Abstract
A bivalent factor H binding protein (fHBP) vaccine for the prevention of disease caused by Neisseria meningitidis serogroup B is currently in clinical development. Since fHBP is also expressed by other meningococcal serogroups, antifHBP antibodies may have bactericidal activity against meningococci independent of serogroup. To begin examining the susceptibility of other meningococcal serogroups to anti-fHBP antibodies, meningococcal serogroup C invasive isolates (n = 116) were collected from the Centers for Disease Control and Prevention's Active Bacterial Core surveillance (ABCs) sites during 2000–2001. These isolates were analyzed for the presence of the fhbp gene. All serogroup C isolates contained the gene, and sequence analysis grouped the proteins into two subfamilies, A and B. Flow cytometry analysis demonstrated that fHBP was expressed on the surface of ∼70% of isolates in vitro with varying levels of expression. fHBP was accessible to antibodies on the cell surface even in the presence of the polysaccharide capsule. Nine isolates from different geographic regions were identified which harboured an identical single nucleotide deletion that could result in a truncated subfamily B fHBP. Analysis by flow cytometry using a polyclonal fHBP antibody preparation revealed that a subpopulation of each of these isolates expressed fHBP. rabbit and non-human primate immune sera generated with bivalent fHBP vaccine were tested for bactericidal activity against a panel of diverse serogroup C clinical isolates using human complement. Sera from both species demonstrated serum bactericidal antibody activity against the serogroup C isolates tested. These promising findings suggest that a bivalent fHBP vaccine may be capable of providing protection against meningococcal disease caused by both serogroup C and B.
Key words: Neisseria meningitidis serogroup C, vaccine, fHBP
Introduction
Meningitis and septicemia caused by Neisseria meningitidis are serious medical problems in both developed and developing countries.1 N. meningitidis is classified by its capsular polysaccharide. While there are several distinct capsular polysaccharide groups or serogroups, only six serogroups, A, B, C, W135, X and Y, are frequently associated with invasive meningococcal disease worldwide. Capsular polysaccharide-based vaccines have been developed and licensed for the prevention of disease caused by serogroups A, C, Y and W135, but there is currently no vaccine that offers broad protection against endemic disease caused by serogroup B. The primary reason a capsular polysaccharide-based vaccine has not been developed for serogroup B is the poor immunogenicity of this capsular polysaccharide. Serogroup B capsular polysaccharide is composed of α-2,8-linked N-acetylneuraminic acid similar to glycans on human cell surfaces.2 As a result, strategies for the development of a vaccine for the prevention of serogroup B disease have focused on non-polysaccharide based antigens.
The search for vaccine antigen candidates capable of eliciting broadly bactericidal immune responses led to the discovery of factor H binding protein (fHBP). fHBP is a lipidated outer membrane protein capable of binding human factor H, a negative regulator of the alternative complement pathway. The fhbp gene is present in all serogroup B meningococcal isolates examined to date and, based on sequence variation, can be divided into two distinct subfamilies, A and B,3,4 based on one classification system or three variants, 1, 2 and 3, based on a separate system.5 A bivalent fHBP vaccine containing a member of each subfamily has been shown to elicit broad bactericidal activity against serogroup B isolates expressing heterologous fHBP6 and is currently in clinical studies as a vaccine against serogroup B disease.7
Limited sequence data suggests that the fhbp gene is also present in other N. meningitidis serogroups.8 Thus, an fHBP-based vaccine could offer protection across other meningococcal serogroups, depending on the prevalence and distribution of the fHBP. To begin to understand the potential impact of an fHBP-based vaccine on other meningococcal serogroups, we analyzed 116 meningococcal serogroup C isolates for the presence of fhbp. These one hundred sixteen invasive clinical isolates were collected during the years 2000–2001 through USA Active Bacterial Core surveillance (ABCs), which is a population and lab-based surveillance system and supported by Centers for Disease Control and Prevention.9 The fhbp sequences were analyzed and fHBP surface expression detected on intact bacteria by flow cytometry. In addition, the bactericidal activity of rabbit and monkey immune sera generated with a bivalent fHBP vaccine against a panel of diverse serogroup C clinical isolates was determined.
Results
Diversity of meningococcal serogroup C isolates.
Of the 116 isolates, 100 (86.2%) belonged to cc11. Within cc11, 77 isolates were ST11; the remaining 23 cc11 isolates belonged to 11 different STs. Within cc11, 37 different fHBP variants were identified. The 16 isolates belonging to clonal complexes other than cc11 were divided between cc103 (five isolates), cc41/44 (four isolates), cc32 (three isolates), cc269 and cc35 with one isolate each and two isolates not assignable to a complex.
fHBP diversity.
