Abstract
In this paper, we present two novel perspectives on the function of the left inferior frontal gyrus (LIFG). First, a structured sequence processing perspective facilitates the search for functional segregation within the LIFG and provides a way to express common aspects across cognitive domains including language, music and action. Converging evidence from functional magnetic resonance imaging and transcranial magnetic stimulation studies suggests that the LIFG is engaged in sequential processing in artificial grammar learning, independently of particular stimulus features of the elements (whether letters, syllables or shapes are used to build up sequences). The LIFG has been repeatedly linked to processing of artificial grammars across all different grammars tested, whether they include non-adjacent dependencies or mere adjacent dependencies. Second, we apply the sequence processing perspective to understand how the functional segregation of semantics, syntax and phonology in the LIFG can be integrated in the general organization of the lateral prefrontal cortex (PFC). Recently, it was proposed that the functional organization of the lateral PFC follows a rostro-caudal gradient, such that more abstract processing in cognitive control is subserved by more rostral regions of the lateral PFC. We explore the literature from the viewpoint that functional segregation within the LIFG can be embedded in a general rostro-caudal abstraction gradient in the lateral PFC. If the lateral PFC follows a rostro-caudal abstraction gradient, then this predicts that the LIFG follows the same principles, but this prediction has not yet been tested or explored in the LIFG literature. Integration might provide further insights into the functional architecture of the LIFG and the lateral PFC.
Keywords: sequence processing, language, cognitive control, inferior frontal gyrus, lateral prefrontal cortex
1. Introduction
In the past decade, a growing number of papers have combined artificial grammar learning (AGL) paradigms with neuroimaging methods such as functional magnetic resonance imaging (fMRI) [1–10], event-related potentials [3] and brain stimulation techniques [11–13] to study structured sequence processing, pattern perception and/or rule learning. In AGL, subjects are exposed to patterned sequences generated from a complex rule system. The subjects are subsequently probed with new examples of sequences that may follow or break the pattern. Interestingly, these studies consistently report the left inferior frontal gyrus (LIFG) to be activated during AGL, whereas other regions involved in AGL vary across studies. However, theories on the functional role of the LIFG remain rather heterogeneous [14]. In addition, a broader view on how these results should be integrated into a general cognitive neuroscience perspective on higher cognition is missing. The first purpose of the paper is to describe the so-called structured sequence processing perspective, inspired by [15] and [9], which provides a framework within which a range of AGL experiments can be integrated. By reviewing the literature on LIFG activation across different cognitive tasks involving processing of sequentially structured stimuli, including structured sequence processing per se (as in AGL), but also language, music and the action domain, we underline the potential of the sequence processing perspective for investigation of LIFG function.
The second purpose of this paper is to bridge the gap between the language literature on the LIFG and the cognitive control literature on lateral prefrontal cortex (PFC) function. We suggest that the structured sequence processing perspective links language and cognitive control functions of the LIFG/PFC. One recent overarching finding on lateral PFC function is a rostro-caudal gradient corresponding to abstraction during cognitive control [16–19]. We use the structured sequence processing perspective to integrate the functional segregation of phonology, syntax and semantics across a rostro-caudal axis in the LIFG with a general rostro-caudal axis in the lateral PFC. We focus on structural sequence processing in the domains of language, action and music as well as on the LIFG, which spans Brodmann areas (BAs) 44, 45 and 47, in the ventral part of the lateral PFC.
2. The structured sequence processing perspective
The structured sequence processing perspective originated in Reber's [20] investigations of human learning of the abstract syntactic structure in natural language using artificial grammars. From this perspective, language is seen as structured sequences of linguistic elements. A sequence is an ordered combination of elements. In a structured sequence, certain elements predict other elements. A structured sequence is thus different from a random sequence, where there are no dependencies between elements. In this paper, we discuss common aspects of temporally extended stimuli, such as speech, music or sequential body movement patterns such as gestures or dance. Importantly, not all stimuli can be described as structured sequences. Examples include visual patterns, natural scenes or stimuli which are non-ordered combinations, such as the ingredients in a salad. We use the structured sequence processing perspective primarily to understand LIFG function. The perspective of language as structured sequences thus highlights common aspects of language and other cognitive domains such as actions and music [5,7,15,21].
The central empirical observation in favour of the structured sequence processing perspective on LIFG function is the overlapping activation of the LIFG during language processing, divided into phonological, syntactic and semantic aspects [22–26], in music [27–31] and action processing [32,33]. We will review this literature further on, but let us first note that these results make sense if there are domain general structured sequence processing mechanisms in the LIFG, used across the domains of language, music and action. In the LIFG, language might thus be processed as sequences of phonemes, syllables, words and sentences; music as rhythmic sequences or tonal sequences of motifs and melodic passages; and grasping movements or dances as sequences of actions.
(a). The structured sequence processing perspective applied to phonology, syntax and semantics
Language displays phonological, syntactic and semantic structure. A common structural feature of these three language domains is adjacent and non-adjacent dependencies between linguistic elements. Phonological structure can be seen as sequences of phonemes, onsets and codas (for recently structured sequence processing perspectives on phonological structure, see [34–36]). Syntactic structure can be seen as sequences of words [4,5,7,9,15,37,38], and morphosyntactic structure can be seen as sequences of word fragments. It has been argued that phonological structure is less complex. For instance, it may involve fewer non-adjacent dependencies compared with syntactic structure [36]. Non-adjacent dependencies between words and expressions can also be analysed as a part of semantic structure. For example, there is discussion on whether anaphora (an expression referring to another within the same text) should be analysed as a part of semantic or syntactic structure [39].
