Abstract
Context
Knowledge of family cancer history is important for assessing cancer risk and guiding screening recommendations.
Objective
To quantify how often throughout adulthood clinically significant changes occur in cancer family history that would result in recommendations for earlier or intense screening.
Design and Setting
Descriptive study examining baseline and follow-up family history data from participants in the Cancer Genetics Network (CGN), a US national population-based cancer registry, between 1999 and 2009.
Participants
Adults with a personal history, family history, or both of cancer enrolled in the CGN through population-based cancer registries. Retrospective colorectal, breast, and prostate cancer screening-specific analyses included 9861, 2547, and 1817 participants, respectively; prospective analyses included 1533, 617, and 163 participants, respectively. Median follow-up was 8 years (range, 0–11 years). Screening-specific analyses excluded participants with the cancer of interest.
Main Outcome Measures
Percentage of individuals with clinically significant family histories and rate of change over 2 periods: (1) retrospectively, from birth until CGN enrollment and (2) prospectively, from enrollment to last follow-up.
Results
Retrospective analysis revealed that the percentages of participants who met criteria for high-risk screening based on family history at ages 30 and 50 years, respectively, were as follows: for colorectal cancer, 2.1% (95% confidence interval [CI], 1.8%–2.4%) and 7.1% (95% CI, 6.5%–7.6%); for breast cancer, 7.2% (95% CI, 6.1%–8.4%) and 11.4% (95% CI, 10.0%–12.8%); and for prostate cancer, 0.9% (95% CI, 0.5%–1.4%) and 2.0% (95% CI, 1.4%–2.7%). In prospective analysis, the numbers of participants who newly met criteria for high-risk screening based on family history per 100 persons followed up for 20 years were 2 (95% CI, 0–7) for colorectal cancer, 6 (95% CI, 2–13) for breast cancer, and 8 (95% CI, 3–16) for prostate cancer. The rate of change in cancer family history was similar for colorectal and breast cancer between the 2 analyses.
Conclusion
Clinically relevant family history of colorectal, breast, and prostate cancer that would result in recommendations for earlier or intense cancer screening increases between ages 30 and 50 years, although the absolute rate is low for prostate cancer.
One of the most effective tools to identify individuals at increased risk of cancer is to ascertain their family history. For example, having 1 or more close relatives with colorectal cancer increases risk from 2-fold to 6-fold.1–3 Individuals at increased risk of colorectal, breast, or prostate cancer due to family history are recommended to begin screening for these cancers earlier and in some cases using more sensitive methods than average risk individuals. Those with family histories suggestive of rare hereditary cancer syndromes that confer much higher risks may require even more intense screening or prevention regimens.4
It is estimated that 22% of individuals have a family history that suggests familial or hereditary predisposition to cancer.5 The Agency for Healthcare Research and Quality recommends that primary care clinicians collect a detailed family cancer history including age at diagnosis for affected first- and second-degree relatives.6 Individuals with increased familial risk should be informed of their risk, offered appropriate preventive and screening strategies, and referred for genetic counseling and testing as indicated.
Little is known about how often clinically important changes in cancer family history occur over time that would render an individual at increased risk and, therefore, a candidate for earlier or intensive screening. To our knowledge, no prior reports have addressed this question. This information is useful for determining how often family histories should be updated by clinicians to inform recommendations for screening. In this study, we quantified how often clinically significant changes in family history of breast, colorectal, or prostate cancer occur throughout adulthood.
METHODS
Study Overview
We examined family history data among individuals enrolled in the Cancer Genetics Network (CGN), a US national registry of individuals with a personal or family history of cancer (predominantly breast, prostate, colorectal, and melanoma) established by the National Cancer Institute in 1998.7 As detailed herein, we assessed changes in participants’ self-reported family history over 2 periods: (1) retrospectively, from birth until enrollment into the CGN using family history data provided at entry and (2) prospectively, from enrollment to time of last completed follow-up survey. We specifically examined changes in family history that would render individuals candidates for earlier and more intense screening for colorectal, breast, or prostate cancer based on current guidelines from the American Cancer Society (ACS) (Table 1).
Table 1.
American Cancer Society (ACS) Criteria and Screening Recommendations for Individuals at Increased Risk Due to Family History
| Cancer Type | Criteria for Family History | ACS Screening Recommendation | Criteria for Exclusion From Current Analysesa |
|---|---|---|---|
| Colorectal | ≥1 FDR diagnosed before age 60 y or ≥2 FDRs diagnosed at any age | Colonoscopy every 5 y, starting at age 40 y or 10 y before youngest case in immediate family, whichever is earlier | History of polyposis; uterine cancer diagnosis |
| Breastb | ≥1 FDR diagnosed before age 30 y or ≥1 FDR and ≥1 SDR diagnosed before age 60 y or ≥2 FDRs with 1 diagnosed before age 60 y and 1 diagnosed before age 70 y | Annual magnetic resonance imaging in addition to mammogram for women aged 35–60 y | Male; ovarian cancer diagnosis |
| Prostate | ≥1 FDR diagnosed before age 65 y | Consider prostate-specific antigen blood test screening starting at age 45 y | Female |
Abbreviations: FDR, first-degree relative; SDR, second-degree relative.