The fhbp gene was detected in all 116 study isolates and 43 unique protein sequences were identified. Phlyogenetic analysis (Fig. 1) revealed that all fHBPs were grouped into one of the two subfamilies observed in a comprehensive prevalence-based study of more than 1,800 invasive disease serogroup B isolates.4,10 In the current analysis of the fhbp gene sequences present in meningococcal serogroup C isolates, there were 57 subfamily A isolates with 11 unique fHBP protein variants, and 59 subfamily B isolates with 32 unique variants. Of the 43 fHBP protein variants detected, 23 are new variants not found among the 198 meningococcus serogroup B fHBP protein variants previously detected. No newly identified variant differed by more than seven amino acids from a previously known variant. The frequency distribution of the sergroup C fHBP protein variants is shown in Figure 2.
Figure 1.
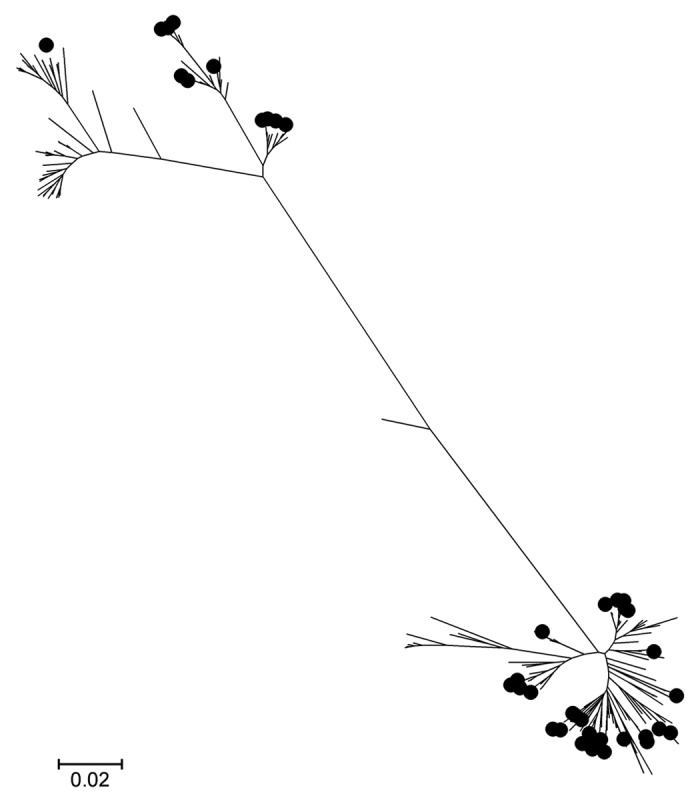
Neighbor-joining Clustalw fHBP tree. The tree incorporates the published 143 meningococcus serogroup B fHBP variants plus 43 serogroup C variants shown in filled circles. The top cluster belongs to fHBP subfamily A and the bottom cluster subfamily B.
Figure 2.
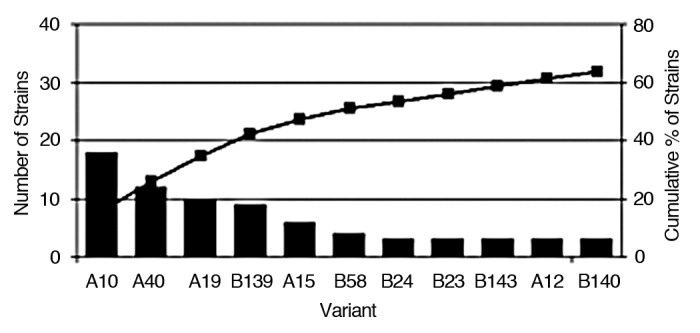
Frequency of the distribution of meningococcus serogroup C fHBP variants. The 11 variants that are represented three or more times are shown. The remaining 32 variants are not shown; 10 of these are represented twice and 22 once. The bars show numbers of isolates of each fHBP variant. The line shows the cumulative of percent meningococcus serogroup C isolates.
Genetic characterization of fhbp truncated sequences.
