Abstract
Despite intensive research, a treatment for diabetic patients that completely restores normoglycemia for an indefinite period of time remains elusive. Although islet transplantation temporarily confers normoglycemia to patients, the lack of a renewable source of insulin-producing β cells hampers the use of this treatment option. Although significant hurdles remain, recent advances in stem cell biology indicate that generation of fully matured β cells from uncommitted progenitor cells, including human embryonic stem cells and induced pluripotent stem cells derived from somatic cell populations, is becoming an achievable goal.
The lack of a renewable source of insulin-producing β cells hampers the use of islet transplantation to treat type 1 diabetes. But enormous advances have been made in generating such cells from human stem cell populations.
With the advent of improved immunosuppressive regimens, islet transplantation has become a feasible treatment option for diabetic patients (Shapiro et al. 2000; Posselt et al. 2010a,b). Unfortunately, the demand for cadaveric islets far outstrips the supply, thus posing a significant obstacle for the ever-increasing list of patients who would undoubtedly benefit from transplanted islets to restore physiological normoglycemia.
Over the last 15 years, stem cells that can differentiate into all cell types of the human body, including insulin-producing β cells, have been identified. Here, I will review the efforts undertaken to manipulate these cells to devise strategies that allow generation of a reliable and renewable supply of human β cells.
IDENTIFICATION AND ISOLATION OF EMBRYONIC STEM CELLS
Following fertilization, mammalian embryos undergo a series of cleavages to form the morula, a ball-like aggregate of cells. Further cell divisions convert the morula into the blastocyst, a cystlike structure with an inner cavity surrounded by cells. The cells at the outer edge of the blastocyst differentiate into the trophoblast layer that constitutes a large part of the placenta and sustains nutrient supply to the embryo. In contrast, the cells of the inner cell mass (ICM) located within the interior of the blastocyst remain pluripotent and give rise to extraembryonic tissue and all cell types of the embryo proper. Seminal work by Martin Evans and Matthew Kaufmann as well as Gail Martin in the early 1980s showed that mouse ICM cells could be isolated and cultured outside the body without losing their pluripotency (Evans and Kaufman 1981; Martin 1981). Because of their ability to mimic the differentiation capacities of ICM cells, the cultured cells were called embryonic stem cells (ESCs). The enormous potential of murine ESCs was shown in subsequent work when it was demonstrated that they could be reintroduced into host blastocysts to give rise to chimeric animals carrying cells derived both from the injected ESCs as well as the host ICM cells. Furthermore, breeding of chimeric animals resulted in offspring carrying only the genetic material of the cultured ESCs, thus indicating that ESCs can contribute to the germline of host animals (Robertson et al. 1986). In 1998, Thomson and colleagues were able to isolate embryonic stem cells (hESCs) from early human embryos (Thomson et al. 1998), thereby setting the stage for subsequent efforts to generate distinct cell types for cell replacement therapies in patients.
DIABETES MELLITUS AS A GOOD MODEL SYSTEM TO TEST THE APPLICABILITY FOR STEM CELL-BASED THERAPIES
Several aspects specific to diabetes suggest that patients suffering from the disease would be good candidates for cell replacement strategies. In type 1 diabetic (T1D) patients, β cells are the main target of an autoimmune attack that results in their destruction and elimination. As a result, T1D patients depend on exogenous insulin to regulate blood glucose, a taxing proposition as optimal control normally present in healthy individuals is hard to achieve. On the other hand, the loss of only one cell type provides a unique opportunity as only this cell type and not a whole organ has to be generated from stem cells. In addition, the critical function of insulin-producing cells is to release the hormone directly into the bloodstream, a function they can fulfill even when not placed into the original location of the pancreatic islet. Although interactions with other endocrine cell types in the pancreatic islet result in optimal regulation of glucose levels, results from islet transplantation studies show that transplantation of insulin-producing cells into several locations, including the liver through injection into the portal vein, allow for efficient and rapid release of insulin into the bloodstream. Therefore, and in contrast to diseases in which damaged cells need to be placed into the correct context of the affected organ, e.g., heart muscle cells or neurons within the brain, cell therapy approaches aimed at restoring normoglycemia in diabetic patients benefit from the fact that stem cell-derived β cells can be inserted in surgically convenient locations and not the pancreas.
STEM CELL TO β-CELL DIFFERENTIATION: THE EARLY YEARS
Over the last decade, numerous groups have tried to generate functional β cells from mammalian stem cell populations. Early efforts focused on mouse ESCs and culture conditions that would guide them toward neural cell types (Lumelsky et al. 2001). The pancreatic endocrine population and neural cells coexpress a large number of markers, suggesting that their function and perhaps mechanisms of differentiation share commonalities. However, a significant divergence, despite the overlapping gene expression, emerges when we consider the origin of these cells within the embryo. Pancreatic cells develop from the endodermal germ layer while neural crest cells are of ectodermal origin. Therefore, in hindsight, it was unlikely that exposing stem cells to conditions that promote the ectodermal lineage would allow them to adopt a “true” β-cell identity that possesses an obligate requirement of passing through the definitive endoderm stage. As a consequence of this strategy, the insulin-producing cells generated by Lumelsky and colleagues did not result in the formation of bona fide β cells but likely cells within the neuronal lineage. Although such cells stained positive for insulin, it was subsequently shown to be a result of uptake of insulin from the culture medium rather than owing to activation of robust insulin transcription (Rajagopal et al. 2003).
