Abstract
The goal of this study was to use bioengineered injectable microgels to enhance the action of bone morphogenetic protein 2 (BMP2) and stimulate cartilage matrix repair in a reversible animal model of osteoarthritis (OA). A module of perlecan (PlnD1) bearing heparan sulfate (HS) chains was covalently immobilized to hyaluronic acid (HA) microgels for the controlled release of BMP2 in vivo. Articular cartilage damage was induced in mice using a reversible model of experimental OA and was treated by intra-articular injection of PlnD1-HA particles with BMP2 bound to HS. Control injections consisted of BMP2 free PlnD1-HA particles, HA particles, free BMP2 or saline. Knees dissected following these injections were analyzed using histological, immunostaining and gene expression approaches. Our results show that knees treated with PlnD1-HA/BMP2 had lesser OA-like damage compared to control knees. In addition, the PlnD1-HA/BMP2-treated knees had higher mRNA levels encoding for type II collagen, proteoglycans, and xylosyltransferase 1, a rate-limiting anabolic enzyme involved in the biosynthesis of glycosaminoglycan chains, relative to control knees (PlnD1-HA). This finding was paralleled by enhanced levels of aggrecan in the articular cartilage of PlnD1-HA/BMP2 treated knees. Additionally, decreases in the mRNA levels encoding for cartilage-degrading enzymes and type X collagen were seen relative to controls. In conclusion, PlnD1-HA microgels constitute a formulation improvement compared to HA for efficient in vivo delivery and stimulation of proteoglycan and cartilage matrix synthesis in mouse articular cartilage. Ultimately, PlnD1-HA/BMP2 may serve as an injectable therapeutic agent for slowing or inhibiting the onset of OA after knee injury.
Keywords: Perlecan, Hyaluronic Acid, Heparan Sulfate, Osteoarthritis, Cartilage Repair, Bone Morphogenetic Protein
1. Introduction
Articular cartilage is a viscoelastic tissue essential for the absorption of shocks and normal distribution of loads. Because of its non-vascularized, non-innervated and sparsely cell populated nature, this tissue displays poor regenerative capacity[1]. Recently, growth factor therapy has emerged as a novel strategy for enhancing chondrogenic differentiation and repairing functional cartilage [2–4]. The heparan sulfate binding growth factor (HBGF) bone morphogenetic protein 2 (BMP2), which plays a critical role in the establishment of normal cartilage during development, also was found to enhance the differentiated phenotype of mesenchymal stem cells in culture [5–7]. In addition, several studies indicate that BMP2 expression is elevated in damaged cartilage during the early stages of spontaneous or instability-induced OA. This increase in BMP2 levels is believed to enhance reparative processes and reactivate morphogenetic pathways including synthesis of extracellular matrix (ECM) components [8,9]. During disease progression, the weakened synthetic machinery of chondrocytes eventually becomes unable to compensate for the degradation of ECM components leading to degenerative OA. Therefore, it is apparent that supplementing BMP2 at the initial stages of OA may have a significant inhibitory effect on the development of OA [3]. Nonetheless, even with the high chondrogenic potency of BMP2, the biggest challenge lies in developing an efficient delivery system to counteract its short half life and rapid degradation in vivo [10,11].
Perlecan/HSPG2 is a heparan sulfate proteoglycan (HSPG) that represents an essential component of cartilage ECM [12–15]. The NH2-terminal portion of perlecan (domain 1 or PlnD1) carries HS chains that bind HBGFs and enhance interaction with their signal transducing receptors [13,16–18]. Thus, PlnD1 can act as a depot for BMP2 storage and controlled release, protect it from proteolytic degradation and potentiate its biological activity [19,20]. Previous studies demonstrate that PlnD1 can be successfully used in vitro to modulate the chondrogenic bioactivity of BMP2 [21]. However, because of its own diffusion and susceptibility to degradation, PlnD1 only can be effectively used as a HBGF reservoir for in vivo cartilage repair if immobilized through conjugation to a larger biocompatible carrier. For this reason, we developed a HBGF delivery system with in vitro chondrogenic activity by conjugating PlnD1 to hyaluronic acid (HA)-based microgels (PlnD1-HA) [2]. HA is a natural component of articular cartilage that functions as a matrix organizer by interacting with other matrix molecules such as aggrecan [22]. HA-based macromolecules are commonly used in the clinic as viscosupplements to enhance joint mobility and provide temporary relief of knee pain by increasing the viscosity and elasticity of synovial fluid [23]. However, HA alone does not promote the regeneration of cartilage ECM and is traditionally not administered in combination with active cartilage repair agents. Thus, palliative HA injection can temporarily alleviate pain and may allow people to maintain some level of function until joint replacement is necessary but in younger people with jobs that require high levels of physical activity, improvement in their ability to perform their duties following HA injection is not well documented [24,25].
Here, we used PlnD1-HA microgels as the carrier for the controlled and slow release of BMP2 into the knee cavity of mice. We tested the efficiency of this system on articular cartilage using the papain-induced model of early OA [26]. The goal of this study was to determine the potential benefits of a single injection of PlnD1 added to HA in potentiating BMP2 activity for the attenuation of early cartilage damage in an experimental model of reversible OA prior to permanent cartilage break-down.
2. Method
2.1. Preparation of PlnD1-HA Microgels
Recombinant human BMP2 (R&D Systems, Minneapolis, MN) stock was prepared at a concentration of 10μg/ml in saline containing 4mM HCl and 0.1% (w/v) mouse albumin (Innovative Research, Novi, MI). Recombinant mouse PlnD1 was expressed by stably transfected kidney cells and purified using an immunoaffinity chromatography approach following established protocols [18,27]. Post-translational modification of PlnD1 by heparan sulfate (HS) was verified by observing a change in its electrophoretic mobility following heparinase I, II, and III and chondroitinase AC treatments [18]. Because the absence of HS results in loss of BMP-2 binding activity only PlnD1 decorated by HS chains was used in this study [21]. PlnD1-HA microgels were prepared as described [2]. Briefly, PlnDI was conjugated to HA microgels via the core protein through a polyethylene glycol (PEG) linker. The aldehyde groups in HA microgels were passivated by glycine and the residual hydrazide groups were allowed to react with a large excess of PEG dial dibutyraldehyde. The generated aldehyde groups were subsequently used for reaction with the lysine amine residues in the core protein. The PlnDI-conjugated HA particles were passivated again with glycine before being used for BMP-2 loading.
The need for PlnD1 bioconjugation to a HA carrier to increase in vivo retention in the articular knee cavity, was tested previously by injecting intra-articularly Alexa 568-labeled PlnD1. In vivo knee imaging showed that PlnD1 was clearly visible for 4–6 hours only when stabilized by a HA carrier (data not shown). Bioconjugation of PlnD1 to HA was performed as described [2]. The presence of glycosaminoglycan modifications was controlled by staining the microgels with Alcian blue. Additionally, the selective binding capacity and release of BMP2 were measured using an ELISA assay as described [2]. Finally, PlnD1-HA microgel bioactivity was evaluated in vitro using a micromass culture system as described [2]. Once the PlnD1-HA microgels passed all these control quality tests, they were extensively rinsed in 70% (v/v) ethanol and saline, pelleted by centrifugation at 3,000 rpm, and resuspended in sterile saline (Fig. 1) at a concentration of 6mg/ml. Approximately 1 mg of PlnD1-HA microgels was combined with 250ng of BMP2. Both PlnD1-HA control and PlnD1-HA/BMP2 mixtures were preincubated for an hour at room temperature on a rocking platform prior to performing the intra-articular injections.
