitivity of insulin-degrading enzyme. Proc Natl Acad Sci 105: 9582–9587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Planque S, Nishiyama Y 2010. Immunological origin and functional properties of catalytic autoantibodies to amyloid beta peptide. J Clin Immunol 30 (Suppl 1): S43–S49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P, Gonzalez-DeWhitt P, Schilling J, Miller J, Hsu D, Greenberg B, Davis K, Wallace W, Lieberburg I, Fuller F 1988. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature 331: 525–527 [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ 1998. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem 273: 32730–32738 [DOI] [PubMed] [Google Scholar]
- Roques BP, Noble F, Dauge V, Fournie-Zaluski MC, Beaumont A 1993. Neutral endopeptidase 24.11: Structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev 45: 87–146 [PubMed] [Google Scholar]
- Rose JB, Crews L, Rockenstein E, Adame A, Mante M, Hersh LB, Gage FH, Spencer B, Potkar R, Marr RA, Masliah E 2009. Neuropeptide Y fragments derived from neprilysin processing are neuroprotective in a transgenic model of Alzheimer’s disease. J Neurosci 29: 1115–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saido TC, Iwata N 2006. Metabolism of amyloid β peptide and pathogenesis of Alzheimer’s disease. Towards presymptomatic diagnosis, prevention and therapy. Neurosci Res 54: 235–253 [DOI] [PubMed] [Google Scholar]
- Saito T, Iwata N, Tsubuki S, Takaki Y, Takano J, Huang SM, Suemoto T, Higuchi M, Saido TC 2005. Somatostatin regulates brain amyloid β-peptide Aβ42 through modulation of proteolytic degradation. Nat Med 11: 434–439 [DOI] [PubMed] [Google Scholar]
- Saito T, Takaki Y, Iwata N, Trojanowski J, Saido TC 2006. Alzheimer’s disease, neuropeptides, neuropeptidase, amyloid-beta peptide metabolism. Sci Aging Knowledge Environ 2003: PE1. [DOI] [PubMed] [Google Scholar]
- Saria A, Hauser KF, Traurig HH, Turbek CS, Hersh L, Gerard C 1997. Opioid-related changes in nociceptive threshold and in tissue levels of enkephalins after target disruption of the gene for neutral endopeptidase (EC 3.4.24.11) in mice. Neurosci Lett 234: 27–30 [DOI] [PubMed] [Google Scholar]
- Schmitz A, Schneider A, Kummer MP, Herzog V 2004. Endoplasmic reticulum-localized amyloid beta-peptide is degraded in the cytosol by two distinct degradation pathways. Traffic 5: 89–101 [DOI] [PubMed] [Google Scholar]
- Selkoe D 2001. Clearing the brain’s amyloid cobwebs. Neuron 32: 177–180 [DOI] [PubMed] [Google Scholar]
- Serretti A, Olgiati P, De Ronchi D 2007. Genetics of Alzheimer’s disease. A rapidly evolving field. J Alzheimers Dis 12: 73–92 [DOI] [PubMed] [Google Scholar]
- Shen Y, Joachimiak A, Rosner MR, Tang W-J 2006. Structures of human insulin-degrading enzyme reveal a new substrate mechanism. Nature 443: 870–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinall H, Song ES, Hersh LB 2005. Susceptibility of amyloid beta peptide degrading enzymes to oxidative damage: A potential Alzheimer’s disease spiral. Biochemistry 44: 15345–15350 [DOI] [PubMed] [Google Scholar]
- Shirotani K, Tsubuki S, Iwata N, Takaki Y, Harigaya W, Maruyama K, Kiryu-Seo S, Kiyama H, Iwata H, Tomita T, et al. 2001. Neprilysin degrades both amyloid beta peptides 1–40 and 1–42 most rapidly and efficiently among thiorphan- and phosphoramidon-sensitive endopeptidases. J Biol Chem 276: 21895–21901 [DOI] [PubMed] [Google Scholar]
- Spencer B, Marr RA, Rockenstein E, Crews L, Adame A, Potkar R, Patrick C, Gage FH, Verma IM, Masliah E 2008. Long-term neprilysin gene transfer is associated with reduced levels of intracellular Abeta and behavioral improvement in APP transgenic mice. BMC Neurosci 9: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Zhou Y, Halabisky B, Lo I, Cho SH, Mueller-Steiner S, Devidze N, Wang X, Grubb A, Gan L 2008. Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer’s disease. Neuron 60: 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi H, Planque S, Nishiyama Y, Szabo P, Weksler ME, Friedland RP, Paul S 2008a. Catalytic antibodies to amyloid beta peptide in defense against Alzheimer disease. Autoimmun Rev 7: 391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi H, Planque S, Sapparapu G, Boivin S, Hara M, Nishiyama Y, Paul S 2008b. Exceptional amyloid beta peptide hydrolyzing activity of nonphysiological immunoglobulin variable domain scaffolds. J Biol Chem 283: 36724–36733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamboli IY, Barth E, Christian L, Siepmann M, Kumar S, Singh S, Tolksdorf K, Heneka MT, Lutjohann D, Wunderlich P, et al. 2010. Statins promote the degradation of extracellular amyloid β-peptide by microglia via stimulation of exosome-associated insulin-degrading enzyme (IDE) secretion. J Biol Chem 285: 37405–37414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Moir RD, Wagner SL 2004. Clearance of Alzheimer’s Abeta peptide: The many roads to perdition. Neuron 43: 605–608 [DOI] [PubMed] [Google Scholar]