Subsequent studies took advantage of the fact that the transcriptional hierarchy of factors regulating β-cell identity during embryogenesis had been well documented, prompting a number of groups to test whether forced expression of such factors, including Pdx1, Pax4, and Nkx2-2, was sufficient to drive ESCs toward the β cell (Blyszczuk et al. 2003; Miyazaki et al. 2004; Shiroi et al. 2005). Although these experiments met with partial success, yielding cells with a measurable level of insulin expression, evidence demonstrating the equivalence of these cells to mature β cells residing in human islets remained lacking.
LESSONS LEARNED FROM EMBRYONIC PANCREAS DEVELOPMENT: MIMICKING ORGANOGENESIS IN CULTURE
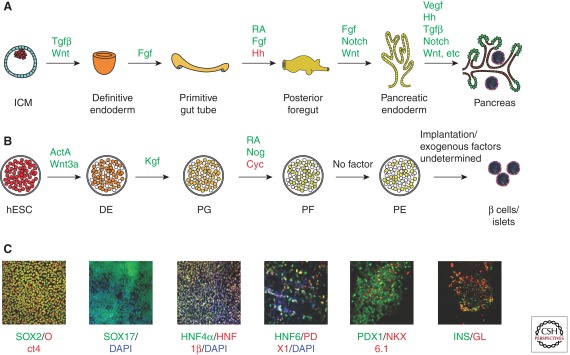
In 2005, the landscape of hESC differentiation into β cells was transformed by D’Amour and colleagues (2005), who insisted that fully matured β cells can only be generated when the culture conditions used replicate embryonic development as closely as possible (Fig. 1). Taking a stepwise approach, this group set out to first differentiate hESCs toward definitive endoderm, a prerequisite for all pancreatic cell types. The use of different model systems to understand early embryonic development had shown that nodal, a soluble molecule of the TGFβ/activin signaling family, was required for mesoderm and definitive endoderm formation during gastrulation, with higher levels of nodal promoting endoderm specification (Conlon et al. 1994; Osada and Wright 1999; Lowe et al. 2001; Vincent et al. 2003). Similarly, sound evidence had been gathered to indicate that Wnt signaling was required for appropriate germ layer formation during gastrulation (Haegel et al. 1995; Liu et al. 1999; Kelly et al. 2004). Based on these observations, D’Amour and colleagues implemented a protocol in which hESCs were exposed to distinct soluble signaling factors. Activin A, another member of the TGFβ signaling family, was used together with Wnt3a to generate cells expressing markers of definitive endoderm (D’Amour et al. 2005, 2006). Furthermore, a critical aspect of these culture conditions was the removal of serum components present in hESC medium that could induce the proliferative and renewal capacity of stem cells (McLean et al. 2007). The approach of using embryonic signals to instruct hESCs to definitive endoderm and subsequently to β cells was validated by several other groups using modifications of this procedure (Jiang et al. 2007; Eshpeter et al. 2008; Mao et al. 2009).
Figure 1.
Signaling pathways controlling embryonic pancreas development and hESC-β-cell differentiation. (A) Depiction of signaling pathways that guide differentiation of pancreatic cells from inner cell mass (ICM) cells present in the blastocyst stage. Pathways with positive activities at the indicated stages are shown in green. Hedgehog signaling (Hh) impairs pancreas organogenesis and is depicted in red during the transition from primitive gut to posterior foregut. (B) Signaling factors used to guide the differentiation from hESCs to pancreatic β cells. Note that while β cells form on transplantation of pancreatic endoderm cells into recipient mice, the factors required to promote full differentiation into functional β cells in cell culture have not been identified. (C) Immunofluorescence images showing the expression of markers for the stages shown in B. The efficiency of cell differentiation is reduced toward the later stages of the protocol with not all cells coexpressing NKX6.1 and PDX1. Also note that the majority of insulin-producing cells derived under cell culture conditions also express glucagon, indicating that they are not fully matured (modified from Guo and Hebrok 2009; reproduced, with permission, from the author).
During embryonic development, numerous signaling cues aid in specification of the definitive endoderm that is progressively segregated into cells carrying distinct differentiation potential along the anterior–posterior axis. Fibroblast growth factor signaling promotes the formation of endodermal cells slated to give rise to organs along the foregut, including the pancreas that forms at the fore-midgut border. Adding Fgf10 or Fgf7 to cultures of differentiating hESCs promotes the formation of foregut endodermal cells. Treatment with retinoic acid and inhibition of Hedgehog signaling further defines the foregut endoderm cells to assume a pancreatic fate.