As expected from the sequence processing perspective of LIFG function, converging evidence suggests that the LIFG—as part of the perisylvian language network—is engaged in semantic, syntactic and phonological processing in natural language [22–24,26]. These activations in the LIFG can thus be seen as resulting from operations on sequences of words or lexical items to produce full sentences. We will call this operation sentence-level processing, as opposed to word-level processing, which involves operations on sublexical items to produce words, i.e. creating words from syllables or phonemes. We will now elaborate on the functional neuroimaging evidence in favour of the structured sequence processing perspective on LIFG function by looking at evidence in domains other than natural language.
3. Neuroimaging of the left inferior frontal gyrus in artificial grammar, music and action processing
(a). Artificial grammar experiments
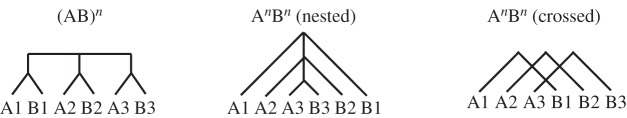
The critical test of the structured sequence processing perspective on LIFG function is whether the LIFG is involved in processing recently learned or acquired sequences, independently of the kind of elements the sequences are built from. These questions have been explored using the AGL paradigm [40,41]. An important advantage of this paradigm compared with natural language paradigms is that it controls for confounding factors such as unsystematic semantic or phonological variability of experimental stimuli [1]. In neuroimaging studies of AGL with grammars that display only adjacent dependencies, BA 44/45 is activated and, moreover, processing of artificial grammars depends causally on activity levels in BA 44/45 [6,9,12,13]. However, it has been shown that this region revealed a significantly increased haemodynamic response to non-adjacent dependencies, when directly compared with adjacent dependencies [1]. In a study by Bahlmann et al. [1], some sequences followed the rule (AB)n, where A and B belonged to two different categories of consonant–vowel syllables (n is the number of elements in sequence; figure 1). In contrast, the rule AnBn generated multiple non-adjacent dependencies between category A and category B syllables. These dependencies were organized in a nested or centre-embedded organization (A1A2A3B3B2B1; figure 1). Several precautions were taken in the experimental procedure to ensure that participants processed the AnBn rule and did not apply alternative strategies such as counting or other simple processing strategies (for details, see [1]).
Figure 1.
The (AB)n grammar, and the AnBn grammar, with two different kinds of organizations of the multiple non-adjacent dependencies. The dependencies are indicated by the drawn lines above the elements.
The direct contrast between processing of multiple non-adjacent versus adjacent sequence processing produced activity in the LIFG (BA 44). Moreover, BA 44 also showed increased haemodynamic response during the processing of centre-embedded compared with adjacent dependencies in an AGL task using visuo-spatial stimuli [2]. A causal connection between activity in the LIFG and processing of multiple non-adjacent dependencies at the end stage of implicit acquisition has recently been demonstrated using transcranial magnetic stimulation (TMS) [12]. In this case, the multiple non-adjacent dependencies were organized in a crossed manner (A1A2A3B1B2B3; figure 1). Both improvements [11,13] and impairments [12] in AGL performance have been observed after brain stimulation of BA 44/45. This suggests a complex causal role of BA 44/45 in a larger structured sequence processing network. Structured sequence processing involves but is not solely processed in the LIFG. AGL studies also report activation in subcortical [1,2,6,42] and other cortical regions (including temporal [9], parietal and occipital cortex [10]). In addition, the right IFG (RIFG) has been implicated [6].
The finding that some sequential patterns engage neuronal populations within the LIFG more than other sequential patterns suggest that the mid-LIFG is a key structured sequence processing region, rather than suggesting a specialization of the LIFG for a particular sequence type. Thus, the LIFG is more recruited during non-adjacent processing than during adjacent processing, but recruitment is not specific to the processing of non-adjacent dependencies. Furthermore, the fact that the mid-LIFG is activated in cognitive contrasts manipulating sequential features such as different grammatical sequential patterns [1,2,43,44] or violations to sequential patterns [6,9,10,12,13] provides further evidence for the involvement of this region in AGL and structured sequence processing in general. This is consistent with other studies reporting activity in the LIFG during processing of syntactically complex sentences when compared with syntactically simpler sentences in natural languages such as English [45], French [46], German [47], Hebrew [48] or Japanese [49] (see [23]).
The relevance of the structured sequence processing perspective for LIFG function is also supported by impaired structured sequence learning, e.g. in agrammatic aphasics, predominantly with lesions in Broca's region [15,50], Broca's aphasics [50] and traumatic brain patients with prefrontal damage [51]. Moreover, the integrity of white matter fibre tracts in the LIFG is associated with AGL performance [52].