Participants with cancer of interest were excluded from each analysis.
Criteria based on predicted lifetime breast cancer risk of at least 20% according to Claus tables.8
Screening Guidelines
Individuals at increased risk of colorectal cancer due to family history are recommended to initiate colonoscopy screening at a younger age and to screen more frequently than those at average risk (Table 1).9 For breast cancer screening, recent studies have demonstrated that breast magnetic resonance imaging (MRI) has greater sensitivity for cancer detection than mammography.10,11 The ACS guidelines recommend addition of annual breast MRI for BRCA1/2 mutation carriers and those with at least a 20% to 25% lifetime risk of breast cancer based on family history (Table 1).12 We used the Claus tables to identify family histories associated with a lifetime breast cancer risk of 20% or greater.13 Having a brother with prostate cancer is associated with a 3-fold increase in risk, and having 2 affected first-degree relatives increases risk 5- to 10-fold.14 The ACS recommends men with 1 or more first-degree relative with prostate cancer before age 65 years to consider prostate-specific antigen (PSA) screening beginning at age 45 years (Table 1).15 We elected to use the ACS guidelines because the ACS provides recommendations for high-risk screening for all 3 cancers of interest (including breast MRI), and these are widely used in clinical practice. Moreover, the ACS guidelines for colorectal and breast cancer screening are identical to those from the National Comprehensive Cancer Network.
Data Collected
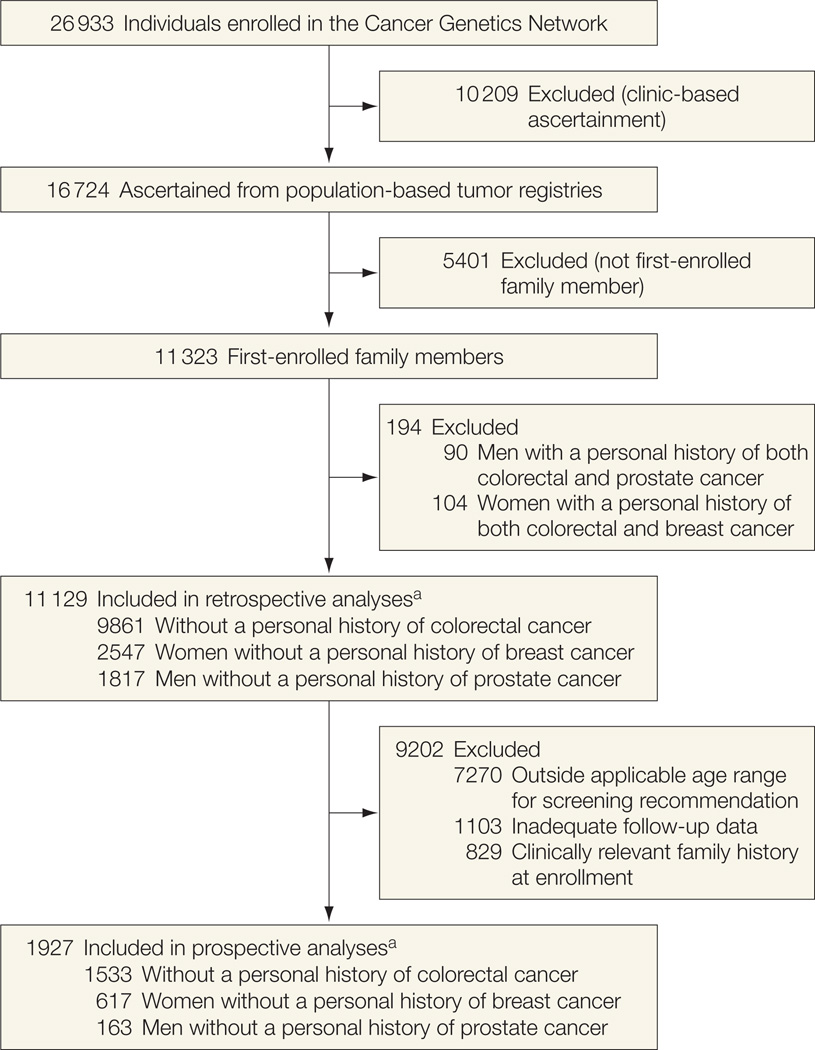
The CGN enrolled 26 933 participants (with family history data on 540 578 family members) at 14 academic research centers across the United States. Institutional review boards at each center approved the study and participants provided written informed consent to be contacted for long-term follow-up. Participants with various types of cancer were ascertained both from local and state tumor registries (ie, population-based ascertainment) and from high-risk cancer clinics. Some centers also recruited cases’ unaffected family members. Only participants ascertained from population-based registries are included in these analyses (n = 16 724). Furthermore, only data from the first enrolled member of each family are included (n = 11 323).