Importantly, the differentiation state of hESCs can be monitored via expression of transcription factors that are expressed at distinct stages during normal embryonic pancreas and β-cell differentiation. For example, cells within the foregut anlage destined to give rise to the pancreas, termed the pancreas progenitors, express Pdx1, Foxa2, and Hnf6. Thus, by performing coimmunofluorescence analysis against a combination of proteins, one can ensure that hESC differentiation has proceeded as planned, thereby allowing for the generation of endocrine progenitor cells and eventually fully differentiated hormone-producing cells. The validity of this approach was shown when pancreas progenitor cells were transplanted into recipient immune-compromised mice. Somewhat surprisingly, considering that hESC-derived pancreas progenitors are transplanted into different, nonpancreatic host tissues, including the fat pad and under the kidney capsule, the in vivo conditions promote the maturation of the progenitor cells into single hormone-positive endocrine cells, including β cells, which produce and release insulin when stimulated with glucose. Even more impressively, these in vivo matured cells are capable of restoring normoglycemia in streptozotocin-treated, diabetic mice and secreted insulin at concentrations similar to what was observed in control mice transplanted with human islets (Kroon et al. 2008). Streptozotocin preferentially depletes murine β cells as it is mainly taken up into cells via the Glut2 glucose transporter expressed in mouse insulin-producing cells. In contrast, human β cells express GLUT1 as the main glucose transporter and consequently are less susceptible to streptozotocin (Eizirik et al. 1994; Hosokawa et al. 2001; Yang and Wright 2002). This experimental “trick” is quite effective as it generates a scenario in which hESC-derived β cells can be evaluated in animals for their ability to regulate glucose levels under physiological conditions as well as under stress, e.g., during a glucose challenge. Importantly, removal of the human cell transplant in streptozotocin-treated mice results in rapid appearance of hyperglycemia and diabetes, strongly supporting the argument that normoglycemia in these animals was regulated by the hESC-derived β cells (Kroon et al. 2008).
SHORTCOMINGS OF CURRENT DIFFERENTIATION PROTOCOLS
Impressive as they are, the current protocols to generate hESC-derived β cells do suffer from several shortcomings. Most notably, the differentiation of fully mature and functional β cells under cell culture conditions has proven elusive (Fig. 1). Implementing current protocols that rely heavily on principles of embryonic development has yielded endocrine cells that appear immature as the vast majority coexpress more than one hormone, e.g., glucagon and insulin. Considering the appearance of functional β cells on transplantation in host animals, it is likely that critical signals present in the in vivo environment are missing in cell culture. For example, it is well known that surrounding tissues including pancreatic mesenchyme and innervating neural cells regulate aspects of pancreas organogenesis (Golosow and Grobstein 1962; Nekrep et al. 2008; Landsman et al. 2011). Therefore, the success achieved with generating essentially pure definitive endoderm populations from hESCs in the absence of cells derived from the mesoderm and ectoderm germ layers might prove detrimental for later stages of pancreas endocrine differentiation. However, the fact that hESC-derived pancreas progenitors can develop into functional β cells on transplantation shows their ability to respond to appropriate cues that promote full differentiation and maturation. Identifying and supplementing these signals to cell culture protocols should allow better replication of the in vivo process.
We must also consider interactions that exist between the developing endocrine and supporting cells during normal islet formation. Pioneering work in Xenopus and mouse established the requirement of endothelial cells in guiding pancreas formation and endocrine and β-cell specification (Lammert et al. 2001; Yoshitomi and Zaret 2004; Nikolova et al. 2006). More recently, Katsumoto and Kume (2011) extended these findings by providing evidence that angioblasts located within the lateral plate mesenchyme are recruited to the endodermal layer to induce formation of Pdx1-positive pancreas progenitors at the onset of organ formation. In the mature organ, a close interaction between pancreatic endocrine cells and endothelial cells is a prerequisite to guarantee efficient release of secreted hormones into the bloodstream. Not surprisingly, hESC-derived pancreas progenitor cells are supported by ingrowing blood vessels on transplantation. Currently, such supporting signals are missing in the in vitro cultures. Supplementing endothelial-derived signals or coculturing hESCs with endothelial cells at specific stages of differentiation during the culture period should be explored to optimize conditions.
Summarily, although current protocols that allow efficient formation of pancreatic progenitors are a great improvement over the initial attempts at generating β cells, critical signals that promote the final stages of differentiation are still missing.
