Abstract
Multidrug-resistant tuberculosis was diagnosed in 21 HIV-negative, nonhospitalized male patients residing in northern Tunisia. A detailed investigation showed accelerated transmission of a Mycobacterium tuberculosis clone of the Haarlem type in 90% of all patients. This finding highlights the epidemic potential of this prevalent genotype.
Keywords: Mycobacterium tuberculosis, Multidrug-resistant, outbreak, Haarlem strain, IS6110 RFLP, Accelerated transmission, genotyping
The ability of multidrug-resistant (MDR) strains of Mycobacterium tuberculosis to cause epidemics and spread globally contrasts with the initial perception that MDR tuberculosis (MDR-TB) has a reduced potential for transmission (1,2). In this respect, the W/Beijing type appears to be most common in humans and accounts for most reported MDR-TB outbreaks (3).
In this report, we provide evidence for the epidemic potential of another worldwide prevalent M. tuberculosis genotype, namely, the Haarlem family (4,5). The identified strain is MDR and has rapidly expanded within immunocompetent and nonhospitalized patients.
The Study
M. tuberculosis isolates were obtained from the Laboratory of Mycobacteriology of the Institut Pasteur de Tunis as part of the National Tuberculosis Surveillance Program. All samples (884 specimens) from patients with suspected TB residing in northern Tunisia (Bizerte) from August 2001 to October 2003 were forwarded to us by the referral regional hospital. This hospital serves a region with 483,086 people and an area of 3,501 km2. The incidence of TB in this area from 2001 to 2002 was 29/100,000 male patients and 11/100,000 female patients. All patients received the standard chemotherapy regimen of the Tunisian National Tuberculosis Program, i.e., 2 months of rifampicin, isoniazid, pyrazinamide, and streptomycin, followed by 4 months of rifampicin and isoniazid (2RHZS/4RH). This regimen was introduced into the region in 1995. Of the 193 M. tuberculosis isolates recovered, 20 were MDR. The corresponding patients were interviewed, and detailed epidemiologic investigations were conducted according to described protocols (6). In April 2004, while the study was in progress, a new MDR case was diagnosed.
Analyses by IS6110 restriction fragment length polymorphism (IS6110 RFLP), ligation-mediated polymerase chain reaction (PCR), and spoligotyping were carried out by using standard protocols (7–9). Typing of the polymorphic GC-rich repetitive sequence (PGRS) with probe MTB484 (1) was conducted according to a previously reported protocol (10), with the exception that DNA was digested with AluI. Isolates were assigned to principal genetic groups according to the polymorphisms in the katG and gyrA genes (11). The following primer pairs were used to sequence rpoB, katG, and pncA gene mutations that confer resistance to rifampicin, isoniazid, and pyrazinamide, respectively: rpoB (5´-ATCACACCGCAGACGTTG-3´, 5´-TGCATCACAGTGATGTAGTCG-3´); katG (5´- CGTCGAAACAGCGGCGCTGA-3´, 5´-CAAGCGCCAGCAGGGCTCTT-3´); and pncA (5´-GGCGCACACAATGATCGGTG-3´, 5´-GCTTTGCGGCGAGCGCTCCA-3´). The recently described single nucleotide polymorphisms in putative M. tuberculosis mutator genes mutT1, mutT2, mutT3, and ogt were investigated with the same protocol reported by Rad et al. (12). DNA sequencing was conducted directly on the purified PCR products by using the Prism Ready Reaction Dye Deoxy Terminator Cycle sequencing kit on an ABI Prism 377 DNA sequencer (Applied Biosystems, Foster City, CA, USA).
Epidemiologic and clinical data indicated that all patients with MDR-TB were male with a mean age of 31 years at diagnosis (Table 1). All were Tunisians and permanently resided in the northern part of the country (Bizerte). All patients were seronegative for HIV with no documented history of travel abroad, and none had a history of immunosuppression, diabetes, or respiratory diseases other than TB. Mapping of the 21 patients with MDR-TB according to their residence sites showed that they were mostly scattered over the northeastern part of the region (surface area ≈1,000 km2) with no concentration in a particular locality (data not shown). Resistance to 5 first-line drugs was observed for most isolates (Table 2).