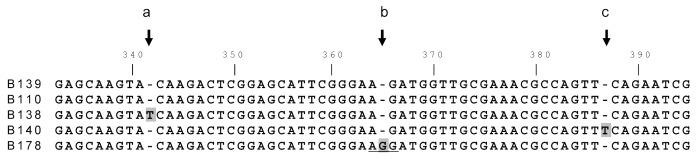
Nine serogroup C isolates encoded a truncated fHBP sequence (B139_001). This variant had a single nucleotide deletion at nucleotide 365 resulting in a frameshift, at amino acid 122 and an early termination following residue 133 (Fig. 3). All nine were ST11 isolates that were not linked by geography as they were present in 5 ABC sites. An identical deletion resulting in the same frameshift and premature truncation was reported in a single meningococcus serogroup B isolate,4 although the two alleles, B139_001 and B110_001, differ at 6 nucleotides in the N-terminal domain. Meningococcus serogroup C isolates carrying variants with the identical deletion at nucleotide 365 but also carrying compensating single nucleotide insertions that restore the correct reading frame within 7–9 amino acids were also identified (B140, 3 isolates in this collection, and B138 [GenBank accession HM588652]), as shown in Figure 3. B138 and B140 are 100% identical to B139 apart from nucleotide 365, as is B178 which carries a G nucleotide at the site of the deletion in B139. In B178 this creates the rare Arg codon AGG in contrast to the AAG (Lys) codon present in virtually all other subfamily B variants. These data suggest that a single deletion event occurred first and was followed by multiple, independent compensating mutations in different isolates. Analysis of porA, porB and fetA sequences for most of these isolates (data not shown) supports the suggestion of a common origin followed by clonal expansion and diversification. The predicted protein that B139_001 encodes encompasses the amino portion of the protein (Fig. 4).
Figure 3.
Alignment of fhbp carrying single nucleotide insertions/deletions. Alignment of fHBP nucleotide sequences in the region surrounding a single nucleotide deletion in B110 and B139 at nucleotide 365 (arrow, position b). B138 and B140 carry the same deletion but each has a compensating insertion of a T after nucleotide 340 (a) or 385 (c), respectively, that restores the correct reading frame within 7–9 amino acids.
Figure 4.
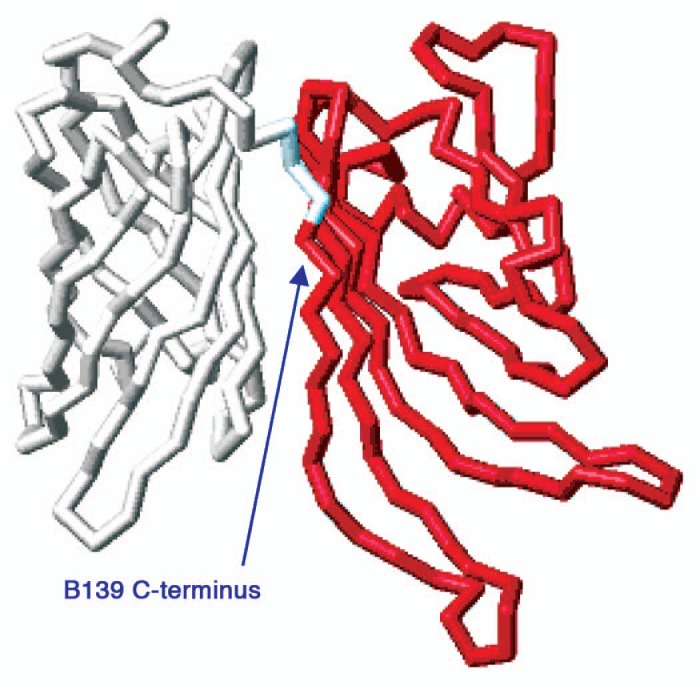
Structural mapping of the B139 sequence. Shown is the top view of the NMr structure of B01 fHBP17 without the N-terminal flexible chain underneath the structure. in red is the region where B139 is aligned with B01, essentially the entire N-domain.
In vitro fHBP and serogroup C polysaccharide capsule expression.
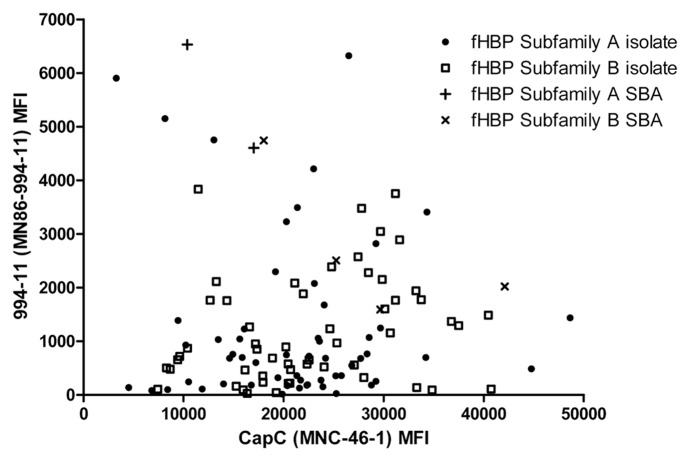
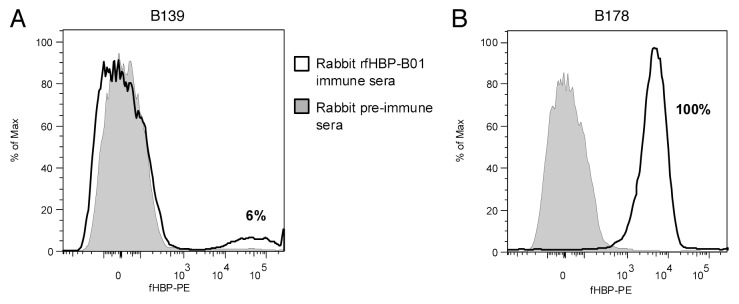
The study isolates were examined for surface expression of fHBP by flow cytometry using mAb MN86-994-11. This antibody recognized fHBP on the surface of 98% of 1,263 meningococcal serogroup B isolates tested.11 For the study isolates, fHBP was detected for 69% of isolates (Fig. 5). Isolates carrying the truncated variant B139 did not demonstrate detectable surface expression of fHBP with this assay. The predicted truncated fHBP would be composed only of the amino-terminal domain. The MN86-994-11 antibody binds to an unknown epitope and thus a negative result in this assay is difficult to interpret. Therefore flow cytometry analysis was also conducted using a rabbit polyclonal antiserum that had been generated by immunization with a subfamily B variant (B01). The nine serogroup C isolates that carried B139 were compared to an isolate that had an otherwise identical nucleotide sequence without the nucleotide deletion at 365 (fHBP B178). In this analysis the isolate that harboured B178 bound the fHBP-B01 polyclonal antiserum (4,875 MFI). Analysis of the nine isolates that harboured the B139 sequence revealed that a sub population of cells in each isolate, between 3 and 21%, did bind to the polyclonal fHBP-B01 antiserum (Fig. 6).