VARIATIONS/MODIFICATIONS OF HESC DIFFERENTIATION PROTOCOLS
Most of the protocols described above rely on two-dimensional culture techniques in which cells are grown as monolayers throughout the differentiation process. However, other groups have used three-dimensional (3D) methods in which ESCs are first aggregated to form embryoid bodies (EB) that have the potential to give rise to cells of all three germ layers, including the endoderm that forms the pancreas (Ku et al. 2004; Kubo et al. 2004). Modifications of the EB culture protocol include separation of cells followed by fluorescence-activated cell sorting (FACS) and reaggregation (Gadue et al. 2006; Gouon-Evans et al. 2006), but the important difference when compared to monolayer cultures is the ability of differentiating cells to interact in a 3D space in EB cultures. Thus, signals provided through cell–cell interactions or via interactions with extracellular matrices are likely more prominent under these conditions. Extracellular interactions have been shown to optimize β-cell functions and proliferation (Hammar et al. 2005; Weber et al. 2008; Parnaud et al. 2009). In addition, 3D culture conditions are likely to strengthen interactions between β cells and could enhance insulin secretory dynamics that may be directly affected by electrical coupling between insulin-producing cells (Speier et al. 2007). A direct comparison of the two-dimensional (2D) monolayer culture with the EB culture was recently performed that revealed accelerated induction of mesendodermal cells in the monolayer cultures compared to the EB approach; however, modifications of the culture conditions resulted in comparable differentiation efficiencies under 2D and EB conditions (Nostro et al. 2011). Future experiments will need to be performed to determine whether 2D versus EB/3D conditions have intrinsic benefits in generating fully matured β cells from human stem cell populations. An important part of these studies will be efforts to control the size of endocrine clusters to optimize culture conditions as recently reported in a collaborative study between stem cell biologists and bioengineers that showed benefits of differentiating hESCs on microcontact printed laminin patches (Van Hoof et al. 2011).
Many of the currently employed differentiation protocols rely on the sequential activation or inhibition of embryonic signaling pathways through treatment with their respective ligands. Alternative approaches have been undertaken to identify biologically active small chemical compounds that can functionally mimic cellular signaling molecules. Mouse ES cells (mESCs) expressing a reporter driven by the Sox17 promoter were used to assess the activity of compounds to differentiate toward the definitive endoderm lineage in the absence of activin A (Borowiak et al. 2009). Two compounds, termed IDE1 and IDE2, were identified under these conditions, and their endoderm-inducing activity was confirmed in hESC cells as well. Molecularly, both compounds phosphorylate Smad2, a critical mediator of activin signaling, and their activity was blocked by the activin receptorlike kinase (ALK) inhibitor SB43125. Endoderm produced from mESCs via IDE1 or IDE2 treatment did incorporate into the developing gut tube of early mouse embryos on transplantation, providing additional evidence for the normal differentiation capacity of these cells. In addition, subsequent treatment of IDE-treated cells with Indolactam V, another small chemical compound that had been shown previously to induce the formation of PDX1+ cells from hESCs (Chen et al. 2009), promoted progression toward the pancreas lineage. Thus, sequential addition of small chemicals is sufficient to direct ESC differentiation toward pancreas and can substitute for endogenous ligands of signaling pathways. Importantly, similar to the endogenous ligands, the chemical compounds also work at specific stages of the process by activating specific signaling pathways critical for each stage.
REPLACING EMBRYONIC ESCs WITH INDUCED PLURIPOTENT STEM CELLS: THE SOMATIC STEM CELL SOURCE
The stem cell field has been taken by storm by the demonstration that the mature state of somatic cells, including human cells, is not permanent but can be reversed toward a progenitor state almost identical to that of ESCs (Takahashi and Yamanaka 2006). Ectopic expression of combinations of only four transcription factors, including cMyc, Oct4, Sox2, Klf4 or cMyc, Oct4, Nanog, and Lin28, appeared to do the trick; human-induced pluripotent stem cells (hiPSCs) emerged on the stem cell horizon (Takahashi et al. 2007; Yu et al. 2007). Since then many modifications of the original protocol have been described, most notably efforts to supplement transcription factors that have oncogenic potential either with chemical compounds (Huangfu et al. 2008), the replacement of viruses for infection with stable proteins (Zhou et al. 2009), or the induction of reprogramming with synthetic, modified messenger RNAs (mRNAs) (Warren et al. 2010).