Fig. 1.
Injectable PlnD1-HA microgels visualized following hydration in saline buffer..
2.2. Intra-articular Injections
Early OA-like damage was induced by injecting intra-articularly 6μl of a 1% (w/v) papain solution prepared in a saline solution containing 5mM L-cysteine in the knee of 10–11 week-old C57BL6/J mice. After waiting 7 days for the OA-like damage to develop, the various test treatments were administered and the knees were allowed to recover for 7 or 14 days, after which the efficiency of each treatment condition to counteract the effects of papain was evaluated following animal sacrifice. Previous in vitro characterization of our delivery system demonstrated that nearly 70% of the initially bound BMP2 was released from PlnD1-HA microgels after 14 days of incubation [2]. Based on this observation, it was assumed that the majority of the bound BMP2 will be released from carrier microgels after a 14-day in vivo incubation period. In the initial study, the usefulness of PlnD1-HA particles plus BMP2 to limit joint damage was compared with growth factor-free PlnD1-HA particles or saline at day 7 post-treatment. In subsequent studies, HA particles or BMP2 alone served as additional control groups, and the knees were dissected 7 days after the treatment injections. Because knee damage induced by HA or BMP2 did not differ significantly from saline controls at day 7, only PlnD1-HA or saline injections served as controls for day 14 histological scorings (n=9/group except for saline followed by saline control injections where n=5). All procedures involving animals were performed in accordance with protocols approved by the IACUC at the University of Delaware.
2.3. Histological Scoring
Knees were fixed in 10% (v/v) formalin and decalcified in a 10% (v/v) formic acid solution. 6μm-thick frontal sections were either stained histologically using a standard Safranin O and Fast Green staining procedure or immunostained (see below) [28]. The stringent conditions used prior and during knee sectioning did not permit us to visualize retention of the microgels in the knee cavity after histological processing. Scoring was done in the four compartments of the knee using a modified semi-quantitative scoring scale as described [29]. Briefly, the scores attributed in this study are: score 0 = normal cartilage, score 0.5 = loss of Safranin O staining with a normal articular surface, score 1 = small fibrillations or roughened articular surface, and score 2 = fibrillations extending into the superficial lamina. For each knee analyzed, 12–15 slides encompassing the entire joint were blinded and scored by two independent observers.
2.4. Immunohistochemistry
Deparaffinized knee sections were treated with Dako (Carpinteria, CA) antigen retrieval solution for 1 hour and blocked overnight with 3% (w/v) BSA and 2% (v/v) goat serum. Primary rabbit antibody [anti-mouse aggrecan (Chemicon International Inc., Temecula, CA) or anti-mouse collagen II (Biodesign International, Saco, ME)] was incubated for 4 hours at 37°C. After washing in PBS, sections were incubated with Alexa 488 conjugated goat anti-rabbit secondary antibody (Invitrogen, Carlsbad, CA) and DRAQ5™ (Biostatus, Leicestershire, United Kingdom) at 37°C for 1 hour, mounted, and viewed under a confocal microscope.
2.5. Quantitative Real-Time PCR
To examine transcriptional changes in cartilage-specific marker expression, mRNA was extracted from knee articular cartilage of both tibiae and femora of 4 papain-damaged knees treated with either PlnD1-HA/BMP2 (combined treatment) or PlnD1-HA (carrier only control) microgels. Mild decalcification was induced in 0.5M EDTA (Sigma-Aldrich, St. Louis, MO) overnight at 4°C. Articular cartilage tissue was microdissected away from subchondral bone prior to RNA extraction with the RNeasy® fibrous tissue mini kit (Qiagen, Valencia, CA) and DNAase treatment (Turbo DNA-free, Ambion Inc, Austin, TX). One μg of mRNA was used to synthesize cDNA using the iScript™ cDNA synthesis kit (BioRad, Hercules, CA) and the quantitative PCR reaction was run in triplicate. Primer sets specific for cartilage markers were purchased from SA Biosciences (Frederick, MD) and included type II and X collagens (COL2a1 and COL10a1); aggrecan (ACAN); perlecan, the major HSPG produced in the pericellular matrix of chondrocytes (HSPG2); and the isoform of xylosyltransferase found in cartilage (XYLT1). In addition, the following primers were used: 1) two aggrecan degrading enzymes, matrix metalloproteinase 3 (MMP3) and aggrecanase-2 (ADAMTS5), and 2) one enzyme primarily responsible for type II collagen breakdown (MMP13) [30,31]. The relative mRNA fold change in PlnD1-HA/BMP2 vs. PlnD1-HA (carrier only) treatments was compared at 1 or 7 days post-injection. These two time points were selected based upon previous in vitro kinetic studies of BMP2 release from PlnD1-HA microgels that occurred in two distinct phases with an initial burst occurring during the first day (10% of cumulative release) followed by a steady controlled release (3.8%/day) that reached a cumulative release of approximately 50% after seven days [2]. For this reason, mRNA analysis was conducted without delay (day 1) during the burst phase to detect transient events responsible for activation of synthesis pathways and in the middle of the steady linear release phase (day 7) before 100% release was achieved. Four knees were used in each biological replicates and two biological replicates were performed. Each sample was run in triplicate, cycle threshold (CT) values were obtained for each gene of interest, and the relative mRNA mean fold change is the results of two biological replicates., This fold change was calculated using the comparative CT method in which the 2 −Δ ΔCT formula normalized target mRNA amount to GAPDH [32]. Differential gene expression results are presented in terms of fold-change (PlnD1HA-BMP2 over PlnD1HA), with 2-fold and 0.5-fold considered significant cutoffs for a relative increase or decrease in gene expression, respectively [3].
2.6. Statistical Analyses
The histological scores obtained at different time points from different knees (n=9) were analyzed using the Kruskal-Wallis statistical test as described on Dr. John H. McDonald’s website (University of Delaware, Newark, DE) [33]. Bonferroni correction was performed for multiple comparisons and p values less than 0.0083 (0.05 divided by 6 groups) and 0.0167 (0.05 divided by 3 groups) were considered significant at day 7 and day 14, respectively. In addition, scores for three experimental groups (PlnD1-HA/BMP2, PlnD1-HA, saline) were compared between day 7 and day 14 using the Kruskall-Wallis test followed by Bonferroni correction and p values below 0.0083 were considered significant.