Figure 5.
Serogroup C capsular polysaccharide and fHBP expression levels on serogroup C isolates. Serogroup C capsular polysaccharide and fHBP expression levels on the 116 serogroup C isolates were analyzed by flow cytometry. The serogroup C capsular polysaccharide MFI values are shown on the X-axis and the fHBP MFI values on the Y-axis. fHBP subfamily A isolates are represented by filled circles or crosses (+) if tested in the SBA. fHBP subfamily B isolates are represented by open squares or x if tested in the SBA.
Figure 6.
Analysis of fHBP expression of a B139 meningococcus serogroup C variant by flow cytometry. A representative B139 isolate (A) and the B178 meningococcus sergroup B isolate (B) were analyzed by flow cytometry for surface expression of fHBP using rabbit rfHBP-B01 immune sera and rabbit pre-immune sera as a negative control. The percentage of cells for each of nine isolates harboring B139 that express fHBP by rabbit rfHBP-B01 immune sera range from 3 to 21%. 100% of the B178 bacteria express fHBP by the rabbit immune sera.
The expression of serogroup C polysaccharide capsule was determined by flow cytometry with the mAb MN86-C-46-1. Figure 5 shows that when both fHBP subfamilies are combined, the level of fHBP accessibility is independent of the level of serogroup C polysaccharide capsule expression (Spearman correlation rs = 0.167, p = 0.074). When the fHBP subfamiles are analyzed separately, there is no correlation between capsule and fHBP subfamily A accessibility (Spearman correlation rs = 0.046, p = 0.735) but there is a weak positive correlation with fHBP subfamily B expression (Spearman correlation rs = 0.288, p 0.027). Thus, as capsule expression increases, fHBP subfamily B accessibility also increases and the meningococcal capsule does not interfere with the binding of antibodies to fHBP expressed on the surface of bacteria.
Bactericidal activity of rabbit and cynomologous macaque immune sera.
Serum bactericidal activity (SBA) assays were developed for two subfamily A and four subfamily B serogroup C isolates that had fHBP expression levels of greater than 1,000 MFI units as measured in the MN86-994-11 FACS assay (Table 1). SBA titers against these isolates for rabbit immune sera are shown in Table 2. These same rabbit immune sera were previously demonstrated to kill 87 serogroup B isolates with diverse fHBP variants.6 All the MnC isolates tested were SBA susceptible and therefore killed by the immune serum regardless of fHBP sequence.
Table 1.
Bacterial surface expression of fHBP of selected meningococcus serogroup C isolates for SBA testing
| Serogroup C isolate | ST (Clonal complex) | fHBP Variant (% identity to vaccine) | fHBP MFIa |
| PMB2060 | 2962 (cc11) | A19 (88%) | 4605 |
| PMB2432 | 11 (cc11) | A68 (88%) | 6532 |
| PMB889 | 11 (cc11) | B149 (92%) | 1592 |
| PMB2486 | 11 (cc11) | B157 (88%) | 2023 |
| PMB3001 | 11 (cc11) | B151 (87%) | 2509 |
| PMB2240 | 34 (cc32) | B24 (86%) | 4744 |
MFI, mean fluorescent intensity.
Table 2.