The impact of the induction of pluripotency in somatic cells is hard to overestimate. Not only do these findings circumvent ethical considerations related to the destruction of human embryos for the generation of stem cell lines, they also raise the hope of generating patient-specific stem cells. In other words, by further optimizing the existing protocols to efficiently generate hiPSCs without viral infection or the use of oncogenic factors, it will be possible to generate stem cell lines from patients suffering from a variety of illnesses, including diabetes, which can be redifferentiated toward desired cell types for retransplantation. Considering that the retransplanted cells have the same genetic makeup, including expression of major histocompatibility complex (MHC) molecules, such an approach carries the potential to alleviate host versus graft rejection. In proof-of-principle experiments, hiPSCs have been generated from diabetic patients and differentiated toward the pancreas lineage (Maehr et al. 2009), thus validating the feasibility of such an approach for potential future treatments. However, although promising, the current methods to generate hiPSCs remain inefficient and expensive, thus hindering the generation of large numbers of patient-specific lines. In addition, recent results indicate that hiPSCs generated from specific cell types retain transcriptional memory of their origin (Ohi et al. 2011). Finally, as has been observed for hESC lines, hiPSC lines display different capacities toward differentiation into specific germ layers, e.g., endoderm versus mesoderm or ectoderm. This may be in part owing to the methods used to generate the lines, but could also reflect intrinsic differences between donors. Although hiPSC technology carries tremendous promise to reshape human cell therapy, additional efforts are required to develop and standardize efficient, cheap, and safe protocols for hiPSC derivation and differentiation.
FATE PLASTICITY WITHIN THE PANCREAS: THE ROLE OF STEM CELLS IN THE DIFFERENTIATED ORGAN
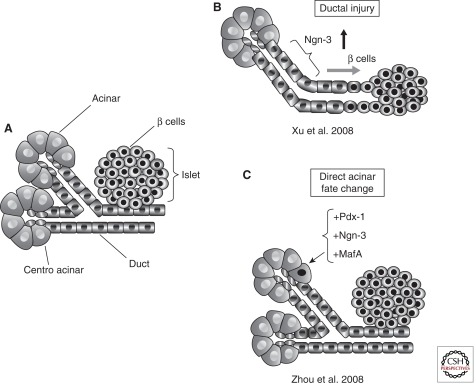
In addition to studies using hESCs and hiPSCs, recent work has shown that nonendocrine cells within the pancreas can transdifferentiate or be reprogrammed toward a β-cell fate, thus revealing remarkable plasticity of adult pancreatic cells (Fig. 2). Introduction of viruses coding for three key transcription factors required during the embryonic formation of pancreas (Pdx1), endocrine (Ngn3), and β cells (MafA) converted mouse enzyme-producing acinar cells into cells displaying key characteristics of β cells (Zhou et al. 2008). The absence of progenitor marker expression during the process of transformation in this study was taken as evidence that the underlying process is one of direct reprogramming, rather than a dedifferentiation event. In contrast, acinar cells are believed to dedifferentiate into a progenitorlike state when pancreatic injury is induced by treatment with caerulein, a cholecystokinin analog that leads to precocious release of digestive enzymes from acinar cells and thus mimics human pancreatitis. On caerulein treatment, acinar cells transiently express Sox9, Foxa2, Pdx1, and Hes1 among others, markers that are abundant during pancreas development but restricted to distinct nonacinar cell types in the adult organ (Jensen et al. 2005; Morris et al. 2010). Intriguingly, dedifferentiated cells also express markers of mature ducts, including CK19. In the absence of sustained injury, dedifferentiated cells regenerate to fully functional acinar cells with normal morphology and marker expression. Although the dedifferentiation phenomenon is transient, these findings show that on injury acinar cells temporarily adopt properties of a progenitorlike cell type normally absent in mature pancreas tissue. The finding that activation of oncogenic Kras signaling during pancreas injury reverts dedifferentiated cells away from the acinar lineage toward neoplastic transformation indicates that the progenitorlike cells can permanently transdifferentiate (Morris et al. 2010).
Figure 2.
Plasticity of adult pancreatic epithelial cells. (A) The adult mammalian pancreas harbors a number of specialized cell types, including the enzyme-producing acinar cells, centroacinar and connecting duct cells, as well as endocrine cells located within the islets of Langerhans. (B) On injury, e.g., duct ligation, duct or duct-associated cells can reactivate Ngn3 and transdifferentiate toward a β-cell state. (C) Acinar cells are reprogrammed toward a β-cell fate on ectopic expression of a set of three transcription factors, Pdx1, Ngn3, and MafA. (Modified from Puri and Hebrok 2010; reproduced, with permission, from the author.)
Evidence for facultative stem cells with endocrine differentiation potential in the mature pancreas comes from studies in which pancreatic duct ligation results in the appearance of Ngn3-positive endocrine and islet cells (Fig. 2) (Inada et al. 2008; Xu et al. 2008; Li et al. 2010). The exact location of the progenitor cells remains unclear as more recent experiments failed to confirm a location within the pancreatic ductal tree (Solar et al. 2009). Considering the above described transient dedifferentiation of acinar cells toward progenitorlike cells with some ductal characteristics, it will be interesting to test whether acinar cells can give rise to facultative stem cells with endocrine differentiation capabilities. Although exploratory at the moment, further experiments could conceivably promote acinar to β-cell transdifferentiation, an intriguing possibility that would make use of the vast majority of cells currently discarded from donor pancreata prepared for islet isolation.