Table 1. Clinical characteristics of 21 patients with multidrug-resistant tuberculosis (MDR-TB), Bizerte, Tunisia, 2001–2004*.
| Patient | Age (y) | Sex | Case history | Isolate used for molecular typing | Initial diagnosis of MDR-TB | Epidemiologic characteristic | Chest radiography |
|---|---|---|---|---|---|---|---|
| P1 | 24 | M | PT | Follow-up, Oct 2001 | Sep 2001 | Brother of patient 14 | Right apical cavity nodular lesion |
| P2 | 26 | M | NC | Initial | Oct 2001 | Same penitentiary as patients 7 and 9 | Left mid-lung nodular opacity with excavation |
| P3 | 25 | M | NC | Initial | Oct 2001 | None apparent | Bi-apical nodular opacity |
| P4 | 62 | M | NC | Initial | Nov 2001 | None apparent | Right apical nodular opacity |
| P5 | 26 | M | PT | Follow-up, Feb 2002 | Sep 2000 | Brother of patient 18 | Diffuse nodular lesions and multiple cavities |
| P6 | 23 | M | PT | Follow-up, Feb 2002 | Feb 2001 | None apparent | Right apical and median bilateral nodular opacity |
| P7 | 24 | M | PT | Follow-up, Mar 2002 | Sep 2000 | Same penitentiary as patients 2 and 9 | Right lobe apical nodular opacity |
| P8 | 27 | M | NC | Initial | Jun 2002 | None apparent | Right apical nodular opacity |
| P9 | 34 | M | PT | Follow-up, Jun 2002 | Aug 2001 | Same penitentiary as patients 2 and 7 | Right apical nodular opacity and left diffuse nodular opacity |
| P10 | 21 | M | NC | Initial | Jun 2002 | None apparent | Right lobe apical nodular opacity and cavity |
| P11 | 42 | M | NC | Initial | Jul 2002 | None apparent | Basal nodular opacity of the right and left lung |
| P12 | 23 | M | NC | Initial | Jul 2002 | None apparent | Left apical cavity and nodular opacity |
| P13 | 29 | M | PT | Follow-up, Aug 2002 | Sep 2001 | None apparent | Bilateral apical and diffuse opacity |
| P14 | 34 | M | NC | Initial | Nov.2002 | Brother of patient 1 | Left apical cavity and nodular lesion |
| P15 | 51 | M | PT | Follow-up, Nov 2002 | ND | None apparent | Bilateral cavity |
| P16 | 17 | M | NC | Initial | Mar 2002 | None apparent | Bilateral nodular infiltration and cavity in the left lung |
| P17 | 17 | M | NC | Initial | May 2003 | Nephew of patient 14 | Wright apical cavity and left lung nodular opacity |
| P18 | 21 | M | NC | Initial | Jun 2003 | Brother of patient 5 | Right apical cavity and nodular opacity |
| P19 | 42 | M | NC | Initial | Jun 2003 | No interview (lost case) | Diffuse nodular opacity and multiple cavities |
| P20 | 53 | M | NC | Follow-up, Oct 2003 | Oct 2002 | None apparent | Right apical cavities and left lobe infiltrate |
| P21 | ND | M | NC | Initial | Apr 2004 | Cousin of patient 14 | ND |
*PT, previously treated; NC, new case; ND, not determined.
Table 2. Laboratory findings and genotyping of multidrug-resistant isolates from 21 tuberculosis patients, Bizerte, Tunisia, 2001–2004*.
| Patient | Smear result | Resistance pattern† | RFLP‡ | Spoligotype | PGG§ | Mutational analysis |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| rpoB | katG | pncA | mutT3 | Ogt | ||||||
| P1 | +++ | HSREZ | 11 | Haarlem3¶ | 2 | S531L+V610M | S315T | A-11C | L209L | T15S |
| P2 | + | HSREZ | 11 | Haarlem3 | 2 | S531L+V610M | S315T | A-11C | L209L | T15S |