At entry, participants completed a baseline questionnaire (in person or via telephone interview or mail) eliciting information about sociodemographic characteristics, detailed personal and 4-generation family cancer history, cancer-related medical history, and smoking history. Participants at most centers indicated their racial and Hispanic background via separate questions permitting participants to select up to 5 categories for racial/ethnic heritage. Participants were contacted annually or biannually to update baseline information. The median follow-up time was 8 years (range, 0–11 years).
Retrospective Analysis
We used family history information reported at enrollment to construct a snapshot of participants’ family cancer history as it changed from birth until CGN enrollment. For each participant, we constructed a data set with an observation corresponding to each year of life up until entry into the CGN, and for each year we evaluated whether he/she had clinically relevant family history. For example, consider a participant who enrolled at age 45 years in 2000, with a father diagnosed as having colorectal cancer in 1985 at age 55 years. Thus, prior to age 30 years (the participant’s age in 1985) the participant was not at increased risk, and at age 30 years the participant would have met criteria for more intensive colorectal cancer screening.
Using this data set, we then calculated the percentages of participants who would have met the elevated risk screening guidelines at each year of age from birth until enrollment in the registry. Ninety-five percent confidence intervals (CIs) for each age-specific estimate were constructed using the binomial distribution. For comparison with the results from the prospective analysis described below, the 10-year rate of those newly meeting high-risk criteria for screening (number of participants per100whodid not meet criteria at the beginning of the interval but met high-risk criteria sometime during 10 years of follow-up) was calculated for each 10-year age interval.
Prospective Analysis
For this analysis, we evaluated how participants’ family cancer history changed prospectively from CGN enrollment through their most recent follow-up. We determined the number of participants who, at baseline, did not meet the criteria for high-risk screening. Among these individuals (a subset of those in the retrospective analysis), we determined how many would have been classified as meeting high-risk status as of their last follow-up. To compute the 10-year rate among individuals for whom changes in their family history resulted in their newly meeting criteria for earlier or more intensive screening, we divided the number who met high-risk criteria by the total person-years of follow-up and multiplied by 1000 to represent the number of participants per 100 who would have changed to meet high-risk status for every 10 years of follow-up. Confidence intervals for these rates were calculated using the Poisson distribution. We examined rates of meeting high-risk screening criteria overall and stratified by participant age at baseline for comparison with the retrospective analysis.
The prospective analyses were restricted on the basis of participant age at enrollment because of the age-specific nature of the cancer screening criteria: colorectal cancer analysis excluded registrants older than 50 years, prostate cancer analysis included men younger than 50 years, and breast cancer analysis included women aged 35 through 60 years (Table 1). Participants with inadequate follow-up data were excluded from the prospective analysis. Overall, about 22% of otherwise eligible participants were excluded because of missing follow-up information (primarily at 1 large center) or an invalid follow-up date.
Each cancer screening–specific analysis excluded participants who reported the cancer of interest at enrollment (for example, the MRI analyses excluded participants with breast cancer) to compensate for the overrepresentation of cancer cases inherent in the tumor registry–based population. These individuals were included in the other screening-specific analyses given that the risks of breast, colorectal, and prostate cancer are relatively independent from each other. Figure 1 depicts the inclusion/exclusion criteria for participants in the retrospective and prospective analyses.
Figure 1.
Inclusion and Exclusion Criteria for Participants in the Retrospective and Prospective Analyses
a Cancer-specific numbers of participants do not sum to total because some female participants in the colorectal cancer analyses were also included in the breast cancer analyses and some male participants in the colorectal cancer analyses were also included in the prostate cancer analyses. Therefore, the total numbers reflect participants included in at least 1 cancer-specific analysis.
RESULTS
Participants
This study used data from individuals enrolled in the CGN registry. Table 2 presents the distribution of demographic characteristics and extent of family cancer history at baseline for the 11 129 participants included in 1 or more of the retrospective cancer screening–specific analyses. Participants included in the prospective analyses are a subset of this population (Figure 1). The age distribution was fairly consistent across analyses, although women in the breast cancer screening analysis tended to be younger than participants included in the comparable colorectal and prostate cancer analyses. The distribution of race and Hispanic ethnicity was comparable across analyses; however, there was less representation in minority groups than in the general population.
Table 2.