CONCERNS SURROUNDING STEM CELL THERAPY
Although the use of human stem cells for the generation of pancreatic β cells, and thus the treatment of a large population of T1D and T2D patients, is an exciting prospect, numerous hurdles need to be overcome to ensure the efficiency of the differentiation process and the safety of the final product. For example, undifferentiated ESCs form teratomas on transplantation into immunocompromised animals, which have been observed on transplantation of hESC-derived pancreas progenitors cultured for ∼12 d in vitro (Kroon et al. 2008). Interestingly, when hESCs were differentiated under similar conditions for an extended period of time (20 d) (Jiang et al. 2007), no teratoma formation was observed in host animals, suggesting a greater degree of differentiated cells and loss for neoplastic transformation. Thus, the risk for teratoma formation can be reduced either through elimination of undifferentiated cells via purification methods or through efficient promotion of differentiation.
Another critical aspect centers on the complement of different cell types present at the final differentiation stage. For example, transplantation of pancreas progenitor cells also results in the development of exocrine pancreas structures, e.g., acinar and duct cells, albeit at much reduced frequency compared to endocrine cells (Kroon et al. 2008). In addition, other endocrine cell types, including glucagon-producing α cells, are likely to be generated as well. Although the entire complement of endocrine cell types similar to that found in human islets is likely beneficial for full function of these structures, the presence of exocrine acinar cells that might release enzymes in an uncontrolled manner is worrisome. Furthermore, as described above, acinar cells can transiently or permanently differentiate into cells with progenitorlike activity under conditions of injury or inflammation, and even develop into neoplastic lesions in the presence of oncogenic mutations (Morris et al. 2010). Although the likelihood of such scenarios is small, extensive tests need to be performed to ensure that nonendocrine cells do not compromise the function of the hESC-derived endocrine cells or pose cancer-related risks.
Other unresolved issues include the immune response that will be directed against hESC-derived cells on transplantation into immune-competent T2D and autoimmune T1D individuals. As addressed in detail elsewhere in this collection, immunosuppressive regimens have been developed over the last few years that now provide significant and long-lasting protection against cadaveric islets that are transplanted into diabetic patients (Posselt et al. 2010a,b). In addition, improved encapsulation devices are currently being developed to not only shelter hESC-derived endocrine cells from the immune insult of the host individual (Vaithilingam et al. 2008), but also prevent escape of potentially tumorigenic cells into the host body.
Finally, it will be critical to ascertain that the hESC/iPSC-derived β cells are truly equivalent to the endogenous counterparts. β cells are highly specialized cells that not only produce and secrete insulin, but do so in a tightly controlled manner. Critical aspects of β-cell function thus include the sensing of physiological glucose levels, the rapid release of stored insulin vesicles, and the immediate cessation of insulin secretion once glucose levels have been normalized. This complex process requires optimal coordination of multiple regulatory processes that exist in endogenous β cells. Extensive efforts need to be undertaken to ensure that the same regulatory mechanisms are intact in stem cell-derived insulin-producing cells to prevent unwanted complications stemming from hypoglycemia caused by inappropriate or prolonged insulin release. Only when we have thoroughly convinced ourselves that the stem cell-derived cells are the true equivalent of the endogenous β cells should transplantation into human diabetic patients become a reality.
OUTLOOK AND CONCLUDING REMARKS
The last decade has seen tremendous efforts and advances in development of protocols that guide the differentiation of human stem cell populations toward pancreatic β cells. In addition, the breakthrough findings that resulted in the formation of human-induced pluripotent stem cells from adult, somatic cells opened up the possibility to generate either patient-specific stem cells or to develop libraries of iPSC lines cataloged for their differentiation capacity as well as immunological makeup. For example, human leukocyte antigen (HLA) typing could be performed to increase the chances of engraftment and reduce the risk of host versus graft reaction, one of the most common posttransplant complications. Nonetheless, whereas iPSC lines might reduce allograft rejections, additional suppression of the immune system will be required for T1D patients suffering from autoimmune defects. Finally, the success with reprogramming somatic cells to iPS cells through activation of only a small set of critical regulators raises the possibility that similar approaches could be used to directly reprogram either stem cells or somatic cells into functional β cells. However, even under the best circumstances, extensive testing of stem cell/somatic cell-derived β cells will need to be performed to show that these “designed” cells fully replicate all functions of the endogenous insulin-producing cells found in the human pancreas. Although we have taken enormous steps toward generating such cells, significant work remains to be done before cell transplantation of stem cell-derived β cells becomes a reality.
ACKNOWLEDGMENTS
I thank members of my laboratory, especially Drs. Sapna Puri, Tingxia Guo, and Grace Wei, for critical comments and suggestions on the article. Research in my laboratory on stem cells is supported by the Juvenile Diabetes Research Foundation (JDRF), the Leona M. and Harry B. Helmsley Charitable Trust, the NIH Beta Cell Biology Consortium (BCBC), and the California Institute of Regenerative Medicine (CIRM).