| P3 | ++ | HSREZ | 12 | Haarlem3 | 2 | S531L+V610M | S315T | T11G (L4W) | L209L | T15S |
| P4 | - | HSREZ | 11 | Haarlem3 | 2 | S531L+V610M | S315T | A-11C | L209L | T15S |
| P5 | - | HSREZ | 12 | Haarlem3 | 2 | S531L+V610M | S315T | WT | L209L | T15S |
| P6 | + | HSREZ | 11 | Haarlem3 | 2 | S531L+V610M | S315T | WT | L209L | T15S |
| P7 | - | HSREZ | 11 | Haarlem3 | 2 | S531L+V610M | S315T | A-11C | L209L | T15S |
| P8 | - | HSRE | 11 | Haarlem3 | 2 | S531L+V610M | S315T | WT | L209L | T15S |
| P9 | + | HSRE | 11 | Haarlem3 | 2 | S531L+V610M | S315T | A-11C | L209L | T15S |
| P10 | - | HSREZ | 11 | Haarlem3 | 2 | S531L+V610M | S315T | WT | L209L | T15S |
| P11 | - | HSREZ | 12 | Haarlem3 | 2 | S531L+V610M | S315T | WT | L209L | T15S |
| P12 | - | HSREZ | 11 | Haarlem3 | 2 | S531L+V610M | S315T | A-11C | L209L | T15S |
| P13 | - | HSRE | ND# | Haarlem3 | 2 | S531L+V610M | S315T | WT | L209L | T15S |
| P14 | - | HRZ | 11 | Haarlem3 | 2 | S531L+V610M | S315T | G insertion (391-392) | L209L | T15S |
| P15 | - | HSREZ | 11 | Haarlem3 | 2 | S531L+V610M | S315T | A-11C | L209L | T15S |
| P16 | ++ | HSR | 12 | Haarlem3 | 2 | S531L+V610M | S315T | T11G (L4W) | L209L | T15S |
| P17 | - | HSREZ | 11 | Haarlem3 | 2 | S531L+V610M | S315T | G insertion (296-297) | L209L | T15S |
| P18 | - | HSREZ | 12 | Haarlem3 | 2 | S531L+V610M | S315T | T11G (L4W) | L209L | T15S |
| P19 | - | HSRE | 9 | Other** | 2 | ΔN (AAC)519 | S315T | G insertion (296-297) | WT | WT |
| P20 | ++ | HSR | 10 | Haarlem3 | 2 | S531L | S315 | WT | L209L | T15S |
| P21 | ++ | HR | 11 | Haarlem3 | 2 | S531L+V610M | S315T | WT | L209L | T15S |
*RFLP, restriction fragment length polymorphism; PGG, principal genetic grouping; WT, wild type (identical to strain H37Rv); ND, not determined. †H, isoniazid; S, streptomycin; R, rifampicin; E, ethambutol; Z, pyrazinamide. ‡Number of IS6110 bands. §Principal genetic grouping according to gyrA and katG polymorphisms (11). ¶Absence of spacers 31 and 33–36. #IS6110 typing was determined by ligation-mediated polymerase chain reaction and the profile was identical to the other outbreak-associated strains. **Absence of spacers 15, 21–24, and 33–36.
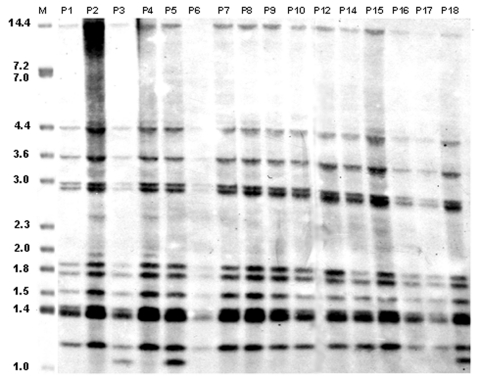
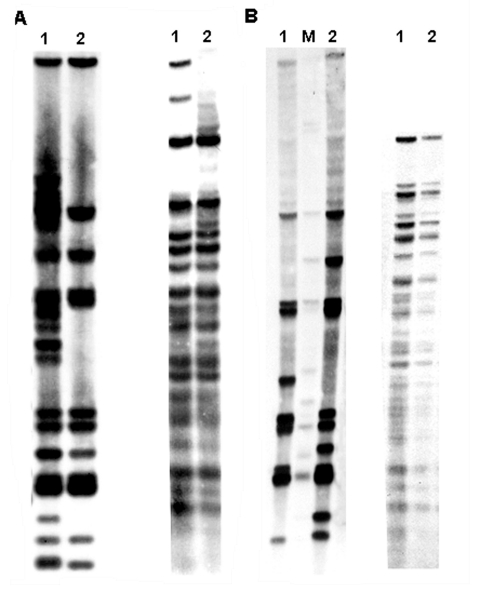
As indicated in Table 1, with the exception of patient P20, the DNA samples subjected to molecular typing were obtained from the initial isolate of all new patients. RFLP showed that 18 patients had nearly identical IS6110 profiles (Figures 1 and 2). The predominant profile (occurring in 13 patients) showed 11 bands, while the remaining 5 patients had an additional IS6110 band. The presence or absence of the additional IS6110 band was not restricted to new or previously treated patients. The RFLP pattern of patient P11, a new patient, clearly showed a mixture of the 12-band profile and some additional IS6110 bands (Figure 2A). Typing of his follow-up culture, which was obtained after 6 months of directly observed short-course therapy, as recommended by the World Health Organization, yielded only the 12-band profile (Figure 2A). Laboratory records and epidemiologic data indicate that this patient likely had a dual infection.