3. Results
3.1. Effect of BMP2-loaded PlnD1-HA Microgels on Damaged Articular Cartilage in Mice
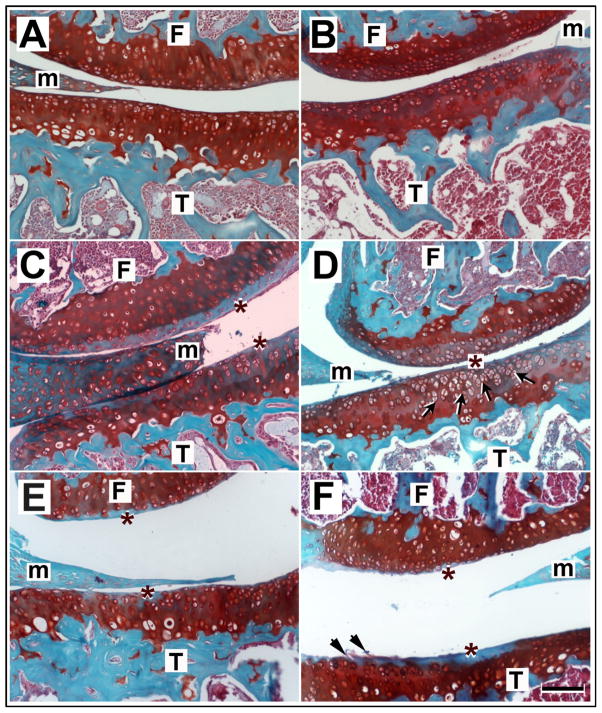
The efficacy of the various treatments in counteracting the damage produced by papain was evaluated by histological scoring (Figs. 2 and 3). Seven days post-treatment, PlnD1-HA/BMP2 injected knees had significantly lesser OA-like damage than the knees treated with PlnD1-HA (p= 1.1 × 10−5, Fig. 3) or the knees treated with saline (p= 1.3 × 10−5, Fig. 3). The majority of coronal knee sections obtained 7 days after a single injection of PlnD1-HA/BMP2 microgels showed a smooth and thick articular cartilage surface with chondrocytic clusters of normal appearance surrounded by intense Safranin-O staining (Fig. 2B). This result was seen in around 90% of the animals tested (8 out of 9 injected knees). Remarkably, severe damage induced by papain including proteoglycan loss and initial delamination of the superficial layer that were observed prior to treatment (day 0, Fig. 1S) were entirely reversed by a single intra-articular injection of PlnD1-HA/BMP2 microgels when analyzed at day 7 post-treatment (Fig. 2B). In addition, the morphology of PlnD1-HA/BMP2 treated knees at day 7 was not distinguishable from knees that were injected twice with a saline solution in place of papain at day minus 7 and in place of the treatment at day 0 (Fig. 2A-B and Fig. 3, p= 0.824).
Fig. 2.
Histological sections of mouse knees processed 7 days post repair or control treatments and stained with Safranin-O and Fast Green. Knees treated with PlnD1-HA/BMP2 showed a normal smooth articular cartilage appearance (B) and showed no OA damage when compared with papain-damaged knees treated with either saline (C), PlnD1-HA (D), HA (E), or BMP2 (F). Proteoglycan depletion indicated by a loss of Safranin-O staining is marked by asterisks. An increase in chondrocyte clusters was observed in the proteoglycan-depleted region of articular cartilage of PlnD1-HA-treated knees (see arrows in D). Such clusters are absent in BMP-2 treated knees where articular cartilage fissures and delamination is observed (see arrowheads in F). No obvious difference is seen between control knees (A) injected twice with saline (in place of papain and the repair treatment) and the papain-injected knees treated with PlnD1- HA/BMP2 microgels (B). T, tibia; F, femur; m, meniscus. Scale bar represents 50 μm
Fig. 3.
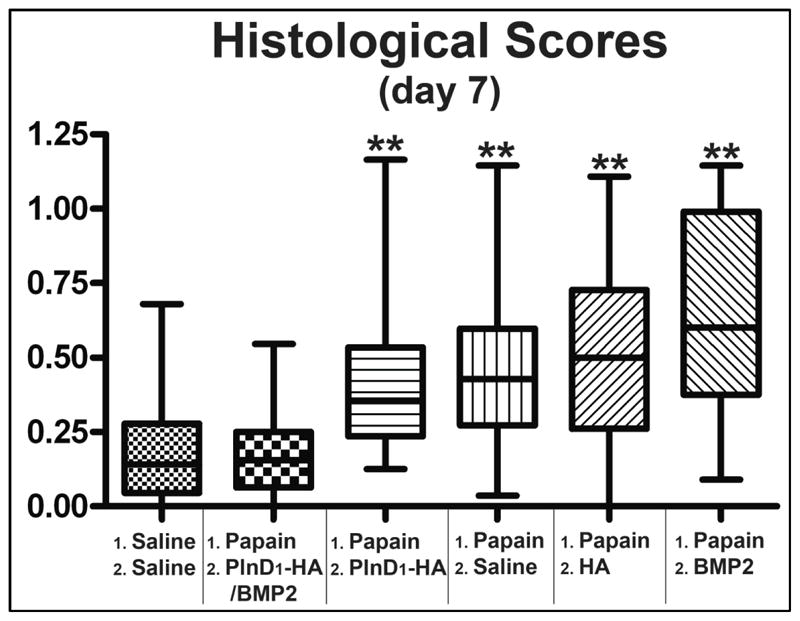
Box and whisker plot showing the median (central line), 25–75 percentile (boxes) and the entire range of scores obtained 7 days after treatment of saline or papain-damaged knees (n=5 for control injected twice with saline; n=9 for all the other groups). ** indicates p<0.001 when compared to PlnD1-HA/BMP2. No statistical difference is seen between the scores obtained in control knees injected with saline twice and the papain-injected knees treated with BMP2-loaded PlnD1-HA particles (p=0.824).
In contrast, control saline (Fig. 2C) and PlnD1-HA (Fig. 2D) treatments of papain-damaged knees resulted in obvious proteoglycan depletion and small fibrillations in approximately 80% (7/9 knees) and 70% (6/9 knees) of the individuals tested, respectively. Additional control treatments consisting of an injection of either HA or BMP2 microgels in the absence of PlnD1 did not reverse proteoglycan loss (Fig. 2E-F, in 7/9 and 8/9 knees respectively). Papain-damaged knees treated with HA (Fig. 2E, with fibrillation in 4/9 knees) had a significantly higher OA score compared to knees treated with PlnD1-HA/BMP2 (Fig. 2B) indicating the lack of chondrogenic repair activity (Fig. 3, p= 2.78 × 10−6). The same observation was made following BMP2 treatment (Fig. 3, BMP2 vs. PlnD1-HA/BMP2; p= 3.2 × 10−7) where scores were slightly more severe and cartilage fibrillation occurred in 7/9 knees (see arrows in Fig. 2F). Interestingly, chondrocyte clusters were seen near the joint surface in the eroding proteoglycan-depleted region of PlnD1-HA treated knees relative to PlnD1-HA control treatments (see arrows in Fig. 2D).