Serum bactericidal activity against serogroup C isolates using serum obtained from rabbits immunized with the bivalent fHBP vaccinea
| SBA titersb | ||||||
| PMB2060 | PMB2432 | PMB889 | PMB2486 | PMB3001 | PMB2240 | |
| Rabbit A | 1408 | 1344 | 2912 | 2592 | 1674 | 315 |
| Rabbit B | 3136 | 4672 | 18624 | 6512 | 928 | 1381 |
| Rabbit C | 960 | 1664 | 1248 | 3775 | 826 | 175 |
| GMT | 1618 | 2186 | 4075 | 3994 | 1087 | 424 |
New Zealand white rabbits were immunized i.m. with 100 µg rfHBP-A and 100 µg rfHBP-B in a formulation with AlPO4 at weeks 0, 4 and 9.
Week 10 SBA titers against six serogroup C isolates are shown. All week 0 SBA titers are below assay detection limit (titer <1:4).
SBA titers were also obtained for cynomologous macaque immune sera (in Table 3). The non human primates elicited bactericidal antibody titers against all the test isolates. These same macaque immune sera were previously shown to have SBA titers against two indicator serogroup B isolates.12
Table 3.
SBA against meningococcus serogroup C isolates using sera obtained from cynomolgus macaques immunized with the bivalent rfHBP vaccinea
| SBA titers | ||||||||||||
| MnC Isolates | ||||||||||||
| PMB2060 | PMB2432 | PMB889 | PMB2486 | PMB3001 | PMB2240 | |||||||
| wk 0 | wk 26 | wk 0 | wk 26 | wk 0 | wk 26 | wk 0 | wk 26 | wk 0 | wk 26 | wk 0 | wk 26 | |
| Monkey Aa | 2 | 272 | 2 | 860 | 2 | 294 | 2 | 161 | 2 | 341 | 5 | 42 |
| Monkey Ba | 2 | 123 | 2 | 366 | 2 | 621 | 2 | 300 | 2 | 288 | 2 | 19 |
| Monkey Ca | 2 | 17 | 2 | 290 | 2 | 338 | 2 | 347 | 2 | 274 | 2 | 50 |
| Monkey Da | 2 | 62 | 2 | 330 | 2 | 384 | 2 | 507 | 2 | 232 | 2 | 18 |
| Monkey Ea | 2 | 32 | 2 | 125 | 2 | 173 | 2 | 535 | 2 | 176 | 2 | 11 |
| GMT | 2 | 65 | 2 | 327 | 2 | 333 | 2 | 340 | 2 | 256 | 2 | 24 |
Cynomolgus macaques were immunized i.m. with 100 µg rfHBP-A and 100 µg rfHBP-B in formulation with AlPO4 at weeks 0, 4 and 24.
Discussion
N. meningitidis fHBP is a promising vaccine candidate for the prevention of meningococcal serogroup B disease. All serogroup B disease isolates contain fhbp and in vitro fHBP surface expression has been detected for 98% of isolates. fHBP protein sequences are conserved and segregate into two distinct subfamiles, A and B3,4 or three variants.5 Previous work demonstrated that a vaccine containing one fHBP from both subfamily A and B induces functional immune responses in mice, rabbits, macaques and humans capable of killing a diverse population of invasive serogroup B isolates and that the level of fHBP expressed by isolates is indicative of whether the strain can be killed in an in vitro SBA.3,6,7,12 The results presented here show that fhbp is also present in all serogroup C clinical isolates that have caused invasive disease in the US in 2000–2001. In nine isolates (8%) fhbp is interrupted by a single nucleotide deletion. The fhbp subfamily distribution within the serogroup C isolates divided approximately equally between the two subfamilies, 57 subfamily A and 59 subfamily B. In contrast, the subfamily distribution in US serogroup B isolates during these years, weighted to account for the epidemic in Oregon,9 was 42% subfamily A and 58% subfamily B,4 and 39% subfamily A and 61% subfamily B in a larger study encompassing the years 2000–2008.16 Compared to our survey of 1,814 serogroup B isolates in the US and Europe,4,10 there was more population diversity with respect to fHBP among the US MnC isolates than among the larger MnB collection: 43 unique fHBP variants within the 116 serogroup C isolates compared to 198 different fHBP variants in the larger serogroup B collection. Of the 43 fHBP variants in the MnC isolates, 23 were new protein sequences not represented among the of 1,814 serogroup B isolates. In the US, during the two years covered by the present study, there were 35 unique variants in 151 (101 without Oregon, which has been experiencing a clonal serogroup B meningococcal outbreak) serogroup B isolates,4 of which 13 were common to both serogroup C and serogroup B isolates. Despite the diversity of fHBP variants found in the serogroup C collection, the fHBP variants had the same distribution within the phylogenetic tree as previously known variants, and no newly described variant differed by more than seven amino acids from those previously described. A subfamily B variant was detected in nine isolates that had a single nucleotide deletion. These isolates did not bind to the fHBP specific mAb; however, a subpopulation of cells did bind to polyclonal antiserum. This warrants further investigation to determine whether this population is expressing full length or truncated fHBP. It is interesting that the deletion was detected at the identical residue in serogroup C and serogroup B isolates. Phylogenetic analysis suggests that a single deletion event occurred first, followed by multiple independent compensating mutations, strongly suggesting that there is selective pressure against the loss of fHBP.