(b). Integration with evidence from music and action
Taken together, the results described earlier suggest that the LIFG is engaged in processing structured sequences whether dependencies are adjacent or non-adjacent, and independently of particular stimulus features of the elements (whether letters, syllables or abstract shapes are used). This is consistent with involvement of the IFG in musical sequences and action sequences. Indeed, as predicted by the structured sequence processing account, the LIFG is activated in musical structured sequence processing [28–30]. Moreover, musicians show increased grey matter density in the LIFG compared with non-musicians [27], and Broca's aphasics are impaired in processing musical sequences [31]. Surrounding areas have also been activated in both musical processing and AGL, in particular the frontal operculum [6,9,53,54] and the anterior insula [6,9,53,54]. Action sequences, action observation and action imitation activate posterior portions of the LIFG in humans [32,33,55,56] and in monkeys [57,58]. Because functional anatomy varies across subjects, one can object to common activations in the LIFG across domains as possible artefacts of group averaging. It is thus important to note that overlaps between activations by sentence processing and sequence processing, in this case artificial grammar processing, can be found on a single subject level [9].
4. A rostro-caudal abstraction gradient in lateral prefrontal cortex
Recent models suggest a rostro-caudal gradient in the lateral PFC for abstraction during cognitive control processes [16,19]. Abstraction is the process of generalization by reducing the information content of a concept or an observable phenomenon, typically in order to retain information that is relevant for a particular purpose. If we take structured sequence processing as an example, then an abstract sequence can be presented as speech, music or an action sequence when focusing on the pattern of adjacent and non-adjacent dependencies between elements. Although we will use this definition, we will however focus on temporal abstraction, defined by Botvinick [17]. Temporally abstract representations span and unite sequences of events over longer time scales than less temporally abstract representations.
(a). Temporal abstraction and cognitive control
The lateral PFC is engaged in cognitive control [59], which refers to the ability to flexibly adapt behaviour in order to achieve goals or intentions. In general, cognitive control is necessary for a variety of processes, such as active maintenance, inhibition, task-switching, decision-making, conflict monitoring, error processing and—interestingly from the structured sequence processing perspective—abstract rule processing. Abstraction is a crucial prerequisite of cognitive control [60], and the ability to process abstract action goals is necessary to act flexibly in changing environments. Some goals require more abstraction than other goals, and the brain has representations at different levels of abstraction. We note that abstraction is rarely defined precisely within the cognitive control literature. Tasks that involve stimulus–response (S–R) mapping (analysed as less abstract), task-switching over longer time scales (more abstract), or task-set maintenance over even longer time scales (most abstract) are however segregated. Thus, temporal abstraction is coherent with the experimental manipulations of abstraction in this literature. Interestingly, recent models summarizing the findings from the cognitive control literature suggest that lower levels of abstraction engage caudal (posterior) subregions, whereas higher levels of abstraction processes engage rostral (anterior) subregions of the lateral PFC. In particular, the dorsal premotor cortex was engaged during the lowest level of abstraction; posterior and middle parts of the inferior frontal sulcus (IFS) were recruited at the middle level; the anterior IFS and orbito-frontal gyrus were engaged during the highest level of abstraction [16,61,62].
An intriguing question now arises: is there a similar rostro-caudal gradient in the ventro-lateral PFC, in particular the LIFG, as in the rest of the lateral PFC? A recent recurrent neural network model focused on modelling hierarchically organized goal-directed behaviour with tasks and subtasks [63]. It was shown that particular properties of connectivity within the network produced a temporal abstraction gradient, such that subtasks that could be accomplished on a shorter time scale were located at the base of the network, and tasks that integrated these subtasks were located at the apex of the network. If connectivity patterns are the sources of the rostro-caudal abstraction gradient in the PFC, then there is no reason to believe that the LIFG would behave differently from the rest of the lateral PFC, because connectivity patterns are similar [18]. The cognitive control literature we have cited on lateral PFC function indeed does not presume that the LIFG would be different. This has however not been discussed within the literature of LIFG function, and the LIFG has not been studied from this perspective. The purpose of the remainder of this paper is to explore the literature with a focus on a possible abstraction gradient in the LIFG, with a focus on temporal abstraction.
(b). Structured sequence processing and temporal abstraction
From the perspective of temporal abstraction, a more abstract sequential representation unites sequences of events over larger time scales. We will now elaborate on this definition in the context of structured sequence processing. Time in this context is logical time or sequence order [21], rather than absolute or physical time (as measured with a global clock). In physical time, a slowly enough pronounced word can take longer to pronounce than a whole sentence containing the same word. If temporal abstraction were defined in physical time, then this word would thus be more temporally abstract than the sentence. However, because temporal abstraction is defined in logical time, a word is always less temporally abstract than a sentence containing the word. The temporal abstraction view on sequence processing is a novel computational or a semiformal approach to artificial grammars, natural language, as well as other cognitive domains with sequential structure (see Jäger & Rogers [64] for more extended formal approaches to sequence processing and an explanation of the rationale). We will now focus on a possible temporal abstraction gradient within the LIFG.
