Abstract
AMPA receptors mediate fast excitatory synaptic transmission in the brain, and are dynamically regulated by phosphorylation of multiple residues within the C-terminal domain. CaMKII phosphorylates Ser831 within the AMPA receptor GluA1 subunit to increase single channel conductance, and biochemical studies show that PKC can also phosphorylate this residue. In light of the discovery of additional PKC phosphorylation sites within the GluA1 C-terminus, it remains unclear whether PKC phosphorylation of Ser831 increases GluA1 conductance in intact receptors. Here, we report that the purified, catalytic subunit of PKC significantly increases the conductance of wild-type GluA1 AMPA receptors expressed in the presence of stargazin in HEK293T cells. Furthermore, the mutation GluA1-S831A blocks the functional effect of PKC. These findings suggest that GluA1 AMPA receptor conductance can be increased by activated CaMKII or PKC, and that phosphorylation at this site provides a mechanism for channel modulation via a variety of protein signaling cascades.
Keywords: AMPA receptor, GluA1, GluR1, phosphorylation, PKC
Introduction
Ligand-gated, AMPA-selective ionotropic glutamate receptors are integral membrane proteins that mediate fast, excitatory neurotransmission.1 The central pore of the AMPA receptor is formed by four large independent subunits that assemble as a homomeric or heteromeric dimer-of-dimers.1,2 Each individual subunit possesses an intracellular carboxy terminal domain, located downstream of the third membrane spanning helix, that contains distinct phosphorylation sites that regulate receptor gating, trafficking and localization.3-7
Biochemical studies have identified Ser831 within the C-terminal domain of the GluA1 subunit as a phosphorylation target of both CaMKII and PKC.4,8-10 GluA1-Ser831 phosphorylation by CaMKII does not appear to increase synaptic localization of GluA1-containing receptors,11-13 but it does increase the recombinant homomeric GluA1 receptor current response to glutamate by increasing single channel conductance.3,14 Although both CaMKII- and PKC-dependent increases in GluA1 activity rely on Ser831,8,15 it is unclear whether PKC modulates the channel in a similar manner to CaMKII. We thus set out to test whether PKC can also increase AMPA receptor conductance via phosphorylation of GluA1-Ser831 in functional AMPA receptors in the presence of accessory TARP subunits. We find that inclusion of the purified, catalytic subunit of PKC in the patch pipette intracellular solution significantly enhances conductance of intact homomeric GluA1 AMPA receptors co-assembled with a TARP accessory subunit.
Results and Discussion
PKC has previously been shown to phosphorylate the GluA1 C-terminus,16 and Roche and colleagues used phosphopeptide mapping to identify GluA1-Ser831 as one specific target residue of PKC phosphorylation.10 Derkach et al. (1999) provided the first evidence that phosphorylation at this site by CaMKII enhances the single channel conductance of GluA1 receptors.3 We recently described the mechanism of action that underlies the CaMKII-dependent increase in GluA1 AMPA receptor conductance. Our data showed that addition of a phosphate group to Ser831 within the GluA1 C-terminus by CaMKII enhances the coupling efficiency of glutamate binding to channel gating.14 While early studies that established GluA1-Ser831 as a substrate for serine/threonine kinases focused on PKC,10 subsequent work has primarily focused on the CaMKII-dependent increase in GluA1 conductance.3,14 PKC phosphorylation of GluA1-Ser831 appears to increase GluA1 currents in a dose-dependent manner,15 but a PKC-dependent conductance increase via Ser831 phosphorylation has not been described.
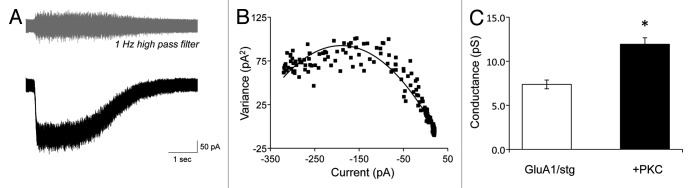
Here, we set out to determine whether the conductance of GluA1 homomeric AMPA receptors in the presence of stargazin can also be increased by phosphorylation of Ser831 by PKC. To do this, we examined whether the purified, catalytic fragment of PKC affects the weighted mean conductance (γMEAN) of GluA1 AMPA receptors expressed in the presence of stargazin in HEK cells. A saturating concentration of glutamate (1 mM) was applied to excised, outside-out macro patches pulled from HEK cells transfected with GluA1 and stargazin (Fig. 1A). In order to obtain conductance measurements, stationary variance analysis was performed using a GluA1 construct in which Leu497 was mutated to tyrosine to block macroscopic desensitization.17 A current response with a graded waveform was generated by slowly washing glutamate from the patch, and γMEAN was determined from variance analysis of the current response (Fig. 1B and C). Inclusion of the purified, catalytic subunit of PKC within the patch pipette solution increased the γMEAN of recombinant homomeric wild-type GluA1-L497Y AMPA receptors in HEK cells from 7.4 ± 0.5 pS (n = 8) to 13.5 ± 1.1 pS (Table 1 and Fig. 1C; n = 14, p < 0.001 by Student’s t-test).