fHBP is accessible to antibody in the presence of capsule in either serogroup C or B.11 Therefore, anti-fHBP sera may have SBA titers against meningococcus regardless of capsule type. To test this hypothesis, six meningococcal serogroup C isolates were used in SBA assays with immune sera from fHBP-immunized animals. The six isolates tested were SBA susceptible. These meningococcal isolates were not diverse with respect to MLST as this collection of invasive serogroup C disease-causing isolates, similar to those reported by others,13 mainly belong to cc11.
Previously, immune sera were generated by immunizing either rabbits or macaques with a bivalent fHBP vaccine containing both a subfamily A and subfamily B protein. The bivalent fHBP vaccine elicits a functional immune response in rabbits capable of killing a collection of serogroup B isolates regardless of fHBP variant, with the major factor for prediction of SBA activity being the level of fHBP expressed on the cell surface.6 Since these antigens have been demonstrated to be present in other meningococcal serogroups,8 fHBP-based vaccines have the potential to be pan-meningococcal vaccines.14 As demonstrated in this study, fHBP is present and expressed on the surface of meningococcal serogroup C isolates, albeit a smaller proportion of isolates than by serogroup B (69% vs. 98%). Moreover, immune sera generated with the bivalent fHBP vaccine killed both serogroup B and C isolates expressing different fHBP variants, thus providing evidence that the bivalent vaccine has the potential to protect across meningococcal serogroups. Additional clinical work will be required to determine the impact of fHBP-based vaccines could have on meningococcal disease as a whole.
Materials and Methods
Serogroup C isolates.
One hundred sixteen invasive clinical meningococcal serogroup C isolates were collected during the years 2000–2001 through USA Active Bacterial Core surveillance (ABCs), which is a population and lab-based surveillance system and supported by Centers for Disease Control and Prevention.9,15
Sequencing.
The fhbp gene in the study isolates was amplified and sequenced as previously described by Murphy, et al. Sequences were deposited in GenBank under accessions HM807448-HM807480. To determine meningococcal genetic lineage, multilocus sequence typing (MLST) was performed and sequence types (STs) assigned according to the protocols at pubmlst.org/neisseria/.
Recombinant fHBP (rfHBP).
Two variants of fHBP, one from each subfamily (A05 and B01), were recombinantly expressed in E. coli as lipoproteins and highly purified using standard chromatographic methodologies essentially as previously described in reference 3.
Generation of serogroup C meningococcal capsule monoclonal antibodies.
A monoclonal antibody (mAb) specific for serogroup C capsule polysaccharide, MNC 46-1, was generated from a fusion with splenocytes that were harvested from mice immunized with serogroup C capsular polysaccharide-CRM conjugate vaccine. Primary fusion screening was done by ELISA with meningococcal serogroup C polysaccharide co-coated with methylated human serum albumin. Subsequent characterization was done on varied meningococcal serogroup C capsule preparations (e.g., de-O-acetyl) and other meningococcal polysaccharide types.
Generation of rabbit rfHBP immune sera.
Preparation of immune sera was previously described in reference 11. New Zealand white rabbits were vaccinated intramuscularly (i.m.) at weeks 0, 4 and 9 with a recombinant bivalent fHBP (A05+B01) vaccine (100 µg of each protein per dose) adsorbed to 250 µg AlPO4 or a recombinant monovalent fHBP (B01). Preimmune (week 0) and immune (week 10) serum samples were obtained for analyses.
Generation of monkey rfHBP immune sera.
Five cynomolgus macaques of both genders ranging in age from 4 to 12 years and weighing 6.32–12.86 kg were used. Before entering the study, macaques were screened for existing antibodies using protein or whole cell ELISAs against rfHBP-A05 or rfHBP-B01. After screening, the macaques with relatively low antibody titers (specific IgG titers <300 units) were vaccinated i.m. at weeks 0, 4 and 24 with a recombinant bivalent rfHBP (A05+B01) vaccine. The vaccine contained 100 µg of each protein and was formulated in 10 mM histidine buffer pH 6.0, 150 mM NaCl, 0.02% Polysorbate 80 and 125 µg of AlPO4. Preimmune (week 0) and immune (week 26) serum samples were obtained for SBA analyses.
Animal care procedures.