Distribution of Participant Characteristics at Cancer Genetics Network Enrollment by Cancer Screening Subgroup (n = 11 129)
| Characteristics | No. (%) of Participants | ||
|---|---|---|---|
| Colorectal (n = 9861) |
Breast (n = 2547) |
Prostate (n = 1817) |
|
| Age, y | |||
| <40 | 642 (7) | 345 (14) | 128 (7) |
| 40–49 | 1510 (15) | 499 (20) | 234 (13) |
| 50–59 | 2332 (24) | 688 (27) | 411 (23) |
| 60–69 | 2658 (27) | 491 (19) | 453 (25) |
| 70–79 | 2404 (24) | 410 (16) | 486 (27) |
| ≥80 | 315 (3) | 114 (5) | 105 (6) |
| Race | |||
| White | 6957 (71) | 1934 (76) | 1398 (77) |
| Black | 431 (4) | 99 (4) | 50 (3) |
| Asian | 518 (5) | 178 (7) | 153 (8) |
| Other | 90 (1) | 43 (2) | 26 (1) |
| Missing | 1865 (19) | 293 (12) | 190 (11) |
| Hispanic ethnicity | |||
| Yes | 774 (8) | 244 (10) | 233 (13) |
| No | 7066 (72) | 1982 (78) | 1374 (75) |
| Missing | 2021 (20) | 321 (12) | 210 (12) |
| No. of affected first-degree relatives | |||
| 0 | 8841 (90) | 2059 (81) | 1645 (91) |
| 1 | 913 (9) | 421 (17) | 156 (9) |
| 2 | 82 (1) | 49 (2) | 13 (1) |
| >2 | 17 (<1) | 13 (1) | 0 |
| Missing | 8 (<1) | 5 (<1) | 3 (<1) |
| No. of affected second-degree relatives | |||
| 0 | 8316 (84) | 1829 (72) | 1606 (88) |
| 1 | 988 (10) | 474 (19) | 120 (7) |
| 2 | 156 (2) | 110 (4) | 12 (1) |
| >2 | 44 (<1) | 32 (1) | 3 (<1) |
| Missing | 357(4) | 102 (4) | 76 (4) |
Retrospective Analyses
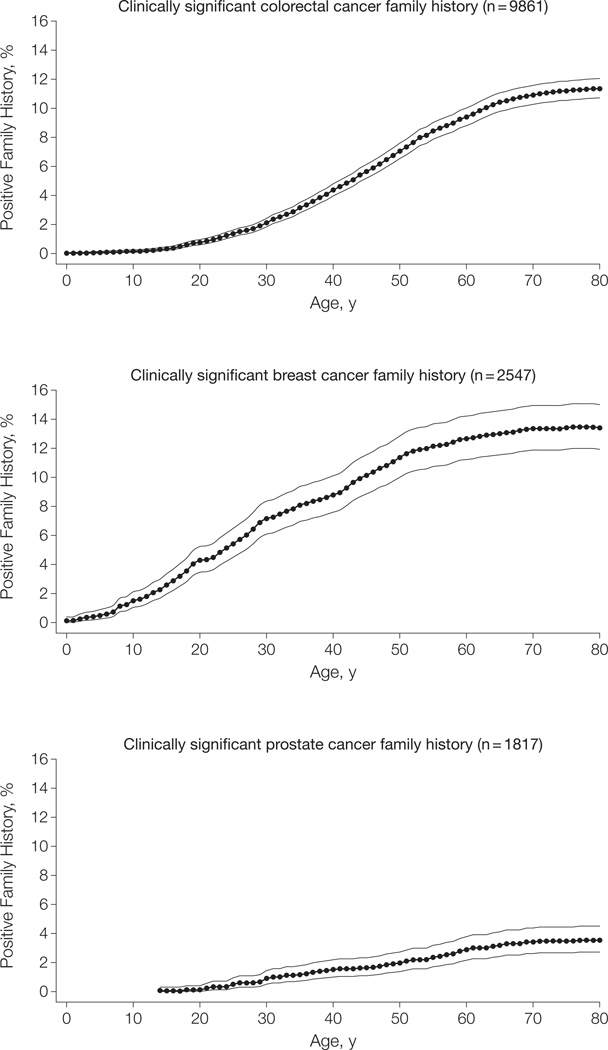
We determined for each year of age from birth through enrollment in the CGN the percentage of registrants who reported clinically relevant family history that would warrant more intense cancer screening for colorectal, breast, and prostate cancer compared with general population guidelines (Figure 2). Each participant contributed data at each yearly point based on retrospective recreation of his/her family history from birth. At age 30 years, 2.1% of participants would have met criteria for early colonoscopy screening (95% CI, 1.8%–2.4%). By age 50 years, this percentage increased to 7.1% (95% CI, 6.5%–7.6%) and peaked at age 70 years at approximately 11%. These estimates are also summarized by decade in Table 3. The 10-year rates for newly meeting high-risk screening criteria based on family history increased from 1 additional person per 100 individuals followed up throughout their 20s, peaking at a rate of 3 additional people per 100 during their 40s.