Footnotes
Editors: Jeffrey A. Bluestone, Mark A. Atkinson, and Peter Arvan
Additional Perspectives on Type 1 Diabetes available at www.perspectivesinmedicine.org
REFERENCES
- Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM 2003. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci 100: 998–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA 2009. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell 4: 348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, et al. 2009. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol 5: 258–265 [DOI] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ 1994. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development 120: 1919–1928 [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE 2005. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23: 1534–1541 [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE 2006. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24: 1392–1401 [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Pipeleers DG, Ling Z, Welsh N, Hellerstrom C, Andersson A 1994. Major species differences between humans and rodents in the susceptibility to pancreatic β-cell injury. Proc Natl Acad Sci 91: 9253–9256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshpeter A, Jiang J, Au M, Rajotte RV, Lu K, Lebkowski JS, Majumdar AS, Korbutt GS 2008. In vivo characterization of transplanted human embryonic stem cell-derived pancreatic endocrine islet cells. Cell Prolif 41: 843–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH 1981. Establishment in culture of pluripotential cells from mouse embryos. Nature 292: 154–156 [DOI] [PubMed] [Google Scholar]
- Gadue P, Huber TL, Paddison PJ, Keller GM 2006. Wnt and TGF-β signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci 103: 16806–16811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golosow N, Grobstein C 1962. Epitheliomesenchymal interaction in pancreatic morphogenesis. Dev Biol 4: 242–255 [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V, Boussemart L, Gadue P, Nierhoff D, Koehler CI, Kubo A, Shafritz DA, Keller G 2006. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol 24: 1402–1411 [DOI] [PubMed] [Google Scholar]
- Guo T, Hebrok M 2009. Stem cells to pancreatic β-cells: New sources for diabetes cell therapy. Endocr Rev 30: 214–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R 1995. Lack of β-catenin affects mouse development at gastrulation. Development 121: 3529–3537 [DOI] [PubMed] [Google Scholar]
- Hammar EB, Irminger JC, Rickenbach K, Parnaud G, Ribaux P, Bosco D, Rouiller DG, Halban PA 2005. Activation of NF-κB by extracellular matrix is involved in spreading and glucose-stimulated insulin secretion of pancreatic β cells. J Biol Chem 280: 30630–30637 [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Dolci W, Thorens B 2001. Differential sensitivity of GLUT1- and GLUT2-expressing β cells to streptozotocin. Biochem Biophys Res Commun 289: 1114–1117 [DOI] [PubMed] [Google Scholar]
- Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA 2008. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 26: 1269–1275 [DOI] [PubMed] [Google Scholar]
- Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S 2008. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci 105: 19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J 2005. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology 128: 728–741 [DOI] [PubMed] [Google Scholar]
- Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS 2007. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 25: 1940–1953 [DOI] [PubMed] [Google Scholar]
- Katsumoto K, Kume S 2011. Endoderm and mesoderm reciprocal signaling mediated by CXCL12 and CXCR4 regulates the migration of angioblasts and establishes the pancreatic fate. Development 138: 1947–1955 [DOI] [PubMed] [Google Scholar]
- Kelly OG, Pinson KI, Skarnes WC 2004. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development 131: 2803–2815 [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. 2008. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26: 443–452 [DOI] [PubMed] [Google Scholar]
- Ku HT, Zhang N, Kubo A, O’Connor R, Mao M, Keller G, Bromberg JS 2004. Committing embryonic stem cells to early endocrine pancreas in vitro. Stem Cells 22: 1205–1217 [DOI] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G 2004. Development of definitive endoderm from embryonic stem cells in culture. Development 131: 1651–1662 [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D 2001. Induction of pancreatic differentiation by signals from blood vessels. Science 294: 564–567 [DOI] [PubMed] [Google Scholar]
- Landsman L, Nijagal A, Whitechurch TJ, VanderLaan RL, Zimmer W, MacKenzie TC, Hebrok M 2011. Pancreatic mesenchyme regulates epithelial organogenesis throughout development. PloS Biol 9: e101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Rukstalis JM, Nishimura W, Tchipashvili V, Habener JF, Sharma A, Bonner-Weir S 2010. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. J Cell Sci 123: 2792–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A 1999. Requirement for Wnt3 in vertebrate axis formation. Nat Genet 22: 361–365 [DOI] [PubMed] [Google Scholar]
- Lowe LA, Yamada S, Kuehn MR 2001. Genetic dissection of nodal function in patterning the mouse embryo. Development 128: 1831–1843 [DOI] [PubMed] [Google Scholar]
- Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R 2001. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 292: 1389–1394 [DOI] [PubMed] [Google Scholar]
- Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA 2009. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci 106: 15768–15773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao GH, Chen GA, Bai HY, Song TR, Wang YX 2009. The reversal of hyperglycaemia in diabetic mice using PLGA scaffolds seeded with islet-like cells derived from human embryonic stem cells. Biomaterials 30: 1706–1714 [DOI] [PubMed] [Google Scholar]