All animal protocols employed in these studies met the established Institutional Animal Care and Use Committee guidelines.
Flow cytometry analysis.
fHBP surface expression for each of the 116 serogroup C isolates was measured using the mAb MN86-994-11 and a polyclonal rabbit antiserum raised against rfHBP subfamily B. MN86-994-11 recognizes an epitope common to both fHBP subfamilies, and was used in flow cytometry following procedures previously described in reference 11. Serogroup C capsule expression was measured using the meningococcal serogroup C capsule polysaccharide specific mAb MNC 46-1. Bacteria were grown to an OD650 of 0.45–0.55 and subsequently fixed in 1% (v/v) paraformaldehyde in 1x PBS (Mediatech Inc., Manassas, VA) for at least 10 min. One hundred µl/well of bacteria were plated into 96-well U-bottom polystyrene plates and washed once in 1% (w/v) BSA in 1x PBS. The mAbs or poly-clonal antisera were added to the bacterial pellets, resuspended and incubated on ice for 30 min. After two washes in 1% BSA/PBS, biotinylated goat anti-mouse IgG (subclasses 1 + 2a + 2b + 3) (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) or biotinylated goat anti-rabbit (Southern Biotech, Birmingham, AL) was added to the cell pellets, resuspended and incubated on ice for 30 min. The cells were washed twice and resuspended in streptavidin-PE (Becton Dickinson, Franklin Lakes, NJ) and incubated on ice for 30 min. After two washes in 1% BSA/PBS, the cell pellets were resuspended in 1% paraformaldehyde. Mouse IgG and rabbit pre-immune sera were included as negative controls. Twenty-thousand events per well were acquired on a BD LSR II flow cytometer and analyzed using FlowJo v7 software. The mean fluorescence intensity (MFI) of the PE channel was determined for each sample after gating on bacterial cells in the logarithmic FSC versus SSC dot plot. A sample was considered positive if the MFI was at least three times the control mouse IgG and over 100.
Serum bactericidal activity (SBA) assay.
SBA assays were performed as previously described in references 11 and 16, with human complement sources. The assays were performed in a 96-well microtiter tissue culture plate. The components consisted of 12.5 µl of Dulbecco's phosphate buffered saline (DPBS; Mediatech Inc., Manassas, VA), 12.5 µl of heat-inactivated (56°C for 30 min) serially diluted (two-fold dilutions) test serum, 10 µl of human complement and 15 µl of DPBS buffer containing approximately 1–1.5 x 103 viable N. meningitidis. The complement source used was screened and shown to have no bactericidal activity for the target bacterial isolate. Following a 30 min incubation of the assay mixture on an orbital shaker set to 700 rpm at 37°C in an incubator containing 5% CO2, 140 µl of DPBS was added to each well. GC media (Northeast Laboratory, Waterville, ME) with Kellogg's supplement mixed 1:1 with normal saline was added into a DPBS pre-wetted and drained Millipore filter plate. A 15 µL aliquot of the diluted assay reaction from each assay well was added to the media in the filter plate. Media was carefully vacuum filtered and the plate placed into a zip-lock bag and incubated at 37°C for approximately 18 h in an incubator containing 5% CO2. A methanol-acetic acid de-stain solution (BioRad Laboratories, Hercules, CA) was added to each well for 2 min and then removed via vacuum filtration. A 0.01% solution of Coomassie blue was added for 2 min and then removed by vacuum filtration. A final de-staining step was performed before the plate was allowed to air-dry. The filter plate was then processed for image and colony counts on an Immunospot reader (Cellular Technology, Cleveland, OH). A serum sample with known bactericidal titer was used as a positive serum control. The serum bactericidal titers were defined as the interpolated reciprocal serum dilution that yielded a 50% reduction in colony forming units (CFU) compared to the CFU in control wells without test sera.
Acknowledgements
The authors would like to thank the Meningococcal Active Bacterial Core surveillance sites for collecting isolates. Work at the University of Pittsburgh was performed by Melina Lenser. We would like to thank also Lynn Miller, Kathryn Mason, Han-Qing Jiang, Kristin Alexander, Lubomira Andrew, Kwok-Leung Lee, Elena Camposano and Xiao-Juan Zhao for their technical assistance, Qing Jiang for statistical analyses, Shuo Lin for expert modeling assistance, Terri Mininni for monoclonal antibodies, Robert Zagursky, Gary Zlotnick and Susan Hoiseth for helpful comments and suggestions during the writing of this manuscript.
Disclosure
CDC received funding (under Cooperative Research and Development Agreements) from Pfizer Vaccine Research and Novartis Vaccines. SLH, DZ, EM, LKM, KUJ and ASA are employees of Pfizer. Dr. Harrison receives funding from the Centers for Disease Control and Prevention and the National Institute of Allergy and Infectious Diseases. He receives research support and lecture fees from Sanofi Pasteur; lecture fees from Novartis Vaccines and Pfizer; and has served as a consultant to GlaxoSmithKline, Novartis Vaccines, Sanofi Pasteur, and Pfizer.