3.2. Effect of PlnD1-HA/BMP2 Microgels on Articular Cartilage Transcript Levels
3.2.1. Cartilage Synthesis Markers
Transcripts levels of ECM components were measured in articular cartilage of papain damaged knees treated with either PlnD1-HA/BMP2 or growth factor free control PlnD1- HA particles (Fig. 4A). As early as one day post-treatment, the mRNA level for the α1 chain of the major fibrillar component of cartilage, type II collagen (COL2a1), was slightly, but significantly, increased 2-fold in cartilage extracted from knees treated with PlnD1-HA/BMP2 relative to control (PlnD1-HA) knees. This positive effect continued with time and a 6-fold increase in type II collagen mRNA levels was seen at day 7 post-treatment in PlnD1-HA/BMP2 treated knees when compared to control (PlnD1-HA) samples.
Fig. 4.
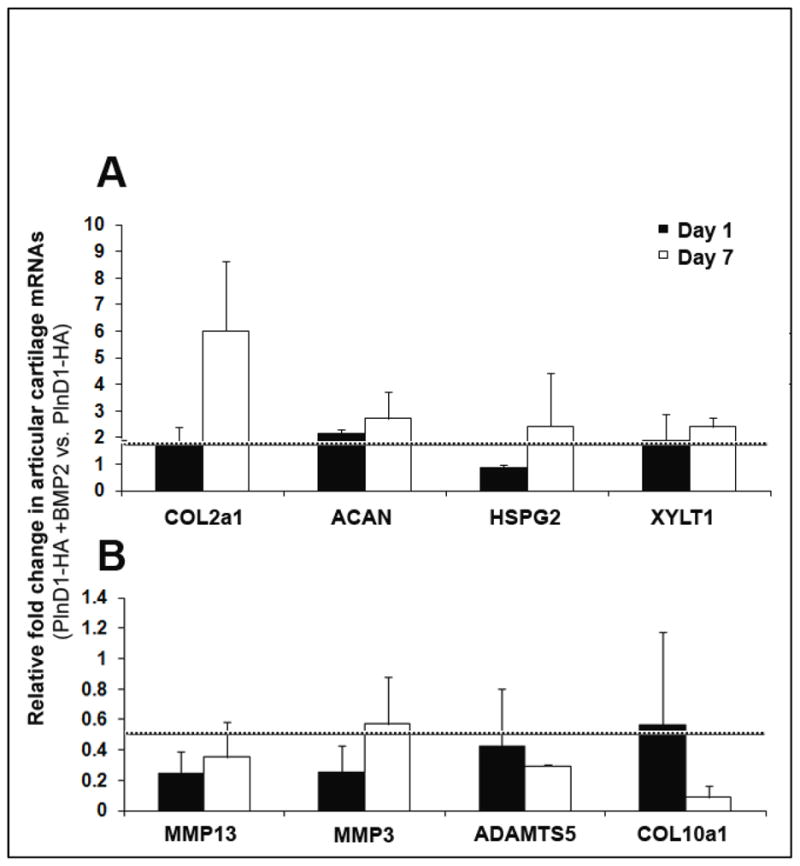
Effect of the combined administration of BMP2 and PlnD1-HA particles on mRNA levels of articular cartilage ECM components and ECM-modifying enzymes. Fold changes in mRNA levels are shown for knees treated with BMP2-loaded PlnD1-HA particles relative to knees treated with growth factor free particles (control PlnD1-HA) on days 1 and 7 following intra-articular injections. mRNA levels of the α1 chain of type II collagen (COL2a1), aggrecan (ACAN), perlecan (HSPG2) and xylosyltransferase 1 (XYLT1) were significantly increased in knees treated with PlnD1-HA/BMP2 compared with control (PlnD1-HA) knees whereas the opposite was seen with matrix degrading enzymes (MMP13, MMP3, and ADAMTS5) and the α1 chain of type X collagen (COL10a1). Each fold change value equal or higher to 2 (above the dashed line in A) and equal or lower to 0.5 (below the dashed line in B) is considered statistically significant, when PlnD1-HA/BMP2 is compared with control PlnD1HA. Error bars represent standard deviations.
Transcript levels of the major cartilage proteoglycan, aggrecan, were about 2.5-fold greater in PlnD1-HA/BMP2 versus control (PlnD1-HA) knees at day 7. We also examined the mRNA levels of perlecan itself, the most abundant HSPG present in cartilage. The relative mRNA level of perlecan was significantly higher in the PlnD1- HA/BMP2 treated knees relative to PlnD1-HA treated knees at day 7. The relative levels of transcripts encoding for the enzyme that initiates glycoaminoglycan chain extension by adding the first sugar group to proteoglycans, xylosyltransferase 1 (XYLT1) [34] also was measured. PlnD1-HA/BMP2 treated knees demonstrated an increase in mRNA encoding for this enzyme in the early post-treatment phase (day 1). The increase remained significant at day 7 when compared to control (PlnD1-HA) knees.
3.2.2. Cartilage degradative enzymes and marker of hypertrophy
We also measured the mRNA levels of degrading enzymes responsible for the breakdown of cartilage ECM components and type X collagen (COL10a1), a marker for chondrocyte hypertrophy and pathological calcification of articular cartilage (Fig. 4B). The mRNA levels of both MMP3 and MMP13 were decreased significantly in the knees treated with PlnD1-HA/BMP2 compared to the control (PlnD1-HA) knees at day 1 and day 7 after treatment injections. The mRNA levels of ADAMTS5 were also significantly decreased at both day 1 and day 7 between the PlnD1-HA/BMP2 treated and the control PlnD1-HA treated knees. The level of transcripts encoding for the α1 chain of type X collagen, was significantly decreased by nearly 5-fold at day 7 in PlnD1- HA/BMP2 treated vs. PlnD1-HA injected knees.
3.3. Comparative Analysis of ECM Protein Distribution in PlnD1-HA/BMP2 treated Knees
3.3.1. Type II collagen Immunoreactivity
Potential changes in the expression pattern of the major fibrillar component, type II collagen, was assessed by comparing immunostained sections of papain-damaged knee sections harvested 7 days after treatment with either BMP2-loaded PlnD1-HA particles or saline (Fig. 5). There was no obvious difference in the intensity of the type II collagen-specific signal detected at the joint interface of PlnD1-HA/BMP2-treated knees (Fig. 5C) and the articular cartilage remaining in knees treated with saline or control PlnD1-HA particles (Fig. 5D and data not shown). Thus, the global decrease of signal seen in control (PlnD1-HA) knees relative to PlnD1-HA/BMP2 can be attributed to a decrease in the amount of articular cartilage tissue present in this region. Conversely, the presence of healthy articular cartilage in the superficial layer of PlnD1-HA/BMP2-treated knees was accompanied by a concomitant increase in the overall extent of collagen type II-positive tissue when compared to control (PlnD1-HA) conditions (compare C and D in Fig. 5).
Fig. 5.

The extent of the type II collagen signal (green) is diminished in the articular cartilage of saline (D) versus PlnD1-HA/BMP2 (C) -treated knees after 7 days of treatment. For each group, consecutive coronal knee sections were stained with Safranin O/Fast Green (A, B) and immunostained with an antibody specifically directed against type II collagen (green in C, D). A and B are the adjacent histological stains for C and D, respectively. DRAQ5™ was used as nuclear stain (blue in C, D). Growth plate (GP) cartilage served as an internal positive control for type II collagen immunodetection. Asterisks indicate the presence of a healthy articular cartilage of high cellularity in the superficial layer of PlnD1-HA/BMP2-treated knees. F, Femur; T, Tibia; m, meniscus. Scale bars represent 100μm.