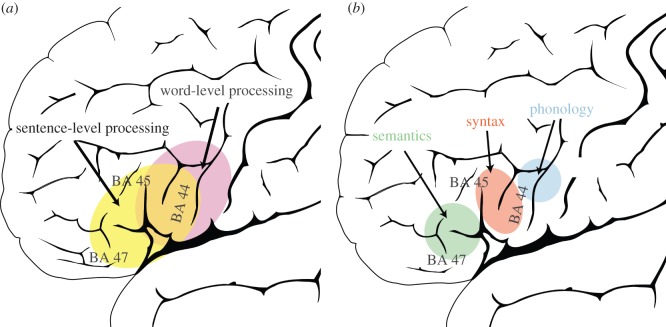
We will start from the observation that sentence-level semantic structure—realized as sequences of phrases or words—has dependencies at a similar or longer timescale compared with syntactic structure, which is mainly realized as sequences of words, and compared with morphosyntactic structure in particular, which is realized as sequences of syllables (e.g. inflectional patterns). Sequences realizing syntactic structure, in turn, have an inherently longer temporal scale than phonological structure, which consists of sequences of syllables and phonemes. The temporal abstraction gradient in the lateral PFC, applied to the perspective on language as sequences of linguistic elements thus predicts a rostro-caudal gradient within the LIFG, where semantic processing is located rostrally to syntactic processing, which in turn is located rostrally to phonological processing (a brief version of this prediction appeared in Koechlin & Jubault [65]). This is the first simplified model that we will compare against the literature. A second, cruder model merely divides sentence-level processing, where there are dependencies between words and phrases, from word-level processing, where there are dependencies between sublexical elements such as phonemes or syllables. The rostro-caudal division in this model is thus between sentence-level processing in the rostral part and word-level or sublexical processing in the caudal part of the LIFG (figure 2).
Figure 2.
The left inferior frontal gyrus (LIFG) is divided into pars opercularis, pars triangularis and pars orbitalis in the anatomical system of nomenclature, which follows the gyri and sulci. These areas roughly correspond to BA 44, 45 and 47, respectively, using cytoarchitectonic nomenclature. We illustrate two simplified models of the rostro-caudal temporal abstraction gradient. (a) Subregions in the LIFG can be segregated between sentence-level processing (processing of sequences of words) versus word-level processing (processing of sequences of sublexical items, e.g. syllables or phonemes). (b) A more fine-grained subdivision in LIFG between semantics, syntax and phonology is depicted. We note that there are word-level processes that engage the anterior LIFG and sentence-level processes that engage the posterior LIFG. The left-hand model suggest that on average word-level processes, in particular on words embedded within sentences, activate more posterior parts of the LIFG than sentence-level processes. The right-hand model should be understood in the same way, mutatis mutandis.
As predicted from the hypothesis of a temporal abstraction gradient in the LIFG, phonological, syntactic and semantic processing are segregated in the rostral–caudal direction (figure 1) [22]. BA 47 has been linked to semantic processing, BA 45 in the middle linked to syntactic processing and the posterior BA 44 connected to phonological processing. The segregation of semantics and phonology was already found in a meta-analysis by Poldrack et al. [25]. We conclude that the segregation of phonology, syntax and semantics in the inferior frontal gyrus is largely supported in large bodies of fMRI/positron emission tomography studies [22,24–26], as well as when intracranial recordings [66] or neurostimulation [67] methods are used. The most clear result is that the anterior part of the LIFG (BA 45/47) is more likely to be activated by sentence-level processing, and the posterior part (BA 44/6) is more likely to be activated by phonologically related tasks, e.g. at a sublexical- or word-processing level. Interestingly, in an fMRI study directly probing sentence-level processing, sentences activated the anterior LIFG (BA 47 and BA 45) more than unstructured word sequences, pointing to a role of anterior LIFG in sentence-level processing [68]. A recent fMRI study on sentence processing gives further evidence for this suggestion. Pallier et al. [46] varied the number of constituents to be integrated in a sentence and found activity in mid (BA 44) and anterior (BA 45) parts of the LIFG, also for sentences, in which all open-class words were replaced by pronounceable non-words (‘Jabberwocky sentences’). Interestingly, only the activation in anterior LIFG increased linearly as a function of constituent integration. This finding is consistent with the hypothesis that the more elements that need to be integrated in a sequence, the more anterior the activation within the LIFG. The finding that the same is true for Jabberwocky sentences suggests that the temporal abstraction gradient is relevant for structured sequences in general, rather than for natural language stimuli in particular. However, in Pallier et al. [46], the constituent size was parametrically varied between 1 and 6, and then jumped to 12 constituents. A paradigm where the parametric modulation of constituent size also covered larger sizes would provide a more direct test of the LIFG gradient that we suggest, also ranging to BA 47. As a note of caution, we would like to mention task complexity as a potential confounding factor for our proposal. If accuracy is lower in task A than in task B, then task A is probably more difficult or complex and this is not always controlled for in the neuroimaging studies we have reviewed.
In summary, there is evidence for a rostro-caudal temporal abstraction gradient when applying the sequence processing perspective to natural language. More abstract structured sequences unite subsequences of events over larger time scales. Neuronal populations that represent/process more temporally abstract structured sequences are located more rostrally. In the mid-LIFG and caudally, syntactic and phonological structure, mainly realized as sequence dependencies at the level of phrases, words or sublexical fragments, are processed. In our perspective, the rostral LIFG is involved in sentence-level processing, integrating less abstract phonological and syntactic processing. The goal is to extract the sentence meaning temporally which includes more temporally abstract dependencies. Interestingly, there is fMRI evidence that gesture (which can be analysed as action sequences) is integrated with speech in the rostral LIFG [69]. This leads us to a discussion on the representations in the anterior LIFG, which may not only be more temporally abstract, but also domain general.