Figure 1.
IS6110 restriction fragment length polymorphism (RFLP) analysis of Mycobacterium tuberculosis isolates from 16 patients associated with the multidrug-resistant tuberculosis outbreak, Bizerte, Tunisia, 2001–2004. Lane M, reference strain MTB14323. Values above each well correspond to each patient as identified in Table 1. Values on the left are in kilobases.
Figure 2.
A) IS6110 restriction fragment length polymorphism (RFLP) analysis (left) and polymorphic GC-rich repetitive sequence (PGRS) typing (right) of patient P11. Lane 1, initial isolate; lane 2, follow-up isolate. B) IS6110 RFLP (left) and PGRS typing (right) of patient P20 (lane 1) compared with patient P3 (lane 2), a typical outbreak-associated patient. Lane M, reference strain MTB14323.
The isolate from patient P13 was typed by ligation-mediated PCR. Its profile was identical to the 18 other MDR isolates. Thus 19 patients with MDR-TB could be clustered according to IS6110-based typing. Effective epidemiologic links were identified for 9 (47%) patients (Table 1). Another similar RFLP pattern was observed for patient P20. It shows 10 IS6110 bands (Figure 2B), 9 of which are common to the 12-band RFLP pattern described for the other isolates. The isolate from patient P19 displayed a 9-band IS6110 profile that was clearly distinct from all the other patients with MDR-TB (data not shown).
With the exception of patient P19, the MDR isolates were identical in their PGRS profile (Figure 2) and spoligotype patterns (Table 2), which is characteristic of the Haarlem3 type (4). Sequence analysis of mutator and drug resistance genes conclusively confirmed that the 19 MDR isolates with nearly identical IS6110 (both 12- and 11-band profiles) are genetically closely related. They all harbor the L209L, T15S, S531L, and S315T mutations in mutT3, ogt, rpoB, and katG genes, respectively (Table 2), whereas mutT1 and MutT2 showed a wild type genotype (data not shown). The occurrence of an additional uncommon mutation in the rpoB gene (V610M) confirmed the clonality of this MDR Haarlem strain since it was present only in 19 patients with MDR-TB. The variability of resistance to pyrazinamide and the mutational profile within the pncA gene (Table 2) strongly suggest that primary transmission from person to person occurred mainly with a strain that was simultaneously resistant to isoniazid and rifampicin.
To extend our analysis of the situation that prevailed in this region, samples from 143 (83%) of 172 patients without MDR strains were spoligotyped. Of these 143 patients, 41 (29%) were female. Overall, 31 (22%) of the 143 patients had Haarlem3 genotype TB. In contrast to the MDR-TB outbreak that involved only men, 6 women had a non-MDR Haarlem3 strain. Aside from the absence of clustering, ligation-mediated PCR typing showed that none of these non-MDR Haarlem3 isolates displayed a profile similar to the 19 MDR isolates involved in the transmission chain. Sequencing of the rpoB gene of 10 isolates randomly selected from the 31 non-MDR Haarlem isolates showed the absence of the outbreak-associated mutation V610M. This finding is strongly indicative of a true clonal expansion and a typical MDR-TB outbreak. The W/Beijing type was absent in the analyzed pool of isolates.
Conclusions
The results indicate that an MDR strain of M. tuberculosis has been actively transmitted among 19 HIV-negative male patients in Tunisia. Several observations indicate that this particular Haarlem strain displays increased transmissibility, virulence, or both. First, the outbreak peaked suddenly within a relatively short period of 21 months; 17 new cases (89%) were reported from September 2001 to June 2003. Inspection of the hospital register for 2000 showed only 3 new patients with MDR isolates, including outbreak-associated patients P5 and P7 (Table 1). Second, no epidemiologic links or contact points could be traced for several patients, which suggests that brief exposure would have been sufficient for effective transmission. Because patients with MDR-TB do not respond to treatment, they may serve as constant sources of transmission. Such a situation is likely to have occurred for the patients with established epidemiologic links. Third, the incidence of TB in the region in which the outbreak occurred is not particularly high. Fourth, patients were seronegative for HIV with no history of treatment causing immunosuppression. Fifth, no AIDS-associated TB outbreak that might have increased the adaptability of the strain within the indigenous population had occurred in the region. Sixth, although the Haarlem strain was MDR, it was able to cause an outbreak in those vaccinated with bacille Calmette-Guérin and in persons who were not hospitalized.