Figure 1. PKC increases γMEAN of homomeric GluA1-L497Y AMPA receptors. (A) Representative macroscopic current response to 1 mM glutamate in an excised outside-out patch recorded under voltage clamp (VHOLD = -60 mV). The upper trace shows the response after high pass filtering, illustrating the increase in membrane current noise during the response time course. (B) Current-variance relationship used to determine γMEAN for the trace shown in (A). (C) Summary of PKC effects on γMEAN of GluA1-LY AMPA receptors expressed in the presence of stargazin in HEK293T cells. *p < 0.001 by Student’s t-test. Data are from 8–14 outside-out patches for each condition.
Table 1. Purified PKC phosphorylates GluA1-Ser831 to enhance γMEAN of AMPA receptors expressed in HEK cells.
| Receptor | Intracellular | # of Channels | γMEAN (pS) | PO | n |
|---|---|---|---|---|---|
| GluA1-LY-SSTS |
Control |
452 ± 84 |
7.4 ± 0.5 |
0.80 ± 0.02 |
8 |
| GluA1-LY-SSTS |
+ PKC/ATP |
570 ± 114 |
13.5 ± 1.1* |
0.70 ± 0.03 |
14 |
| GluA1-LY-SSTS |
+PKC |
572 ± 166 |
8.6 ± 0.8 |
0.82 ± 0.07 |
6 |
| GluA1-LY-ASAA |
Control |
356 ± 134 |
9.8 ± 1.6 |
0.60 ± 0.07 |
6 |
| GluA1-LY-ASAA |
+ PKC/ATP |
666 ± 121 |
17.7 ± 1.8* |
0.72 ± 0.07 |
6 |
| GluA1-LY-AAAA |
Control |
600 ± 90 |
8.7 ± 2.1 |
0.70 ± 0.08 |
8 |
| GluA1-LY-AAAA | + PKC/ATP | 371 ± 124 | 8.4 ± 1.8 | 0.65 ± 0.04 | 6 |
Weighted mean unitary conductance, γMEAN, was determined using variance analysis of current responses obtained from transfected HEK cells. All mutant GluA1 subunits contained the L497Y mutation to block desensitization, and the S845A mutation to block endogenous PKA phosphorylation at this site. In addition, alanine was substituted for other putative serine/threonine PKC phosphorylation targets (GluA1-Ser831 and/or, Ser818 and GluA1-Thr840) in order to isolate effects of PKC at GluA1-Ser831. All AMPA receptors were coexpressed with stargazin. Values are mean ± SEM; n is the number of outside-out patches studied at a holding potential of -60 mV. *p < 0.01 by one way ANOVA, with Tukey’s post hoc test. Open probability (PO) was calculated as the ratio of the maximal macroscopic current to the product of the fitted unitary current and number of channels. Neither the number of channels nor PO was significantly affected by inclusion of PKC in the intracellular solution (by one-way ANOVA).