- Martin GR 1981. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci 78: 7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean AB, D’Amour KA, Jones KL, Krishnamoorthy M, Kulik MJ, Reynolds DM, Sheppard AM, Liu H, Xu Y, Baetge EE, et al. 2007. Activin A efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells 25: 29–38 [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yamato E, Miyazaki J 2004. Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes 53: 1030–1037 [DOI] [PubMed] [Google Scholar]
- Morris JPt, Cano DA, Sekine S, Wang SC, Hebrok M 2010. β-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest 120: 508–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrep N, Wang J, Miyatsuka T, German MS 2008. Signals from the neural crest regulate β-cell mass in the pancreas. Development 135: 2151–2160 [DOI] [PubMed] [Google Scholar]
- Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, et al. 2006. The vascular basement membrane: A niche for insulin gene expression and β cell proliferation. Dev Cell 10: 397–405 [DOI] [PubMed] [Google Scholar]
- Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, Micallef SJ, Park IH, Basford C, Wheeler MB, et al. 2011. Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development 138: 861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, et al. 2011. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat Cell Biol 13: 541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada SI, Wright CV 1999. Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development 126: 3229–3240 [DOI] [PubMed] [Google Scholar]
- Parnaud G, Hammar E, Ribaux P, Donath MY, Berney T, Halban PA 2009. Signaling pathways implicated in the stimulation of β-cell proliferation by extracellular matrix. Mol Endocrinol 23: 1264–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posselt AM, Bellin MD, Tavakol M, Szot GL, Frassetto LA, Masharani U, Kerlan RK, Fong L, Vincenti FG, Hering BJ, et al. 2010a. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant 10: 1870–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posselt AM, Szot GL, Frassetto LA, Masharani U, Tavakol M, Amin R, McElroy J, Ramos MD, Kerlan RK, Fong L, et al. 2010b. Islet transplantation in type 1 diabetic patients using calcineurin inhibitor-free immunosuppressive protocols based on T-cell adhesion or costimulation blockade. Transplantation 90: 1595–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Hebrok M 2010. Cellular plasticity within the pancreas—Lessons learned from development. Dev Cell 18: 342–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal J, Anderson WJ, Kume S, Martinez OI, Melton DA 2003. Insulin staining of ES cell progeny from insulin uptake. Science 299: 363. [DOI] [PubMed] [Google Scholar]
- Robertson E, Bradley A, Kuehn M, Evans M 1986. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature 323: 445–448 [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV 2000. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343: 230–238 [DOI] [PubMed] [Google Scholar]
- Shiroi A, Ueda S, Ouji Y, Saito K, Moriya K, Sugie Y, Fukui H, Ishizaka S, Yoshikawa M 2005. Differentiation of embryonic stem cells into insulin-producing cells promoted by Nkx2.2 gene transfer. World J Gastroenterol 11: 4161–4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, et al. 2009. Pancreatic exocrine duct cells give rise to insulin-producing β cells during embryogenesis but not after birth. Dev Cell 17: 849–860 [DOI] [PubMed] [Google Scholar]
- Speier S, Gjinovci A, Charollais A, Meda P, Rupnik M 2007. Cx36-mediated coupling reduces β-cell heterogeneity, confines the stimulating glucose concentration range, and affects insulin release kinetics. Diabetes 56: 1078–1086 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872 [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM 1998. Embryonic stem cell lines derived from human blastocysts. Science 282: 1145–1147 [DOI] [PubMed] [Google Scholar]
- Vaithilingam V, Sundaram G, Tuch BE 2008. Islet cell transplantation. Curr Opin Organ Transplant 13: 633–638 [DOI] [PubMed] [Google Scholar]
- Van Hoof D, Mendelsohn AD, Seerke R, Desai TA, German MS 2011. Differentiation of human embryonic stem cells into pancreatic endoderm in patterned size-controlled clusters. Stem Cell Res 6: 276–285 [DOI] [PubMed] [Google Scholar]
- Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ 2003. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev 17: 1646–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al. 2010. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7: 618–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber LM, Hayda KN, Anseth KS 2008. Cell-matrix interactions improve β-cell survival and insulin secretion in three-dimensional culture. Tissue Engineering Part A 14: 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, D’Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. 2008. β cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132: 197–207 [DOI] [PubMed] [Google Scholar]
- Yang H, Wright JR Jr 2002. Human β cells are exceedingly resistant to streptozotocin in vivo. Endocrinology 143: 2491–2495 [DOI] [PubMed] [Google Scholar]
- Yoshitomi H, Zaret KS 2004. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development 131: 807–817 [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. 2007. Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA 2008. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455: 627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, et al. 2009. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4: 381–384 [DOI] [PMC free article] [PubMed] [Google Scholar]