Figure 2.
Results of Retrospective Analyses: Percentage of Participants With Clinically Significant Cancer Family History by Participant Age
Solid lines indicate 95% confidence intervals.
Table 3.
Results From Retrospective Analyses: Participants With Clinically Significant Cancer Family History by Age (n = 11 129)
| Cancer Type by Age at Enrollment, y |
Participants With Family History |
|
|---|---|---|
| No. (%) [95% CI] | 10-Year Ratea |
|
| Colorectal (n = 9861) | ||
| 20 | 79 (0.8) [0.6–1.0] | |
| 30 | 207 (2.1) [1.8–2.4] | 1 |
| 40 | 434 (4.4) [4.0–4.8] | 2 |
| 50 | 700 (7.1) [6.5–7.6] | 3 |
| 60 | 927 (9.4) [8.8–10.0] | 2 |
| 70 | 1075 (10.9) [10.3–11.6] | 2 |
| 80 | 1124 (11.4) [10.7–12.1] | 1 |
| Breast (n = 2547) | ||
| 20 | 110 (4.3) [3.5–5.2] | |
| 30 | 183 (7.2) [6.1–8.4] | 3 |
| 40 | 227 (8.9) [7.6–10.1] | 2 |
| 50 | 290 (11.4) [10.0–12.8] | 3 |
| 60 | 321 (12.6) [11.2–14.2] | 1 |
| 70 | 339 (13.3) [11.9–14.9] | 1 |
| 80 | 341 (13.4) [11.9–15.0] | 0 |
| Prostate (n = 1817) | ||
| 20 | 2 (0.1) [0–0.4] | |
| 30 | 16 (0.9) [0.5–1.4] | 1 |
| 40 | 27 (1.5) [1.0–2.2] | 1 |
| 50 | 36 (2.0) [1.4–2.7] | 1 |
| 60 | 53 (2.9) [2.2–3.8] | 1 |
| 70 | 62 (3.4) [2.6–4.4] | 1 |
| 80 | 64 (3.5) [2.7–4.5] | 0 |
Abbreviation: CI, confidence interval.
The 10-year rate was calculated as the number of participants who newly met increased-risk criteria per 100 followed up for 10 years.
Results from the retrospective breast cancer analysis show a pattern similar to the colorectal cancer analysis, with steady increases in the percentage of participants who would have met criteria for MRI screening through early and middle age, from 7.2% (95% CI, 6.1%–8.4%) of women at age 30 years to 11.4% (95% CI, 10.0%–12.8%) at age 50 years. After age 60 years, the percentage leveled off at about 13%. The 10-year rates for newly meeting high-risk criteria for screening were fairly even over the decades from ages 20 to 50 years at 3, 2, and 3 additional persons per 100 followed up for 10 years among women aged 20 to 29, 30 to 39, and 40 to 49 years, respectively.
Although the retrospective prostate cancer analysis indicated similar findings of increasing family history until age 60 years, the overall percentage of men who would have met criteria for early PSA screening was much lower at only 0.9% (95% CI, 0.5%–1.4%) of men at age 30 years, and it increased only to 2.0% (95% CI, 1.4%–2.7%) by age 50 years. This could reflect the low incidence of prostate cancer among men younger than 65 years, the family history criterion that prompts PSA screening. As in the colorectal and breast cancer analyses, the increase in the percentage of men with clinically relevant family history leveled off around age 60 years at about 3%; similarly, the 10-year rates for newly meeting high-risk screening criteria were lower for each age interval compared with the rates for colorectal and breast cancer, remaining constant at 1 additional person who met high-risk screening criteria per 100 followed up for 10 years for men aged 20 through 70 years.
Prospective Analyses
The prospective analyses assessed clinically relevant changes in family cancer history from baseline (enrollment in the CGN) to the most recent follow-up (Table 4). The prospective colorectal cancer analysis included data from 1533 participants who were younger than 50 years at enrollment, did not have a personal history of colorectal cancer, and met the other eligibility criteria described previously. During follow-up, 15 registrants reported changes in family history that altered their risk, resulting in a 10-year rate of 1 (95% CI, 0–6) additional person (who becomes eligible for enhanced screening) per 100 participants followed up for 10 years. The age-specific results suggest that more family history changes occur during the 30s (10-year rate, 2 per 100; 95% CI, 0–7) than the 40s (10-year rate, 1 per 100; 95% CI, 0–6), although the CIs are fairly wide and overlapping.
Table 4.