(c). Domain general functions of the left inferior frontal gyrus
The function of the LIFG is not restricted to sequence processing. This region is also engaged in functions such as conflict resolution in semantic discrimination tasks [70], analogical [71] and deductive reasoning [72]. TMS [73], patient [74], and combined patient and fMRI [75] studies suggest a causal involvement of this region in interference suppression. Although these studies do not support a temporal abstraction gradient in the LIFG, some of them [71] support a domain generality abstraction gradient in this region. Bunge et al. [71] showed that analogy reasoning in the language domain recruits anterior parts of the LIFG (BA 47) and left fronto-polar cortex (BA 10) in comparison with a semantic retrieval task. Finding analogies between concepts requires more abstraction, following our definition, compared with retrieving the exemplar concepts. Moreover, BA 47 in the most rostral part of the LIFG, and BA 10—which is one step more rostral—have been proposed as core regions for abstract deductive reasoning [72,76]. Abstract logical arguments activated BA 10/47 [72,76]. Logical judgements might be regarded as more abstract than abstract linguistic judgements, which activated BA 45 [76]. From a broader perspective, several lines of research indicate that caudal–lateral PFC regions are engaged in domain specific cognitive control processes, whereas rostral–lateral PFC regions are domain general [77–79]. For instance, Sakai et al. [79] showed that caudal–lateral PFC regions (BA 6v/44d) were more strongly activated during verbal tasks in comparison with spatial tasks. In contrast, sustained activity during task preparation for both verbal and spatial tasks was found in rostral–lateral PFC regions (right hemispheric BA 47, extending into BA 45a). These findings demonstrate that the type of stimulus to be processed seems to influence caudal frontal regions more than rostral frontal regions. Possibly, the rostro-caudal temporal abstraction gradient should thus be extended to a domain generality abstraction gradient (figure 3). This abstraction gradient, built on the notion of abstraction as processes generalizing some concept by reduction of information, thus making more abstract representations more domain general. In the case of domain generality, particular stimulus dimensions are reduced in order to arrive at domain general, abstract representations.
Figure 3.
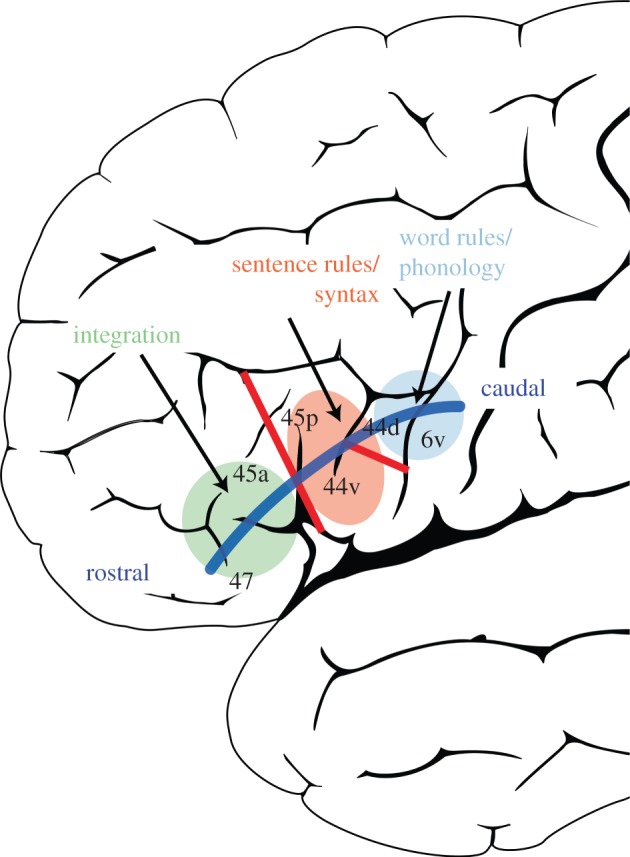
We propose a rostro-caudal abstraction gradient within the left inferior frontal gyrus in terms of two parallel but intertwined abstraction features, integrating the temporal abstraction gradient from figure 2 with an abstraction gradient of domain generality. The red lines in this figure correspond to BA 44 being subdivided into a ventral and dorsal part (44v and 44d) and BA 45 comprising an anterior and posterior part (45a and 45p), based on receptor architectonic parcellations [80]. These divisions can be used to describe a rostro-caudal gradient in more detail. In this model, 44d and 6v are engaged in phonological processing (light blue), which instantiates sequence rules at the word level; 44v and 45p are engaged in processing e.g. syntactic rules at the word and sentence processing level and perhaps also semantic rules at the sentence level; and 45a and BA 47 are engaged in the integration of these processes as well as information from other domains (such as gesture information) with the goal of extracting the meaning (light green) at longer time scales.
In the example of sentence-level processing, temporally abstract and domain general representations of sentence processing may integrate domain specific representations such as semantic structure and gestures (some initial evidence is provided in Willems et al. [69]). These domain general representations may include aspects of world knowledge and pragmatic information as well. This is supported by an fMRI study contrasting correct sentences versus sentences with semantic violations (the capital of Germany is sour) or world knowledge violations (the capital of Germany is Stockholm), which showed an overlap of these two contrasts in the anterior LIFG [81]. The same area was activated when the content of speech was incongruent with pragmatic information (voice characteristics), compared against pragmatically congruent sentences [82].