Like Ser831, GluA1-Ser818 and -Thr840 have also been established as PKC phosphorylation sites.18-20 Thus, in order to study the effects of PKC at GluA1-Ser831 in isolation, a mutant GluA1 AMPA receptor construct was used that expressed alanine substitutions at both Ser818 and Thr840. In addition, GluA1-Ser845 was mutated to alanine to prevent phosophorylation by endogenous PKA. For simplicity, this mutant is referred to as GluA1-LY-ASAA (GluA1-L497Y,S818A,Ser831,T840A,S845A). PKC also enhanced the conductance of the GluA1-LY-ASAA mutant receptor. When the purified, catalytic fragment of PKC was included in the patch pipette solution, γMEAN was increased from 9.8 ± 1.6 pS (n = 6) to 17.7 ± 1.8 pS (Table 1; n = 6, p < 0.01 by ANOVA). Although GluA1-Ser818 and Thr840 were mutated to alanine to prevent any competing functional effects of PKC at those residues, it is still possible that the increased conductance could result from PKC acting at other targets within the GluA1 C-terminus or associated proteins such as stargazin. To ensure that the increased conductance was indeed a result of PKC phosphorylation of GluA1-Ser831, this residue was mutated to alanine to block phosphorylation at Ser831 (GluA1-L497Y,S818A,S831A,T840A,S845A or GluA1-LY-AAAA). PKC did not induce a significant change in conductance of GluA1-LY-AAAA receptors in the presence of stargazin (8.7 ± 2.1 pS; n = 8), as compared with control (Table 1; 8.4 ± 1.8 pS; n = 6; p > 0.05 by ANOVA). To ensure that the observed effects of PKC on conductance were due to its catalytic activity and not an effect of PKC binding to GluA1, we repeated the experiments on wild-type GluA1 receptors using an intracellular solution that contained PKC but lacked ATP. PKC is catalytically active, but the lack of ATP will prevent the kinase from phosphorylating its substrates, including GluA-Ser831. PKC in the absence of ATP had no significant effect on the conductance of GluA1-LY receptors with wild-type C-terminal (Table 1; 8.6 ± 0.8 pS; n = 6), as compared with recordings performed in the presence of ATP and PKC (7.4 ± 0.5 pS; n = 8; p > 0.05). These results establish that the PKC-mediated conductance increase results from PKC-catalyzed phosphorylation of GluA1-Ser831.
Previous studies that examined the mechanism by which GluA1-Ser831 phosphorylation augments channel activity have focused on phosphorylation at this site by CaMKII but not PKC. However, the conductance increase described in these studies could depend on binding of CaMKII to the C-terminal tail. If so, PKC phosphorylation of GluA1-Ser831 would not have increased receptor conductance. Phosphomimetic mutations inserted in place of GluA1-Ser831 mimic the CaMKII-mediated conductance increase, suggesting that the presence of a negatively charged group at GluA1-Ser831 alone can induce the structural changes necessary to increase conductance. Data presented here further support the idea that Ser831 phosphorylation, by either PKC or CaMKII, can enhance AMPA receptor conductance.3,14 In addition, these data confirm that GluA1 Ser831 is a substrate for PKC in functional full length receptors.
Examining this phenomenon is of particular importance in light of the report that PKC may be as physiologically relevant for GluA1 phosphorylation at Ser831 as CaMKII.21 While it has been suggested that A-kinase-anchoring protein 79 (AKAP79) targeting of PKC to GluA1 via synapse-associated protein 97 (SAP97) is not important in regulation of GluA1 in some systems,22 the data presented here show that PKC can phosphorylate GluA1 to enhance conductance. Indeed, AKAP79 binds and concentrates PKC near GluA1 via the mutual interaction of AKAP79 and GluA1 with the scaffolding protein SAP97.15 This mechanism makes PKC a more potent regulator of GluA1 phosphorylation at this site than CaMKII. However, active CaMKII can bind and phosphorylate SAP97 leading to uncoupling of AKAP79 from GluA1 subunits,23 which likely helps to stabilize CaMKII phosphorylation of GluA1-Ser831 (Fig. 2). Thus a complex interplay between the AMPA receptor, CaMKII, PKC and their scaffolding proteins exists. Data showing that either PKC or CaMKII can enhance AMPA receptor conductance by phosphorylating GluA1-Ser831 suggest that phosphorylation of GluA1-Ser831 could provide a common end point for enhancing the activity of AMPA receptors via multiple signaling pathways within neurons. These findings highlight the versatile nature of the GluA1 C-terminus and reveal multiple mechanisms by which phosphorylation at Ser831 could regulate synaptic function in the central nervous system.