Results From Prospective Analyses: Rate of Change to High-Risk Screening Criteria During Follow-up (n = 1927)
| Cancer Type by Age at Enrollment, y |
Participants Without Family History at Enrollment |
Occurrences of Family History During Follow-up, No. |
10-Year Rate (95%CI)a |
|
|---|---|---|---|---|
| No. | Follow-up, y | |||
| Colorectal | ||||
| 18–29 | 72 | 511 | 0 | 0 (0–4) |
| 30–39 | 359 | 2444 | 4 | 2 (0–7) |
| 40–49 | 1102 | 8021 | 11 | 1 (0–6) |
| Overall | 1533 | 10 976 | 15 | 1 (0–6) |
| Breast | ||||
| 35–39 | 66 | 482 | 0 | 0 (0–4) |
| 40–49 | 223 | 1649 | 7 | 4 (1–10) |
| 50–59 | 328 | 2361 | 6 | 3 (0–9) |
| Overall | 617 | 4493 | 13 | 3 (0–9) |
| Prostate | ||||
| 18–29 | 18 | 134 | 1 | 7 (3–14) |
| 30–39 | 35 | 219 | 1 | 5 (2–12) |
| 40–49 | 110 | 782 | 2 | 3 (0–9) |
| Overall | 163 | 1135 | 4 | 4 (1–10) |
Abbreviation: CI, confidence interval.
The 10-year rate was calculated as the number of participants who newly met increased-risk criteria per 100 followed up for 10 years.
The prospective breast MRI analysis included 617 women aged 35 through 65 years. The overall rate of newly meeting criteria for more intensive screening was 3 (95% CI, 0–9) additional women per 100 followed up for 10 years, with 10-year rates of 0 (95% CI, 0–4) per 100 among women aged 35 to 39 years (when MRI screening starts), 4 (95% CI, 1–10) per 100 for women aged 40 to 49 years, and 3 (95% CI, 0–9) per 100 for women aged 50 to 59 years.
The prospective PSA screening analysis included 163 men younger than 50 years, and the rate of newly meeting criteria for more intensive screening was 4 (95% CI, 1–10) additional men per 100 followed up for 10 years. The age-specific breakdown for the prostate analysis results was similar to the earlier finding that the rate of change in family cancer history is higher in early adulthood than in middle adulthood, with the 10-year rate of newly meeting criteria for more intensive screening of 7 (95% CI, 3–14) per 100 for men younger than 30 years and 5 (95% CI, 2–12) per 100 for men aged 30 to 39 years compared with 3 (95% CI, 0–9) per 100 for men aged 40 to 49 years.
The rates of newly meeting criteria for more intensive screening for colorectal and breast cancer obtained in the retrospective analyses were within the 95% CIs for the comparable age-specific results of the prospective analyses presented in Table 4. However, for prostate cancer, the rates of having first-reported family history that would meet criteria for more enhanced screening in the retrospective analyses were substantially lower than those obtained in the prospective analysis. For comparison with the retrospective rates of change to high-risk status between ages 30 and 50 years, the overall 20-year rates from the prospective analyses are 2 (95% CI, 0–7) per 100 for colorectal cancer, 6 (95% CI, 2–13) per 100 for breast cancer, and 8 (95% CI, 3–16) per 100 for prostate cancer.
COMMENT
We evaluated the rate at which family cancer history changes over time with respect to indicating the need for earlier or intensified screening for colorectal, breast, and prostate cancer. Although family history of cancer was based on self-report, other groups have confirmed the high quality of self-reported data.16 Participants with incomplete family history information (such as unknown age of cancer diagnosis for a relative) were included, as would be the case in the clinical setting, where determination of the need for cancer screening is based on information provided by the patient. However, we acknowledge that participants with a cancer diagnosis may be more motivated to provide accurate family history in a study setting than in a clinic setting.
We used 2 complementary approaches with distinct sets of family history data obtained within the same study population: retrospectively, from birth to enrollment, and prospectively, from enrollment to last follow-up, in a large cohort of individuals followed up to 10 years. Both analyses demonstrate that clinically relevant family history changes substantially during early and middle adulthood, particularly for colorectal and breast cancer, for which the percentage recommended for high-risk screening increases 1.5- to 3-fold between ages 30 and 50 years. Results of the 2 prostate cancer analyses were not in complete agreement, possibly because of the limited data available, particularly for the prospective analysis.
We have taken several steps to design our analyses so the results could be applied to the general US population, although the CGN registry is underrepresented in participants from racial and ethnic minorities and estimates in these populations may differ. For these analyses, we included approximately two-thirds of the registrants who were ascertained via population-based tumor registries. To attenuate the influence of our higher-than-average-risk population, we excluded participants who had a diagnosis of the cancer of interest from the screening-specific analyses. Thus, by excluding these participants, who are more likely to have clinically relevant family history, our results may represent an underestimate of the true rate of change in family history; however, we cannot rule out that our results are overestimates due to the elevated cancer risk level inherent in our population. Scheuner et al5 found that the percentage of adult women who met criteria for genetic evaluation for hereditary breast cancer was similar but lower than ours (2%–4%), but this is not surprising given that their measure was indication for genetic evaluation as opposed to indication for MRI.