5. Cytoarchitectonic, receptor architectonic and myeloarchitectonic structure in bilateral inferior frontal gyrus
We have suggested a functional gradient in the LIFG, and would now like to discuss one possible neural substrate of this gradient: the local structural differences in the different regions of the LIFG. A recent investigation into the topography of the LIFG revealed further subdivisions of this area into a dorsal and ventral BA 44 and an anterior and posterior BA 45, using receptor architectonic parcellation techniques (figure 3) [80]. Cytoarchitectonic sections coincide with receptor architectonic and myeloarchitectonic sections, also in Broca's area [80,83]. BA 47 and BA 45 are more similar than BA 45 and 44, in terms of cytoarchitectonics (in particular, layer IV is granular in the two more anterior areas [84–86]). We would now like to discuss what functional significance these coinciding sections of anatomical structure can be expected to have. The argument against strong functional significance of these divisions is that central properties of cortical circuits, such as asymmetrical connections between pyramidal cells in different layers, are homogenous over the brain. Other properties, such as pyramidal cell size and dendritic complexity, change gradually in the anterior–posterior direction [87]. Another important observation is that patches of cortex can change their function, such as in the congenitally blind [88]. Thus, it has been suggested that long-range connectivity to other areas is rather what determines the function of a particular patch of cortex or a brain-wide network as a whole. Although this might be true, the question remains whether it is a coincidence that particular areas are systematically wired together.
Let us consider the functional location of phonological processing in the posterior LIFG, as one node in a larger perisylvian language network. There are two possible explanations for the consistent location of this node in the posterior rather than anterior LIFG over subjects: (i) cyto-, recepto- and myeloarchitechtonic properties (node properties or local connectivity) and (ii) the relation of the node to other nodes in the brain-wide network, in which it is included (global connectivity properties). In the current state of the literature, we see no basis for rejecting either of the putative explanations outright. For instance, although the anatomical micro- and mesoscopic structure, e.g. of connectivity within a cortex patch, might not exclude the possibility of a certain area performing many different functions, there might be a bias to perform one function over another. After millions of iterations in the course of development, such a bias might result in computationally specific representations in a cortical patch as a function of its local anatomical structure. For instance, modelling has shown that neural networks involving a so-called bottleneck in terms of connectivity are better at learning to perform a task that involves multiple time scales, compared with fully connected networks [89]. Such task–connectivity interactions for optimal performance might be relevant locally, within a cortex patch, as well as globally, within brain-wide networks.
The posterior and anterior part of the IFG differs in terms of both local and global connectivity. Tractography from voxels in the anatomical regions of BA 44/45 differentiated two main pathways connecting BA 44/45 with posterior language areas [90,91]. First, a dorsal pathway, connecting BA 44 with the posterior temporal lobe via the superior longitudinal fasciculus, a tract of particular importance for processing syntactic structures [7]. Second, a ventral pathway, connecting BA 45 with the anterior temporal lobe via the extreme capsule, has been shown to play a functional role in language processing [92]. In summary, there is independent evidence for a rostro-caudal gradient and/or parcellation of the LIFG, from anatomy and connectivity alone. Our proposal is a rostro-caudal gradient at a gross anatomical level. This might be implemented in gradual transitions or stepwise transitions at the level of BAs, or a mixture of both. For instance, gradual changes in the cytoarchitecture of layer IV in LIFG have been observed [93]. Results reported in Amunts et al. [80] also indicate gradual changes in receptor architectonics. The borders between BAs are however also present in many of the receptor systems studied in Amunts et al. [80]. When describing the full function of the LIFG or PFC, the many connections to posterior brain structures must be considered. None of the brain regions we have been discussing performs one function in isolation, but can be recruited for different purposes by different networks. We would like to emphasize the importance of thinking in terms of networks, such as the extended network for structured sequence processing described in §3a.
The proposed implementation of structured sequence processing in the brain includes (but is not limited to) the LIFG. In our impression, there is not enough functional data to make a systematic review on a rostro-caudal gradient in the RIFG. Thus, our proposal and cited evidence will at least apply to the LIFG, whereas parts of the evidence might apply also to the RIFG. A recent receptor architectonic study revealed different distribution of receptor density between the LIFG and the RIFG [80]. Moreover, a cross-species comparison of primate brains (including humans) suggests a hemispheric asymmetry of the PFC, such that relative size of the left PFC increased more than the right PFC [94] during human evolution. These micro- and macro-anatomical differences between the left and right hemisphere, together with the relatively small number of functional studies investigating the RIFG, caused us to restrict our hypothesis to the LIFG.
6. Conclusion
In summary, we suggest that the rostro-caudal abstraction gradient in the LIFG is a part of a general rostro-caudal abstraction gradient in the lateral PFC. Stimuli with a strong sequential component, such as language, music and action sequences, are processed mainly in the LIFG, rather than the rest of the lateral PFC. We have discussed empirical support for the structured sequence processing perspective on LIFG function across cognitive domains, whereas support for the rostro-caudal abstraction gradient in the LIFG mainly comes from the language domain and the cognitive control literature.