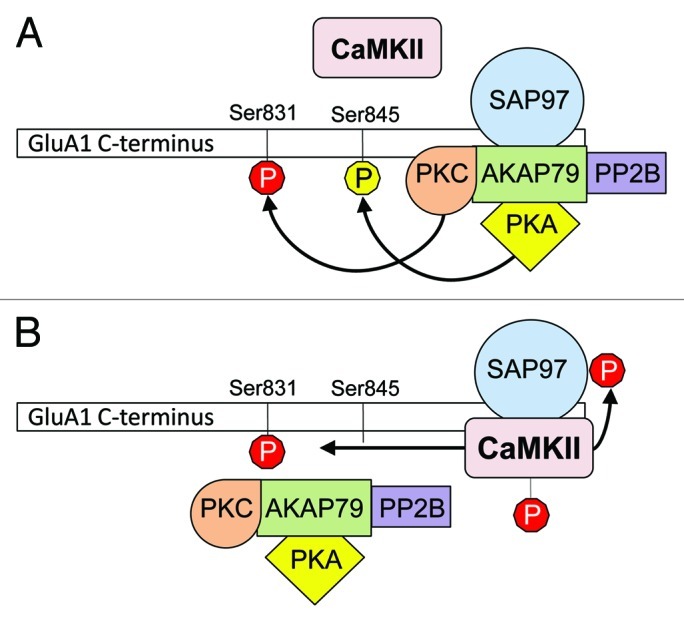
Figure 2. Scaffolding proteins mediate PKC-dependent vs. CaMKII-dependent GluA1-Ser831 phosphorylation. (A) AKAP79 localizes PKA and calcineurin (PP2B) to the GluA1 C-terminus via SAP97 to allow phosphorylation and dephosphorylation of Ser845 by PKA and PP2B, respectively.15,27 AKAP79, with SAP97, also promote phosphorylation of GluA1 at Ser831 by PKC. (B) When CaMKII is activated by autophosphorylation, it binds and phosphorylates SAP97, which disrupts phosphorylation of GluA1 by PKC and dephosphorylation of GluA1-Ser845 by PP2B. Phosphorylation at Ser831 is then mediated primarily by CaMKII rather than PKC.15,23 Our results suggest that phosphorylation of GluA1-Ser831 by either PKC or CaMKII14 can increase AMPA receptor conductance.
Materials and Methods
Molecular biology
The coding region of rat GRIA1 gene was placed in the CMV-based mammalian expression vector pRK5 (BD PharMingen), and this construct was used for transient expression of GluA1 in HEK293T cells. The GluA1-S818A, S831A, T840A and S845A mutations, as well as the non-desensitizing mutation L497Y, were inserted into GluA1 constructs using the QuikChange mutagenesis method according to the Stratagene protocol; cDNA sequences were verified by DNA sequencing (SeqWright, Fisher Scientific). Stargazin (rat) was contained within an IRES-EGFP vector,24 to allow for identification of transiently transfected cells.
Maintenance and transfection of HEK 293T cells
All cell biology reagents were from Gibco (Invitrogen) unless otherwise stated. Human embryonic kidney cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal calf serum, 100 units/ml of penicillin, and 100 ug/ml of streptomycin on polystyrene culture dishes (or 8 mm glass goverslips coated with poly-d-lysine) in a humidified atmosphere of 5% CO2, 95% O2, at 37°C. To protect against excitotoxicity by endogenous glutamate in the media, transfected cells were grown in DMEM supplemented with 200 µM NBQX (2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione, a non-selective AMPA receptor antagonist; Tocris). Trypsin-EDTA (0.05%) was used to passage cells. Cells plated on coverslips contained in 24-well tissue culture plates were transfected using FuGENE6 (Invitrogen) according to the manufacturer’s protocol.
Recording and analysis of macroscopic currents from excised membrane patches
Outside-out membrane patches were excised from transiently-transfected HEK cells using thick-walled borosilicate micropipettes (1.5 mm OD, 0.86 mm ID; World Precision Instruments) filled with internal solutions comprised of (in mM) 110 gluconic acid, 110 CsOH, 30 CsCl, 4 NaCl, 5 HEPES, 4.37 EGTA, 2.1 CaCl2, 2.27 MgCl2, 0.1 spermine (Sigma-Aldrich), 4 ATP, 0.3 GTP; for some experiments ATP was omitted from the internal solution. The pH was adjusted to 7.3 with CsOH. For some experiments the intracellular solution was supplemented with the catalytic fragment of PKC purified from rat brain (20 nM; Sigma-Aldrich). Pipettes had a tip resistance of 4–6 MΩ. External recording solution for all experiments were comprised of (in mM) 150 NaCl, 10 HEPES, 3 KCl, 1 CaCl2, 1 MgCl2, pH 7.4; 310–330 mOsm. Currents were recorded at room temperature (23°C) at a holding potential (VHOLD) of -60 mV with an HEKA EPC9 amplifier, filtered at 5 kHz (-3dB), and digitized with a sampling rate of 20 kHz.