Other studies have documented that the majority of patients do not receive adequate familial cancer risk assessment in primary care settings.8,17–20 Family health history data are more likely to be collected at the initial clinic visit and are not adequately updated during follow- up visits.21 These analyses are relevant principally to primary care clinicians, who are often the first (or only) to initiate collection of family health history and are the main source of referral for cancer screening, although they are also relevant for other physicians who provide care to patients over many years. For this analysis, we only examined breast, colorectal, and prostate cancer status and age at diagnosis among first- and second-degree relatives. Subsequent updates require determining only whether there have been any new diagnoses among living first- and second-degree relatives. These questions could be easily added to standard patient questionnaires filled out during routine physician visits. Electronic medical record systems could be designed to prompt physicians for periodic updates and alert them to patients who now meet criteria for high-risk screening.
In our study, we found a 5% chance that an individual’s colorectal cancer screening recommendation would change between ages 30 and 50 years based on family history and that 4% of women would become candidates for MRI screening. Although there is disagreement in the medical community regarding the merits of screening for prostate cancer, family history of prostate cancer also increased over these age ranges. If a patient’s family history is not updated during early and middle adulthood, the opportunity may be missed to intervene with earlier or more intensive screening that maximizes the likelihood of detecting cancer at an early, treatable stage.
Our study has several limitations. First, our determination of whether an individual would meet criteria that would represent an increased risk level and therefore would be recommended for earlier or intensive screening was based solely on reported changes in family history of cancer over time. We did not address how many additional cancers would be expected to be detected if appropriate screening based on the changing family history was performed. Factors such as an individual’s personal medical history or prior cancer screening results were not considered, and these issues also may change during adulthood and need to be assessed by clinicians. Second, our study did not evaluate indications for genetic risk assessment, although obtaining an accurate family history is also critical for identifying patients who merit further genetic evaluation to reduce cancer morbidity in their families. Third, our study did not assess whether participants who met criteria for high-risk screening based on family history and had recommendations for screening made by their physicians actually had additional screening performed.
CONCLUSION
In summary, we found that family history of breast and colorectal cancer becomes increasingly relevant in early adulthood, highlighting the need to obtain a comprehensive family history at this time. We also found that family histories change significantly between the ages of 30 and 50 years. We therefore recommend that family history should be updated at least every 5 to 10 years to appropriately inform recommendations for cancer screening.
Acknowledgments
Funding/Support: The CGN is supported by grants U01CA078284, U24CA078134, U24CA078164, U24CA078156, U24CA078148, U24CA078146, U24CA078174, U24CA078157, and U24CA078142 and contract HHSN2612007440000C from the National Cancer Institute.
Role of the Sponsor: The funding organization was involved in the design and conduct of the CGN resource but not in the design and conduct of the study reported in this article, nor in the collection, analysis, and interpretation of the data or preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Finkelstein had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr Ziogas and Ms Horick contributed equally.
Study concept and design: Ziogas, Kinney, Lowery, Domchek, Isaacs, Griffin, Anton-Culver, Strong, Kasten, Finkelstein, Plon.
Acquisition of data: Ziogas, Horick, Kinney, Lowery, Domchek, Isaacs, Moorman, Edwards, Hill, Berg, Tomlinson, Anton-Culver, Strong, Kasten, Finkelstein.
Analysis and interpretation of data: Ziogas, Horick, Kinney, Lowery, Domchek, Isaacs, Moorman, Hill, Anton-Culver, Finkelstein, Plon.
Drafting of the manuscript: Ziogas, Horick, Kinney, Lowery, Domchek, Isaacs, Hill, Strong, Kasten, Plon.
Critical revision of the manuscript for important intellectual content: Ziogas, Kinney, Lowery, Domchek, Isaacs, Griffin, Moorman, Edwards, Hill, Berg, Tomlinson, Anton-Culver, Finkelstein, Plon.
Statistical analysis: Ziogas, Horick, Anton-Culver, Finkelstein.
Obtained funding: Ziogas, Anton-Culver, Strong, Finkelstein.
Administrative, technical, or material support: Horick, Kinney, Domchek, Isaacs, Moorman, Hill, Tomlinson, Strong, Kasten, Finkelstein.
Study supervision: Horick, Tomlinson, Anton-Culver, Strong, Finkelstein, Plon.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Additional Contributions:We acknowledge Scott Rogers, MPH, and Barbara H. Guest, MSW, MPH, Epidemiology and Genetics Research Program, Division of Cancer Control and Prevention Sciences, National Cancer Institute, for their contributions to the conception and design of the CGN. No compensation was received.