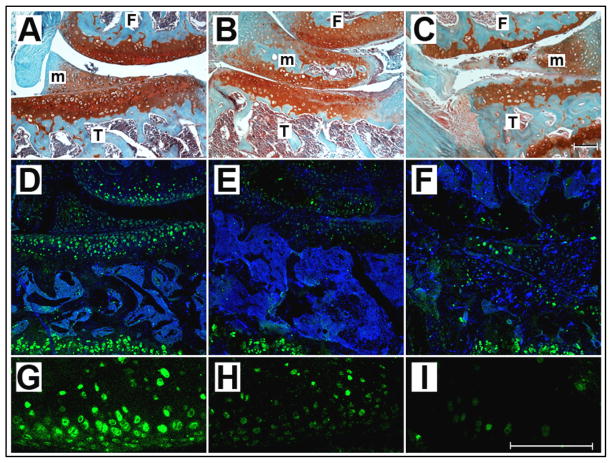
3.3.2. Aggrecan Immunoreactivity
To correlate the higher levels of aggrecan transcripts in PlnD1-HA/BMP2 treated articular cartilage with corresponding protein expression, we performed immunolabeling of treated knees with an antibody directed against the aggrecan molecule (Fig. 6). The articular cartilage of knees treated with PlnD1-HA/BMP2 (Fig. 6D and G) showed higher expression of aggrecan than control knees treated with PlnD1-HA (Fig. 6, compare D and G to E and H) or saline (Fig. 6, compare D and G to F and I). In contrast, aggrecan signal in growth plate cartilage remained unchanged among all three experimental conditions and was used a positive control for antibody immunoreactivity (Fig. 6D-F).
Fig. 6.
Articular cartilage of knees treated with PlnD1-HA/BMP2 showed increased expression of aggrecan relative to control (PlnD1-HA or saline) knees after 7 days of treatment. For each group, consecutive coronal knee sections were stained with Safranin O/Fast Green (A-C) and immunolabeled with an antibody specifically directed against aggrecan (D–F). The aggrecan-specific immune signal (green) was noticeably increased in knees treated with PlnD1-HA/BMP2 (D and G) when compared with control knees treated with PlnD1-HA (E and H) or saline (F and I). A, B, C are the adjacent histological stains for D, E and F, respectively. DRAQ5™ was used as nuclear stain (blue in D-F). Growth plate (GP) cartilage served as an internal positive control for aggrecan immunodetection in osteoarthritic knees that displayed severe depletion of proteoglycans at their articular cartilage surface (see arrows in panel D-F). Scale bars represent 100μm for either A-F or G-I. F, Femur; T,Tibia, m, meniscus.
3.4. PlnD1-HA Microgels Prolong BMP2 Cartilage Repair Activity in vivo
To determine if PlnD1-HA microparticles can prolong the chondrogenic effect of BMP2 on articular cartilage over a longer period of time, we compared the histological appearance of papain-damaged knees dissected 14 days after a single intra-articular injection of either PlnD1-HA/BMP2, PlnD1-HA, or saline (Fig. 7B-D). The analysis of the histological scores showed that PlnD1-HA/BMP2-treated knees had lesser OA-like damage than the knees of the two control (PlnD1-HA and saline) groups (Fig. 7A). Although PlnD1-HA/BMP2 and PlnD1-HA treatments significantly increased between day 7 and day 14, these scores followed the same trends as the scores obtained after 7 days of treatment and PlnD1-HA/BMP2 treated knees at day 14 still displayed significantly less damage than knees treated with either PlnD1-HA or saline (p= 6.10−9 and p=0.005, respectively).
Fig. 7.
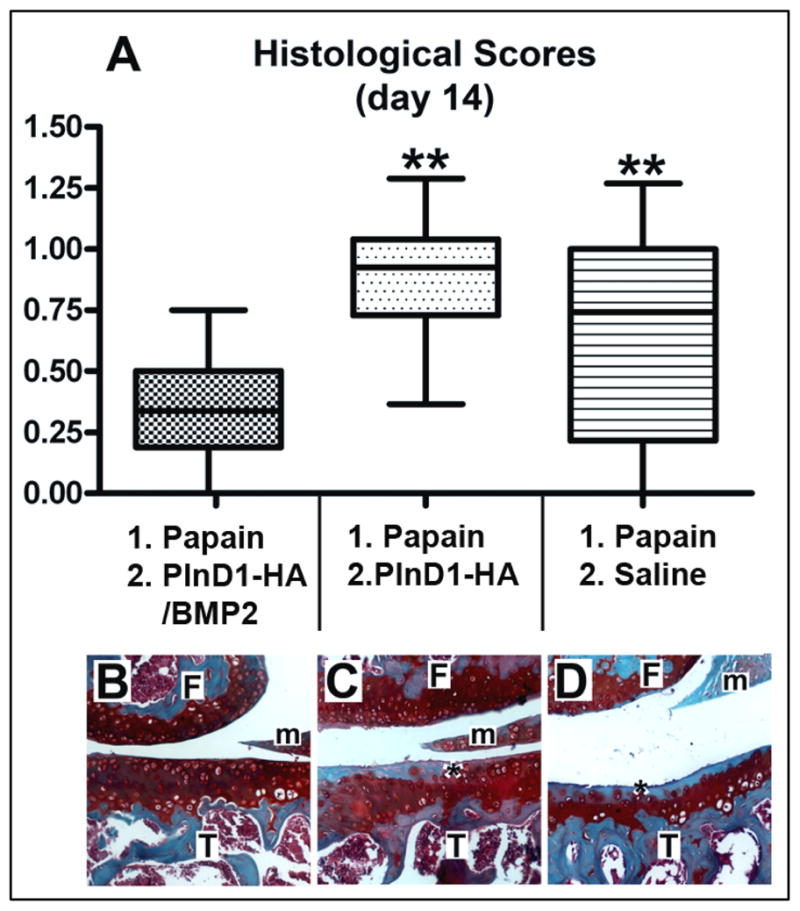
Box and whisker plot showing the median (central line), 25 to 75 percentile (boxes) and the entire range of scores obtained 14 days after treatment of papain-damaged knees (A, n=9 for each group). ** indicates p<0.001 when compared to PlnD1-HA/BMP2. Representative histological sections from papain-damaged mouse knees processed 7 days post repair (B, PlnD1-HA/BMP2) or control (C, PlnD1-HA; D, saline) treatments and stained with Safranin-O and Fast Green. Proteoglycan depletion in the superficial articular cartilage layer is marked by an asterisk. Femur; T,Tibia, m, meniscus.