The rostro-caudal abstraction gradient hypothesis predicts similar regional brain activation for all the sequential aspects of the domains of language, music and action sequences as well as with abstract sequence-processing paradigms such as AGL. If a rostro-caudal abstraction gradient turns out to also apply to the LIFG as we have proposed, then we have furthered our understanding of the organization of the frontal cortex substantially. Thus, a possible future direction for this line of research is to integrate experimental paradigms from language research with paradigms from cognitive control research in order to elucidate common neural substrates in the lateral PFC, in particular the organization of the lateral PFC into a rostro-caudal gradient and perhaps a dorsal–ventral gradient as well. The AGL paradigm could be used to test the rostro-caudal abstraction gradient. For instance, in sequences spanning longer time scales, the temporal abstraction gradient predicts that longer sequence dependencies activate areas more rostrally than shorter sequence dependencies. The domain generality gradient hypothesis predicts that sequence processing where some element a has to be treated the same irrespective of how it is presented (for instance, in the visual or auditory modality) will produce more rostral activation compared with sequence processing where no generalization across the input domain is needed. These hypotheses about the functional subdivisions of the lateral PFC remain critical objectives for future investigations.
Acknowledgements
This work was supported by the Max Planck Institute for Psycholinguistics, the Donders Institute for Brain, Cognition and Behaviour, Centre for Cognitive Neuroimaging, Radboud University Nijmegen, the Swedish Research Council, Hedlunds Stiftelse, the Stockholm County Council, the Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, the University of California at Berkeley and Leopoldina—National Academy of Science (grant no. LPDS 2009-20). We thank Gustav Nilsonne and Martin Eriksson for comments on an earlier version of the paper. We also thank the three anonymous reviewers for their very helpful comments and suggestions.
References
- 1.Bahlmann J., Schubotz R. I., Friederici A. D. 2008. Hierarchical artificial grammar processing engages Broca's area. NeuroImage 42, 525–534 10.1016/j.neuroimage.2008.04.249 (doi:10.1016/j.neuroimage.2008.04.249) [DOI] [PubMed] [Google Scholar]
- 2.Bahlmann J., Schubotz R. I., Mueller J. L., Koester D., Friederici A. D. 2009. Neural circuits of hierarchical visuo-spatial sequence processing. Brain Res. 1298, 161–170 10.1016/j.brainres.2009.08.017 (doi:10.1016/j.brainres.2009.08.017) [DOI] [PubMed] [Google Scholar]
- 3.Dominey P. F., Hoen M., Blanc J. M., Lelekov-Boissard T. 2003. Neurological basis of language and sequential cognition: evidence from simulation, aphasia and ERP studies. Brain Lang. 86, 207–225 10.1016/S0093-934X(02)00529-1 (doi:10.1016/S0093-934X(02)00529-1) [DOI] [PubMed] [Google Scholar]
- 4.Folia V., Forkstam C., Ingvar M., Hagoort P., Petersson K. M. 2011. Implicit artificial syntax processing: genes, preference, and bounded recursion. Biolinguistics 5, 105–132 [Google Scholar]
- 5.Folia V., Uddén J., De Vries M. H., Forkstam C., Petersson K. M. 2011. Artificial language learning in adults and children. Lang. Learn. 60, 188–220 10.1111/j.1467-9922.2010.00606.x (doi:10.1111/j.1467-9922.2010.00606.x) [DOI] [Google Scholar]
- 6.Forkstam C., Hagoort P., Fernandez G., Ingvar M., Petersson K. M. 2006. Neural correlates of artificial syntactic structure classification. NeuroImage 32, 956–967 10.1016/j.neuroimage.2006.03.057 (doi:10.1016/j.neuroimage.2006.03.057) [DOI] [PubMed] [Google Scholar]
- 7.Friederici A. D., Bahlmann J., Heim S., Schubotz R. I., Anwander A. 2006. The brain differentiates human and non-human grammars: functional localization and structural connectivity. Proc. Natl Acad. Sci. USA 103, 2458–2463 10.1073/pnas.0509389103 (doi:10.1073/pnas.0509389103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friederici A. D., Steinhauer K., Pfeifer E. 2002. Brain signatures of artificial language processing: evidence challenging the critical period hypothesis. Proc. Natl Acad. Sci. USA 99, 529–534 10.1073/pnas.012611199 (doi:10.1073/pnas.012611199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersson K. M., Folia V., Hagoort P. 2010. What artificial grammar learning reveals about the neurobiology of syntax? Brain Lang. 120, 83–95 10.1016/j.bandl.2010.08.003 (doi:10.1016/j.bandl.2010.08.003) [DOI] [PubMed] [Google Scholar]
- 10.Petersson K. M., Forkstam C., Ingvar M. 2004. Artificial syntactic violations activate Broca's region. Cogn. Sci. 28, 383–407 10.1207/s15516709cog2803_4 (doi:10.1207/s15516709cog2803_4) [DOI] [Google Scholar]
- 11.de Vries M. H., Barth A. R. C., Knecht S., Zwitserlood P., Floeel A. 2010. Electrical stimulation of Broca's area enhances implicit learning of an artificial grammar. J. Cogn. Neurosci. 22, 2427–2436 10.1162/jocn.2009.21385 (doi:10.1162/jocn.2009.21385) [DOI] [PubMed] [Google Scholar]
- 12.Uddén J.Language as structured sequences: a causal role for Broca's region in sequence processing. 2012. PhD thesis, Department of Clinical Neuroscience. Karolinska Institute, Stockholm. See http://hdl.handle.net/10616/40842 .