REFERENCES
- 1.American Cancer Society. Cancer Facts and Figures 2007. Atlanta, GA: American Cancer Society; 2007. [Accessed December 13, 2010]. http://www.cancer.org/acs/groups/content/@nho/documents/document/caff2007pwsecuredpdf.pdf. [Google Scholar]
- 2.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994;331(25):1669–1674. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 3.Burt RW, DiSario JA, Cannon-Albright L. Genetics of colon cancer: impact of inheritance on colon cancer risk. Annu Rev Med. 1995;46:371–379. doi: 10.1146/annurev.med.46.1.371. [DOI] [PubMed] [Google Scholar]
- 4.Hampel H, Stephens JA, Pukkala E, et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology. 2005;129(2):415–421. doi: 10.1016/j.gastro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Scheuner MT, McNeel TS, Freedman AN. Population prevalence of familial cancer and common hereditary cancer syndromes: the 2005 California Health Interview Survey. Genet Med. 2010;12(11):726–735. doi: 10.1097/GIM.0b013e3181f30e9e. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi N, Wilson B, Santaguida P, et al. Collection and Use of Cancer Family History in Primary Care: Evidence Report/Technology Assessment No. 159. Rockville, MD: Agency for Healthcare Research and Quality; 2007. AHRQ publication 08-E001. [PMC free article] [PubMed] [Google Scholar]
- 7.Anton-Culver H, Ziogas A, Bowen D, et al. The Cancer Genetics Network: recruitment results and pilot studies. Community Genet. 2003;6(3):171–177. doi: 10.1159/000078165. [DOI] [PubMed] [Google Scholar]
- 8.Sweet KM, Bradley TL, Westman JA. Identification and referral of families at high risk for cancer susceptibility. J Clin Oncol. 2002;20(2):528–537. doi: 10.1200/JCO.2002.20.2.528. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society. Colorectal cancer early detection. [Accessed December 13, 2010]; http://www.cancer.org/Cancer/ColonandRectumCancer/MoreInformation/ColonandRectumCancerEarlyDetection/colorectal-cancer-early-detection-a-c-s-recommendations.
- 10.Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008;148(9):671–679. doi: 10.7326/0003-4819-148-9-200805060-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kuhl C, Weigel S, Schrading S, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol. 2010;28(9):1450–1457. doi: 10.1200/JCO.2009.23.0839. [DOI] [PubMed] [Google Scholar]
- 12.Saslow D, Boetes C, Burke W, et al. American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 13.Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73(3):643–651. doi: 10.1002/1097-0142(19940201)73:3<643::aid-cncr2820730323>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Bratt O, Garmo H, Adolfsson J, et al. Effects of prostate-specific antigen testing on familial prostate cancer risk estimates. J Natl Cancer Inst. 2010;102(17):1299–1301. doi: 10.1093/jnci/djq265. [DOI] [PubMed] [Google Scholar]
- 15.American Cancer Society. Prostate cancer: early detection. [Accessed December 13, 2010]; http://www.cancer.org/Cancer/ProstateCancer/MoreInformation/Prostate CancerEarlyDetection/prostate-cancer-early-detectionacs-recommendations.
- 16.Airewele G, Adatto P, Cunningham J, et al. Family history of cancer in patients with glioma: a validation study of accuracy. J Natl Cancer Inst. 1998;90(7):543–544. doi: 10.1093/jnci/90.7.543. [DOI] [PubMed] [Google Scholar]
- 17.Tyler CV, Jr, Snyder CW. Cancer risk assessment: examining the family physician’s role. J Am Board Fam Med. 2006;19(5):468–477. doi: 10.3122/jabfm.19.5.468. [DOI] [PubMed] [Google Scholar]
- 18.Sifri RD, Wender R, Paynter N. Cancer risk assessment from family history: gaps in primary care practice. J Fam Pract. 2002;51(10):856. [PubMed] [Google Scholar]
- 19.Qureshi N, Wilson B, Santaguida P, et al. Collection and use of cancer family history in primary care. Evid Rep Technol Assess (Full Rep) 2007;(159):1–84. [PMC free article] [PubMed] [Google Scholar]
- 20.Burke W, Culver J, Pinsky L, et al. Genetic assessment of breast cancer risk in primary care practice. Am J Med Genet A. 2009;149A(3):349–356. doi: 10.1002/ajmg.a.32643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acheson LS, Wiesner GL, Zyzanski SJ, Goodwin MA, Stange KC. Family history-taking in community family practice: implications for genetic screening. Genet Med. 2000;2(3):180–185. doi: 10.1097/00125817-200005000-00004. [DOI] [PubMed] [Google Scholar]