- Tsubuki S, Takaki Y, Saido TC 2003. Dutch, Flemish, Italian, Arctic mutations of APP and resistance of Abeta to physiologically relevant proteolytic degradation. Lancet 361: 1957–1958 [DOI] [PubMed] [Google Scholar]
- Tucker HM, Kihiko-Ehmann M, Wright S, Rydel RE, Estus S 2000. Tissue plasminogen activator requires plasminogen to modulate amyloid-beta neurotoxicity and deposition. J Neurochem 75: 2172–2177 [DOI] [PubMed] [Google Scholar]
- Turner AJ 1998. Neprilysin. In Handbook of proteolytic enzymes (ed. Barret AJ, Rawlings ND, Woessner JF), pp. 1080–1085 Academic, San Diego [Google Scholar]
- Turner AJ 2004. Neprilysin. In Handbook of proteolytic enzymes (ed. Barret AJ, Rawlings ND, Woessner JF), pp. 419–426 Academic, London [Google Scholar]
- Turner AJ, Nalivaeva NN 2007. New insights into the roles of metalloproteinases in neurodegeneration and neuroprotection. Int Rev Neurobiol 82: 113–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AJ, Isaac RE, Coates D 2001. The neprilysin (NEP) family of zinc metalloendopeptidases: Genomics and function. Bioessays 23: 261–269 [DOI] [PubMed] [Google Scholar]
- Van Nostrand WE, Cunningham DD 1987. Purification of protease nexin II from human fibroblasts. J Biol Chem 262: 8508–8514 [PubMed] [Google Scholar]
- Van Nostrand WE, Porter M 1999. Plasmin cleavage of the amyloid beta-protein: Alteration of secondary structure and stimulation of tissue plasminogen activator activity. Biochemistry 38: 11570–11576 [DOI] [PubMed] [Google Scholar]
- Van Nostrand WE, Wagner SL, Suzuki M, Choi BH, Farrow JS, Geddes JW, Cotman CW, Cunningham DD 1989. Protease nexin-II, a potent antichymotrypsin, shows identity to amyloid beta-protein precursor. Nature 341: 546–549 [DOI] [PubMed] [Google Scholar]
- Van Wart HE, Birkedal-Hansen H 1990. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci 87: 5578–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekrellis K, Ye Z, Qiu WQ, Walsh D, Hartley D, Chesneau V, Rosner MR, Selkoe DJ 2000. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J Neurosci 20: 1657–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivel KS, Zhang X, Meckler X, Cheng H, Lee S, Gong P, Lopes KO, Chen Y, Iwata N, Yin KJ, et al. 2008. Evidence that CD147 modulation of beta-amyloid (Abeta) levels is mediated by extracellular degradation of secreted Abeta. J Biol Chem 283: 19489–19498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DS, Iwata N, Hama E, Saido TC, Dickson DW 2003. Oxidized neprilysin in aging and Alzheimer’s disease brains. Biochem Biophys Res Commun 310: 236–241 [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J 2003. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med 9: 453–457 [DOI] [PubMed] [Google Scholar]
- Yamin R, Bagchi S, Hildebrant R, Scaloni A, Widom RL, Abraham CR 2007. Acyl peptide hydrolase, a serine proteinase isolated from conditioned medium of neuroblastoma cells, degrades the amyloid-beta peptide. J Neurochem 100: 458–467 [DOI] [PubMed] [Google Scholar]
- Yamin R, Zhao C, O’Connor PB, McKee AC, Abraham CR 2009. Acyl peptide hydrolase degrades monomeric and oligomeric amyloid-beta peptide. Mol Neurodegener 4: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR, Xiao Q, Hsu FF, Turk JW, Xu J, et al. 2006a. Matrix metalloproteinase-9 degrades amyloid-β fibrils in vitro and compact plaques in situ. J Biol Chem 281: 24566–24574 [DOI] [PubMed] [Google Scholar]
- Yan SD, Xiong WC, Stern DM 2006b. Mitochondrial amyloid-β peptide: Pathogenesis or late-phase development? J Alzheimers Dis 9: 127–137 [DOI] [PubMed] [Google Scholar]
- Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X, Bateman R, Song H, Hsu FF, Turk J, et al. 2006. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J Neurosci 26: 10939–10948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Li L, Leissring MA 2009. Insulin-degrading enzyme is exported via an unconventional protein secretion pathway. Mol Neurodegener 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Lee HG, Perry G, Smith MA 2007. Alzheimer disease, the two-hit hypothesis: An update. Biochim Biophys Acta 1772: 494–502 [DOI] [PubMed] [Google Scholar]