4. Discussion
Despite its established anabolic effect during both chondrogenesis and the pathogenesis of OA, BMP2 usefulness in cartilage repair has been limited due to its short in vivo half life and known side effects when administered at high doses [2,35]. Indeed, when injected under its soluble form into the knee cavity it rapidly loses its bioactivity due to clearance through systemic passive diffusion/clearance, and/or inhibition via specific BMP antagonists and proteases [10,11,36,37]. Additionally, burst induction from repetitive high dose injections or sustained overexpression through adenoviral genetic insertion of TGFβ family members are known to result in adverse side effects on adjacent joint tissues including induction of an inflammatory response, synovial fibrosis, and formation of de novo osteophytes at sites of tendon insertions or at the periosteal joint margins [35,38,39]. The objective of the current study was to highlight the benefits of controlled BMP2 delivery via PlnD1-HA microgels for the inhibition of cartilage break-down during reversible OA. The use of an early model of OA for testing the in vivo repair effect of controlled BMP2 release was favored because 1) less severe preclinical models are better systems to evaluate novel therapies effective at slowing disease progression prior to severe cartilage damage [40], and 2) BMP antagonists are upregulated during advanced stages of OA [37].
Consistent with previous reports, the single administration of soluble BMP2 in the current study neither induced side effects (expansion of the synovial membrane/subchondral bone sclerosis/osteophyte formation etc.) nor enhanced cartilage repair. For this reason, PlnD1 bearing HS chains was bioconjugated to a biocompatible carrier (HA microgel) to potentiate/prolong BMP2 action after injection in mouse knee cavities. In our system, binding of BMP2 to HS chains could serve two roles as it both protects BMP2 from being degraded and may enhance its opportunity to bind with cellular receptors through the formation of functional ternary complexes in a similar fashion to that described for FGF2 [41,42]. Although it is not well understood if the folded core protein portion of PlnD1 itself plays a direct role in the modulation of BMP2 activity, it is important for: 1) covalent conjugation to the carrier microgels due to the presence of surface lysines and 2) proper spacing of at least two appropriately spaced surface HS chains that facilitate the controlled release of BMP2 [2]. Recently, another member of the TGFβ superfamily, Activin A, was found to specifically bind to perlecan via HS chains interactions that regulate its localization within tissues [43]. Although Activin A binds HS of perlecan via its cleavable N-terminal pro-region, BMP-2 and BMP-4 were reported to interact with proteoglycans in a HS-dependent fashion through a highly conserved region of their mature NH2-terminal portion [44]. This form of non-covalent highly specific binding is important in retaining the growth factor bioactivity that could easily be compromised through covalent chemical bonds and is likely to increase its half life, as demonstrated by prolonged in vitro and in vivo effects [2]. Indeed, injection of BMP2 in combination with PlnD1-HA microgels carrying HS chains in papain-damaged knees significantly improved histological scores when compared to all other treatments including free BMP2.
The significant score improvement observed in PlnD1-HA/BMP2 treated knees observed at day 7 was still evident, albeit diminished, 14 days post-injection. These results extend previous in vitro findings that PlnD1-HA potentiates BMP2 and helps in spatial and temporal presentation of BMP2 for approximately two weeks [2]. It can be speculated that the release kinetics of BMP2 from PlnD1-HA microparticles are governed by the equilibrium between the endogenous BMP2 released from the degrading cartilage matrix and those bound to the PlnD1-HA particles. Such dynamic model would support stimulation of repair pathways and prevention of interaction between active BMP2 molecules and their natural antagonists. While this idea requires further investigation, it is clear from the data obtained during a two-week period that PlnD1-HA microgels injected in the absence of BMP2 are not responsible for knee damage worsening and that injection of these microgels in combination with BMP2 induces an anabolic response in articular cartilage.
Comparative analysis of mRNA levels in articular cartilage following treatment with PlnD1-HA microgels in the presence or absence of BMP2 indicates that BMP2 release from these biomaterials rapidly increases the relative level of transcripts encoding for both aggrecan and its modifying enzyme (XYLT1) during cartilage repair processes [34]. These initial transcriptional events soon were followed by a significant increase in the relative levels of both type II collagen and perlecan mRNAs. The fact that BMP2 efficiently delivered via PlnD1-HA microgels increased the steady state of mRNA encoding for ECM components, strongly implies that the repair mechanisms involved under our experimental conditions primarily consist of de novo synthesis of cartilage matrix by resident chondrocytes. In addition, the small but significant decrease of the relative mRNA levels encoding for MMP3 and MMP13 upon PlnD1-HA/BMP2 treatment suggests that our BMP2 delivery system also may reduce early OA onset by inhibiting articular cartilage matrix degradation. This data contrasts with other reports in which BMP2 stable overexpression via adenoviral integration leads to an initial catabolic response by chondrocytes and boosts matrix turnover [3]. Thus, the lack of catabolic effect accompanied by increased matrix synthesis under our treatment conditions indicates that controlled delivery of BMP2 induces a seemingly exclusive anabolic effect that may protect new and resident cartilage against further destruction by MMPs. Consistent with our results, in vitro studies conducted in chondrocyte progenitors demonstrated that the close homolog of BMP2, BMP4, can strongly down-regulate MMP3 and MMP13 gene expression [45]. In comparison, the relatively low but significant decrease of MMP3 and MMP13 gene expression obtained under our experimental conditions may be attributed to the low amount of chondrocytic stem cells present in adult articular cartilage.
The significant and continuous increase in aggrecan transcript levels during the course of the experiments is accompanied by a transcriptional inhibition of ADAMTS5, the aggrecan-specific protease [30]. Whereas up-regulation of Adamts5 gene expression is a well-accepted indicator of early disease progression, moderate down-regulation of ADAMTS5 transcripts during the initial phase post injection actually may be important for normal cartilage turnover and the creation of space for organized deposition of newly synthesized ECM components [3]. Finally, reduction of both MMP13 and type X collagen mRNA levels in treated versus control (PlnD1-HA) knees indicated that the global reparative effect of PlnD1-HA/BMP2 biomatrices on articular cartilage do not activate developmental program associated with cartilage growth plate terminal differentiation [30,36]. Altogether, our gene expression data shows that BMP2 delivered through PlnD1-HA triggers both anabolic (by increasing the transcription of proteoglycans and type II collagen) and protective responses (by lowering matrix degradation).
The replenishment of proteoglycans such as aggrecan with large negatively charged polysaccharide chains is essential for the restoration of the viscoelastic properties of normal functional hyaline cartilage with ability to resist compressive loads [30]. Therefore, our aggrecan expression data strongly favors a reversion of the early OA damage induced by papain. While Col2a1 gene expression was significantly upregulated, signal specific for type II collagen protein remained unchanged in proteoglycan-depleted regions of the articular cartilage superficial layer. During early OA, the loss of aggrecan is initiated at the joint surface and progresses to the deeper zones before degradation of the collagen fibrillar meshwork [30]. Similarly, the original collagen matrix might not have been destabilized under our experimental conditions and the lack of obvious change in the intensity of type II collagen-specific signal among treatments is likely due to the difficulty of visualizing de novo expression above baseline levels [30]. This idea is supported by the fact that the type II collagen triple helix is remarkably stable (half-life in cartilage ≥100 years) [46].