Among the identified M. tuberculosis strain families (4,5), the W/Beijing type has been associated with outbreaks or microepidemics worldwide (3). The Haarlem strain family appears to be widespread (4), but its ability to cause outbreaks has been reported only twice, once in Argentina (13) and once in the Czech Republic (14). The distinctive feature of the present Haarlem MDR-TB outbreak is its accelerated transmission compared with the first 2 MDR-TB outbreaks.
Alterations within DNA repair genes (mutator genes) are thought to favor the emergence of MDR strains with an increased adaptability (12). In this respect, both W/Beijing and Haarlem strains accumulated mutations within their putative mutator genes. Widespread MDR strains might also benefit from their intrinsic adaptability (15). From an epidemiologic point of view, TB programs must conduct extensive surveillance of MDR strains of M. tuberculosis strain families because they might cause serious outbreaks.
Acknowledgments
We thank Fethi Diouani for mapping the MDR cases, Maherzia Ben Fadhel for sequencing, and Rob M. Warren for thoughtfully reviewing the manuscript.
This study was supported by the United Nations Development Program/World Bank/World Health Organization Special Program for Research and Training in Tropical Diseases (TDR).
Biography
Dr. Mardassi is head of a research group at the Institut Pasteur de Tunis. His research interests include the molecular epidemiology of M. tuberculosis and gene expression within the mycobacterial host cell.
Footnotes
Suggested citation for this article: Mardassi H, Namouchi A, Haltiti R, Zarrouk M, Mhenni B, Karboul A, et al. Tuberculosis due to resistant Haarlem strain, Tunisia. Emerg Infect Dis [serial on the Internet]. June 2005 [date cited]. http://dx.doi.org/10.3201/eid1106.041365
References
- 1.Espinal MA. The global situation of MDR-TB. Tuberculosis (Edinb). 2003;83:44–51. 10.1016/S1472-9792(02)00058-6 [DOI] [PubMed] [Google Scholar]
- 2.Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362:887–99. 10.1016/S0140-6736(03)14333-4 [DOI] [PubMed] [Google Scholar]
- 3.Glynn JR, Whiteley J, Bifani PJ, Kremer K, van Soolingen D. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg Infect Dis. 2002;8:843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filliol I, Driscoll JR, van Soolingen D, Kreiswirth BN, Kremer K, Valetudie G, et al. Global distribution of Mycobacterium tuberculosis spoligotypes. Emerg Infect Dis. 2002;8:1347–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kremer K, van Soolingen D, Frothingham R, Haas WH, Hermans PW, Martin C, et al. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J Clin Microbiol. 1999;37:2607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munsiff SS, Bassof T, Nivin B, Li J, Sharma A, Bifani P, et al. Molecular epidemiology of multidrug-resistant tuberculosis, New York City, 1995–1997. Emerg Infect Dis. 2002;8:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prod'hom G, Guilhot C, Gutierrez MC, Varnerot A, Gicquel B, Vincent V. Rapid discrimination of Mycobacterium tuberculosis complex strains by ligation-mediated PCR fingerprint analysis. J Clin Microbiol. 1997;35:3331–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren R, Richardson M, Sampson S, Hauman JH, Beyers N, Donald PR, et al. Genotyping of Mycobacterium tuberculosis with additional markers enhances accuracy in epidemiological studies. J Clin Microbiol. 1996;34:2219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sreevatsan S, Pan X, Stockbauer KE, Connell ND, Kreiswirth BN, Whittam TS, et al. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci U S A. 1997;94:9869–74. 10.1073/pnas.94.18.9869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rad ME, Bifani P, Martin C, Kremer K, Samper S, Rauzier J, et al. Mutations in putative mutator genes of Mycobacterium tuberculosis strains of the W-Beijing family. Emerg Infect Dis. 2003;9:838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritacco V, Di Lonardo M, Reniero A, Ambroggi M, Barrera L, Dambrosi A, et al. Nosocomial spread of human immunodeficiency virus-related multidrug-resistant tuberculosis in Buenos Aires. J Infect Dis. 1997;176:637–42. 10.1086/514084 [DOI] [PubMed] [Google Scholar]
- 14.Kubin M, Havelkova M, Hynccicova I, Svecova Z, Kaustova J, Kremer K, et al. A multidrug-resistant tuberculosis microepidemic caused by genetically closely related Mycobacterium tuberculosis strains. J Clin Microbiol. 1999;37:2715–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson DI. Persistence of antibiotic resistant bacteria. Curr Opin Microbiol. 2003;6:452–6. 10.1016/j.mib.2003.09.001 [DOI] [PubMed] [Google Scholar]