Stationary variance analysis of macroscopic currents was performed as previously described.25,26 Briefly, variance (σ2) can be related to the macroscopic (I) and unitary current (i) amplitude by
| σ2 = i I – (I2 / N) | (1) |
where N is the number of channels. Nonlinear least squares fitting of this equation to the data was used to determine the unitary current and number of channels. The unitary current is a weighted mean average of all single channel conductance levels.26 Chord conductance (γNOISE) was determined assuming a 0 mV reversal potential.
Statistical methods
Results are expressed as mean ± SEM. Statistical analysis of pair wise or multiple comparisons were performed using Student’s t-test or ANOVA with Tukey’s post hoc test. p < 0.05 was considered to be statistically significant. Power of all statistical tests was at least 0.8.
Acknowledgments
This work was supported by the NIH (NS068464). The authors thank Drs Roger Colbran and Steve Tavalin for their insightful comments.
Glossary
Abbreviations:
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- PKC
protein kinase C
- CaMKII
Ca2+/calmodulin dependent protein kinase II
- TARP
transmembrane AMPA receptor regulatory protein
- SAP97
synapse-associated protein 97
- AKAP79
A-kinase-anchoring protein 79
- PP2B
calcineurin
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/18648
References
- 1.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–96. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–56. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA. 1999;96:3269–74. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272:32727–30. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–43. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 6.Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, et al. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–8. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- 7.Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–5. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 9.Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–33. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- 10.Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–88. doi: 10.1016/S0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 11.Boehm J, Malinow R. AMPA receptor phosphorylation during synaptic plasticity. Biochem Soc Trans. 2005;33:1354–6. doi: 10.1042/BST20051354. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–7. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 13.Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–43. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, et al. Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci. 2011;14:727–35. doi: 10.1038/nn.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tavalin SJ. AKAP79 selectively enhances protein kinase C regulation of GluR1 at a Ca2+-calmodulin-dependent protein kinase II/protein kinase C site. J Biol Chem. 2008;283:11445–52. doi: 10.1074/jbc.M709253200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackstone C, Murphy TH, Moss SJ, Baraban JM, Huganir RL. Cyclic AMP and synaptic activity-dependent phosphorylation of AMPA-preferring glutamate receptors. J Neurosci. 1994;14:7585–93. doi: 10.1523/JNEUROSCI.14-12-07585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern-Bach Y, Russo S, Neuman M, Rosenmund C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron. 1998;21:907–18. doi: 10.1016/S0896-6273(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 18.Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–25. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, et al. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–87. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HK, Takamiya K, Kameyama K, He K, Yu S, Rossetti L, et al. Identification and characterization of a novel phosphorylation site on the GluR1 subunit of AMPA receptors. Mol Cell Neurosci. 2007;36:86–94. doi: 10.1016/j.mcn.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks IM, Tavalin SJ. Ca2+/calmodulin-dependent protein kinase II inhibitors disrupt AKAP79-dependent PKC signaling to GluA1 AMPA receptors. J Biol Chem. 2011;286:6697–706. doi: 10.1074/jbc.M110.183558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshi N, Langeberg LK, Scott JD. Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat Cell Biol. 2005;7:1066–73. doi: 10.1038/ncb1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikandrova YA, Jiao Y, Baucum AJ, Tavalin SJ, Colbran RJ. Ca2+/calmodulin-dependent protein kinase II binds to and phosphorylates a specific SAP97 splice variant to disrupt association with AKAP79/150 and modulate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptor (AMPAR) activity. J Biol Chem. 2010;285:923–34. doi: 10.1074/jbc.M109.033985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating. Neuron. 2007;55:905–18. doi: 10.1016/j.neuron.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin R, Banke TG, Mayer ML, Traynelis SF, Gouaux E. Structural basis for partial agonist action at ionotropic glutamate receptors. Nat Neurosci. 2003;6:803–10. doi: 10.1038/nn1091. [DOI] [PubMed] [Google Scholar]
- 26.Traynelis SF, Jaramillo F. Getting the most out of noise in the central nervous system. Trends Neurosci. 1998;21:137–45. doi: 10.1016/S0166-2236(98)01238-7. [DOI] [PubMed] [Google Scholar]
- 27.Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD. Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J Neurosci. 2002;22:3044–51. doi: 10.1523/JNEUROSCI.22-08-03044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]