5. Conclusions
In summary, we demonstrated that single injection of BMP2 complexed to PlnD1-HA based microgels in papain-damaged knees can increase the mRNA levels of articular cartilage matrix components, reverse proteoglycan loss and cartilage erosion, and inhibit cartilage degradative pathways and hypertrophy. Although our experimental design allowed us to study the early effects of bioactive microgels, one of the limitations of this study is that papain is a reversible model of OA that prevents the exploration of the long term effect of single or multiple injections of active microgels. Indeed, self repair mechanisms occur and replenishment of proteoglycans become visible around one month post-papain injection [26]. During the two-week period of our studies, histological scores obtained with PlnD1-HA/BMP2 increased between day 7 and day 14, suggesting the need for a second injection at or before day 14 to prolong the repair effect of BMP2-releasing microgels. However, because of the reversibility of the papain model during the time window that follows a second set of injections this idea is not testable using the current experimental design and will require the use of more severe animal models in which duration of the experiment is longer to test whether the observed anabolic response can overcome wear caused by altered knee biomechanics and normal daily activities. Importantly, our data shows that BMP2 only promotes a strong and sustained anabolic response on compromised cartilage when delivered in a controlled manner that mimics its natural mode of release from the cartilage matrix. Thus, covalent modification of HA with bioactive molecular complexes of native cartilage may constitute a new promising therapeutic option to control the anabolic response of articular cartilage chondrocytes and slow degradative processes in patients susceptible to develop OA at relatively young age. Problems associated with knee injury in young patients exposed to intense daily activities (athletes, military trainees, etc.) include the formation of fibrocartilage at sites of injury followed by progressive degeneration and development of severe OA [47]. Future studies will investigate if multiple injections (weekly intra-articular injections over a period of at least 1.5 months during the acute degradative phase preceding full-thickness articular cartilage damage) of BMP2-releasing PlnD1-HA microgels can help preserve the normal structure of hyaline cartilage and slow disease progression in more severe instability-induced models of knee OA. Yet, recent recommendations for the use of preclinical models in the study and treatment of OA [40] pointed out that less severe animal models (such as papain) are required to better evaluate potential therapies for use in human OA as overly severe experimental models (surgical knee destabilization, iodoacetate intra-articular knee injections) may only constitute tools to study mechanisms involved in irreversible disease progression. In conclusion, the current study shows for the first time that the PlnD1-conjugated, HA-based microgels can enhance BMP2 bioactivity in vivo and are promising injectable materials for the targeted delivery of HBGFs without the initiation of side effects often seen following repetitive administration of growth factors.
Supplementary Material
Acknowledgments
This work was supported by NIH P20-RR016458 (to C. B. Kirn-Safran & M. C. Farach- Carson) and ARRA supplement (to C. B. Kirn-Safran), and NIH R01 DC008965 (to X. Jia). The authors would like to thank Dr. Kirk J. Czymmek (UD-Biological Sciences and Delaware Biotechnology Institute) for his help with tissue imaging, Mrs. Julie Mis Hoffman (UD,OLAM) for assistance with animal husbandry, and Dr. John H. McDonald (UD-Biological Sciences) for assistance with statistical analysis. We also thank Mr. Dylan A. Lowe for assistance with scoring of histological sections. We are grateful to Drs. Esmeralda N. Blaney Davidson and Peter M. Van der Kraan for valuable advice relative to mouse intra-articular injections and articular cartilage quantitative PCR. We are indebted to David Tuke and Sue Seta (OLAM) for their technical help with the initial settings of mouse knee intra-articular injections. We also thank Drs. Erica M. Selva and William R. Thompson for fruitful discussions relative to the role of xylosyltransferases in proteoglycan metabolism, and immunostainings of adult bone tissue, respectively. The author wish to acknowledge Genzyme for the generous gift of the HA starting material.
Footnotes
Conflict of interest
One or more co-authors of the present manuscript have been named as inventors of one or more U.S. patents and/or applications relating to the technology described in the manuscript.
References
- 1.van der Kraan PM, Buma P, van Kuppevelt T, van den Berg WB. Interaction of chondrocytes, extracellular matrix and growth factors: relevance for articular cartilage tissue engineering. Osteoarthritis Cartilage. 2002;10:631–7. doi: 10.1053/joca.2002.0806. [DOI] [PubMed] [Google Scholar]
- 2.Jha AK, Yang W, Kirn-Safran CB, Farach-Carson MC, Jia X. Perlecan domain I-conjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via BMP-2 release. Biomaterials. 2009;30:6964–75. doi: 10.1016/j.biomaterials.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaney Davidson EN, Vitters EL, van Lent PL, van de Loo FA, van den Berg WB, van der Kraan PM. Elevated extracellular matrix production and degradation upon bone morphogenetic protein-2 (BMP-2) stimulation point toward a role for BMP-2 in cartilage repair and remodeling. Arthritis Res Ther. 2007;9:R102. doi: 10.1186/ar2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quintana L, Muinos TF, Genove E, Del Mar Olmos M, Borros S, Semino CE. Early tissue patterning recreated by mouse embryonic fibroblasts in a three-dimensional environment. Tissue Eng Part A. 2009;15:45–54. doi: 10.1089/ten.tea.2007.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–47. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney Int. 2000;57:2207–14. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt B, Ringe J, Haupl T, Notter M, Manz R, Burmester GR, Sittinger M, Kaps C. BMP2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture. Differentiation. 2003;71:567–77. doi: 10.1111/j.1432-0436.2003.07109003.x. [DOI] [PubMed] [Google Scholar]
- 8.Blaney Davidson EN, Vitters EL, van der Kraan PM, van den Berg WB. Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis. 2006;65:1414–21. doi: 10.1136/ard.2005.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dell'Accio F, De Bari C, El Tawil NM, Barone F, Mitsiadis TA, O'Dowd J, Pitzalis C. Activation of WNT and BMP signaling in adult human articular cartilage following mechanical injury. Arthritis Res Ther. 2006;8:R139. doi: 10.1186/ar2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimni ME. Polypeptide growth factors: targeted delivery systems. Biomaterials. 1997;18:1201–25. doi: 10.1016/s0142-9612(97)00050-1. [DOI] [PubMed] [Google Scholar]
- 11.Babensee JE, McIntire LV, Mikos AG. Growth factor delivery for tissue engineering. Pharm Res. 2000;17:497–504. doi: 10.1023/a:1007502828372. [DOI] [PubMed] [Google Scholar]
- 12.Rodgers KD, Sasaki T, Aszodi A, Jacenko O. Reduced perlecan in mice results in chondrodysplasia resembling Schwartz-Jampel syndrome. Hum Mol Genet. 2007;16:515–28. doi: 10.1093/hmg/ddl484. [DOI] [PubMed] [Google Scholar]