References
- 1.WHO, author. Control of Epidemic Meningococcal Disease. Geneva: World Heather Organization; 1998. [Google Scholar]
- 2.Hayrinen J, Jennings H, Raff HV, Rougon G, Hanai N, Gerardy-Schahn R, et al. Antibodies to polysialic acid and its N-propyl derivative: binding properties and interaction with human embryonal brain glycopeptides. J Infect Dis. 1995;171:1481–1490. doi: 10.1093/infdis/171.6.1481. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infection & Immunity. 2004;72:2088–2100. doi: 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy E, Andrew L, Lee KL, Dilts DA, Nunez L, Fink PS, et al. Sequence Diversity of the Factor H Binding Protein Vaccine Candidate in Epidemiologically Relevant Strains of Serogroup B Neisseria meningitidis. J Infect Dis. 2009;200:379–389. doi: 10.1086/600141. [DOI] [PubMed] [Google Scholar]
- 5.Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, et al. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med. 2003;197:789–799. doi: 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang HQ, Hoiseth SK, Harris SL, McNeil L, Zhu D, Tan C, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine. 2010;28:6086–6093. doi: 10.1016/j.vaccine.2010.06.083. [DOI] [PubMed] [Google Scholar]
- 7.Marshall H, Nissen MD, Richmond P, Lambert SB, Roberton DM, Jones T, et al. 16th International Pathogenic Neisseria Conference. Rotterdam, The Netherlands; 2008. A randomized, placebo-controlled, double-blind, phase 1 trial of ascending doses of meningococcal group B rLP2086 vaccine in healthy adults; p. 213. 2008. [Google Scholar]
- 8.Beernink PT, Caugant DA, Welsch JA, Koeberling O, Granoff DM. Meningococcal Factor H-Binding Protein Variants Expressed by Epidemic Capsular Group A, W-135 and X Strains from Africa. The Journal of Infectious Diseases. 2009;199:1360–1368. doi: 10.1086/597806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, et al. Changes in Neisseria meningitidis disease epidemiology in the United States: implications for prevention of meningococcal disease. Clin Infect Dis. 1998;50:184–191. doi: 10.1086/649209. [DOI] [PubMed] [Google Scholar]
- 10.Zlotnick G, Hoiseth SK, Jiang HQ, et al. 10th European Meningococci Disease Society (EMGM) meeting Manchester. UK; 2009. Epidemiology of the serogroup B Neisseria meningitidis (MnB) Factor H binding protein in strains samples from Spain and Germany in the Years 2001–2006. [Google Scholar]
- 11.McNeil LK, Murphy E, Zhao XJ, Guttmann S, Harris SL, Scott AA, et al. Detection of LP2086 on the cell surface of Neisseria meningitidis and its accessibility in the presence of serogroup B capsular polysaccharide. Vaccine. 2009;27:3417–3421. doi: 10.1016/j.vaccine.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 12.Anderson AS, Xu R, Tan C, et al. 16th International Pathogenic Neisseria Conference. Rotterdam, The Netherlands: 2008. Functional cross-reactive antibodies are elicited by a Group B Neisseria meningitidis bivalent recombinant lipidated LP2086 vaccine in Cynomologous macaques. [Google Scholar]
- 13.Brehony C, Wilson DJ, Maiden MCJ. Variation of the factor H-binding protein of Neisseria meningitidis. Microbiology. 2009;155:4155–4169. doi: 10.1099/mic.0.027995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Cohn A, Comanducci M, Andrew L, Zhao X, MacNeil JR, et al. 17th International Pathogenic Neisseria Conference (IPNC) Banff, Canada: 2010. Prevalence and genetic diversity of candidate vaccine antigens among invasive Neisseria meningitidis isolates in the United States. 2010. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention, author. Active Bacterial Core Surveillance. [July 29 2010]. Available at: www.cdc.gov/abcs/index.html.
- 16.Liu X, Wang S, Sendi L, Caulfield MJ. High-throughput imaging of bacterial colonies grown on filter plates with application to serum bactericidal assays. Journal of Immunological Methods. 2004;292:187–193. doi: 10.1016/j.jim.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Mascioni A, Bentley BE, Camarda R, Dilts DA, Fink P, Gusarova V, et al. Structural Basis for the Immunogenic Properties of the Meningococcal Vaccine Candidate LP2086. J Biol Chem. 2009;284:8738–8746. doi: 10.1074/jbc.M808831200. [DOI] [PMC free article] [PubMed] [Google Scholar]