- 13.Farach-Carson MC, Carson DD. Perlecan--a multifunctional extracellular proteoglycan scaffold. Glycobiology. 2007;17:897–905. doi: 10.1093/glycob/cwm043. [DOI] [PubMed] [Google Scholar]
- 14.Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–8. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- 15.Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fassler R. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–22. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirn-Safran C, Farach-Carson MC, Carson DD. Multifunctionality of extracellular and cell surface heparan sulfate proteoglycans. Cell Mol Life Sci. 2009;66:3421–34. doi: 10.1007/s00018-009-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French MM, Gomes RR, Jr, Timpl R, Hook M, Czymmek K, Farach-Carson MC, Carson DD. Chondrogenic activity of the heparan sulfate proteoglycan perlecan maps to the N-terminal domain I. J Bone Miner Res. 2002;17:48–55. doi: 10.1359/jbmr.2002.17.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang WD, Gomes RR, Jr, Alicknavitch M, Farach-Carson MC, Carson DD. Perlecan domain I promotes fibroblast growth factor 2 delivery in collagen I fibril scaffolds. Tissue Eng. 2005;11:76–89. doi: 10.1089/ten.2005.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takada T, Katagiri T, Ifuku M, Morimura N, Kobayashi M, Hasegawa K, Ogamo A, Kamijo R. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003;278:43229–35. doi: 10.1074/jbc.M300937200. [DOI] [PubMed] [Google Scholar]
- 20.Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem. 1996;237:295–302. doi: 10.1111/j.1432-1033.1996.0295n.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang W, Gomes RR, Brown AJ, Burdett AR, Alicknavitch M, Farach-Carson MC, Carson DD. Chondrogenic differentiation on perlecan domain I, collagen II, and bone morphogenetic protein-2-based matrices. Tissue Eng. 2006;12:2009–24. doi: 10.1089/ten.2006.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knudson CB, Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12:69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 23.Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:154–62. doi: 10.1016/j.joca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Chevalier X, Jerosch J, Goupille P, van Dijk N, Luyten FP, Scott DL, Bailleul F, Pavelka K. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69:113–9. doi: 10.1136/ard.2008.094623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jazrawi LM, Rosen J. Intra-articular hyaluronic acid: potential treatment of younger patients with knee injury and/or post-traumatic arthritis. Phys Sportsmed. 2011;39:107–13. doi: 10.3810/psm.2011.05.1900. [DOI] [PubMed] [Google Scholar]
- 26.van der Kraan PM, Vitters EL, van de Putte LB, van den Berg WB. Development of osteoarthritic lesions in mice by "metabolic" and "mechanical" alterations in the knee joints. Am J Pathol. 1989;135:1001–14. [PMC free article] [PubMed] [Google Scholar]
- 27.Costell M, Mann K, Yamada Y, Timpl R. Characterization of recombinant perlecan domain I and its substitution by glycosaminoglycans and oligosaccharides. Eur J Biochem. 1997;243:115–21. doi: 10.1111/j.1432-1033.1997.t01-1-00115.x. [DOI] [PubMed] [Google Scholar]
- 28.Kang QK, LaBreck JC, Gruber HE, An YH. Histological Techniques for Decalcified Bone and Cartilage. In: An YH, Martin KL, editors. Handbook of Histology Methods for Bone and Cartilage. Humana Press Inc; Totowa: 2003. pp. 209–219. [Google Scholar]
- 29.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5:94–103. doi: 10.1186/ar630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;12:19–32. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- 32.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald J. Handbook of Biological Statistics. Sparky House Publishing; Baltimore: 2009. [Google Scholar]
- 34.Venkatesan N, Barre L, Magdalou J, Mainard D, Netter P, Fournel-Gigleux S, Ouzzine M. Modulation of xylosyltransferase I expression provides a mechanism regulating glycosaminoglycan chain synthesis during cartilage destruction and repair. Faseb J. 2009;23:813–22. doi: 10.1096/fj.08-118166. [DOI] [PubMed] [Google Scholar]
- 35.Blaney Davidson EN, Vitters EL, van Beuningen HM, van de Loo FA, van den Berg WB, van der Kraan PM. Resemblance of osteophytes in experimental osteoarthritis to transforming growth factor beta-induced osteophytes: limited role of bone morphogenetic protein in early osteoarthritic osteophyte formation. Arthritis Rheum. 2007;56:4065–73. doi: 10.1002/art.23034. [DOI] [PubMed] [Google Scholar]
- 36.Glasson SS, Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–8. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 37.Tardif G, Pelletier JP, Boileau C, Martel-Pelletier J. The BMP antagonists follistatin and gremlin in normal and early osteoarthritic cartilage: an immunohistochemical study. Osteoarthritis Cartilage. 2009;17:263–70. doi: 10.1016/j.joca.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 38.van Beuningen HM, Glansbeek HL, van der Kraan PM, van den Berg WB. Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage. 1998;6:306–17. doi: 10.1053/joca.1998.0129. [DOI] [PubMed] [Google Scholar]
- 39.Bakker AC, van de Loo FA, van Beuningen HM, Sime P, van Lent PL, van der Kraan PM, Richards CD, van den Berg WB. Overexpression of active TGF-beta-1 in the murine knee joint: evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthritis Cartilage. 2001;9:128–36. doi: 10.1053/joca.2000.0368. [DOI] [PubMed] [Google Scholar]
- 40.Poole R, et al. Blake S, Buschmann M, Goldring S, Laverty S, Lockwood S, Matyas J, McDougall J, Pritzker K, Rudolphi K, van den Berg W, Yaksh T. Recommendations for the use of preclinical models in the study and treatment of osteoarthritis. Osteoarthritis Cartilage. 2010;18(Suppl 3):S10–6. doi: 10.1016/j.joca.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 41.Vlodavsky I, Miao HQ, Medalion B, Danagher P, Ron D. Involvement of heparan sulfate and related molecules in sequestration and growth promoting activity of fibroblast growth factor. Cancer Metastasis Rev. 1996;15:177–86. doi: 10.1007/BF00437470. [DOI] [PubMed] [Google Scholar]
- 42.Whitelock JM, Melrose J, Iozzo RV. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47:11174–83. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, Shimono C, Norioka N, Nakano I, Okubo T, Yagi Y, Hayashi M, Sato Y, Fujisaki H, Hattori S, Sugiura N, Kimata K, Sekiguchi K. Activin A binds to perlecan through its pro-region that has heparin/heparan sulfate binding activity. J Biol Chem. 2010;285:36645–55. doi: 10.1074/jbc.M110.177865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohkawara B, Iemura S, ten Dijke P, Ueno N. Action range of BMP is defined by its N-terminal basic amino acid core. Curr Biol. 2002;12:205–9. doi: 10.1016/s0960-9822(01)00684-4. [DOI] [PubMed] [Google Scholar]
- 45.Otto TC, Bowers RR, Lane MD. BMP-4 treatment of C3H10T1/2 stem cells blocks expression of MMP-3 and MMP-13. Biochem Biophys Res Commun. 2007;353:1097–104. doi: 10.1016/j.bbrc.2006.12.170. [DOI] [PubMed] [Google Scholar]
- 46.Maroudas A, Palla G, Gilav E. Racemization of aspartic acid in human articular cartilage. Connect Tissue Res. 1992;28:161–9. doi: 10.3109/03008209209015033. [DOI] [PubMed] [Google Scholar]
- 47.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.