- 13.Uddén J., et al. 2008. The inferior frontal cortex in artificial syntax processing: an rTMS study. Brain Res. 1224, 69–78 10.1016/j.brainres.2008.05.070 (doi:10.1016/j.brainres.2008.05.070) [DOI] [PubMed] [Google Scholar]
- 14.Fitch W. T., Friederici A. D. 2012. Artificial grammar learning meets formal language theory: an overview. Phil. Trans. R. Soc. B 367, 1933–1955 10.1098/rstb.2012.0103 (doi:10.1098/rstb.2012.0103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christiansen M. H., Louise Kelly M., Shillcock R. C., Greenfield K. 2010. Impaired artificial grammar learning in agrammatism. Cognition 116, 382–393 10.1016/j.cognition.2010.05.015 (doi:10.1016/j.cognition.2010.05.015) [DOI] [PubMed] [Google Scholar]
- 16.Badre D., D'Esposito M. 2009. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat. Rev. Neurosci. 10, 659–669 10.1038/nrn2667 (doi:10.1038/nrn2667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botvinick M. M. 2008. Hierarchical models of behavior and prefrontal function. Trends Cogn. Sci. 12, 201–208 10.1016/j.tics.2008.02.009 (doi:10.1016/j.tics.2008.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuster J. M. 1997. The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. Philadelphia, PA: Lippincott-Raven [Google Scholar]
- 19.Koechlin E., Hyafil A. 2007. Anterior prefrontal function and the limits of human decision-making. Science 318, 594–598 10.1126/science.1142995 (doi:10.1126/science.1142995) [DOI] [PubMed] [Google Scholar]
- 20.Reber A. S. 1967. Implicit learning of artificial grammars. J. Verbal Learn. Verbal Behav. 6, 855–863 10.1016/S0022-5371(67)80149-X (doi:10.1016/S0022-5371(67)80149-X) [DOI] [Google Scholar]
- 21.Petersson K. M. 2008. On cognition, structured sequence processing, and adaptive dynamical systems. Proc. Am. Inst. Phys. 1060, 195–199 10.1063/1.3037051 (doi:10.1063/1.3037051) [DOI] [Google Scholar]
- 22.Bookheimer S. 2002. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu. Rev. Neurosci. 25, 151–188 10.1146/annurev.neuro.25.112701.142946 (doi:10.1146/annurev.neuro.25.112701.142946) [DOI] [PubMed] [Google Scholar]
- 23.Indefrey P. 2011. Neurobiology of syntax. In The Cambridge encyclopedia of the language sciences (ed. Hogan P. C.), pp. 835–838 Cambridge, UK: Cambridge University Press [Google Scholar]
- 24.Lindenberg R., Fangerau H., Seitz R. J. 2007. ‘Broca's area’ as a collective term? Brain Lang. 102, 22–29 10.1016/j.bandl.2006.11.012 (doi:10.1016/j.bandl.2006.11.012) [DOI] [PubMed] [Google Scholar]
- 25.Poldrack R. A., Wagner A. D., Prull M. W., Desmond J. E., Glover G. H., Gabrieli J. D. E. 1999. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage 10, 15–35 10.1006/nimg.1999.0441 (doi:10.1006/nimg.1999.0441) [DOI] [PubMed] [Google Scholar]
- 26.Vigneau M., Beaucousin V., Hervé P. Y., Duffau H., Crivello F., Houdé O., Mazoyer B., Tzourio-Mazoyer N. 2006. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage 30, 1414–1432 10.1016/j.neuroimage.2005.11.002 (doi:10.1016/j.neuroimage.2005.11.002) [DOI] [PubMed] [Google Scholar]
- 27.Gaser C., Schlaug G. 2003. Gray matter differences between musicians and nonmusicians. Ann. N.Y. Acad. Sci. 999, 514–517 10.1196/annals.1284.062 (doi:10.1196/annals.1284.062) [DOI] [PubMed] [Google Scholar]
- 28.Janata P., Grafton S. T. 2003. Swinging in the brain: shared neural substrates for behaviors related to sequencing and music. Nat. Neurosci. 6, 682–687 10.1038/nn1081 (doi:10.1038/nn1081) [DOI] [PubMed] [Google Scholar]
- 29.Maess B., Koelsch S., Gunter T. C., Friederici A. D. 2001. Musical syntax is processed in Broca's area: a MEG study. Nat. Neurosci. 4, 540–545 10.1038/87502 (doi:10.1038/87502) [DOI] [PubMed] [Google Scholar]
- 30.Patel A. D. 2003. Language, music, syntax and the brain. Nat. Neurosci. 6, 674–681 10.1038/nn1082 (doi:10.1038/nn1082) [DOI] [PubMed] [Google Scholar]
- 31.Patel A. D., Iversen J., Wassenaar M., Hagoort P. 2008. Musical syntactic processing in agrammatic Broca's aphasia. Aphasiology 22, 776–789 10.1080/02687030701803804 (doi:10.1080/02687030701803804) [DOI] [Google Scholar]
- 32.Hamzei F., Rijntjes M., Dettmers C., Glauche V., Weiller C., Büchel C. 2003. The human action recognition system and its relationship to Broca's area: an fMRI study. NeuroImage 19, 637–644 10.1016/S1053-8119(03)00087-9 (doi:10.1016/S1053-8119(03)00087-9) [DOI] [PubMed] [Google Scholar]
- 33.Molnar-Szakacs I., Iacoboni M., Koski L., Mazziotta J. C. 2005. Functional segregation within pars opercularis of the inferior frontal gyrus: evidence from fMRI studies of imitation and action observation. Cereb. Cortex 15, 986–994 10.1093/cercor/bhh199 (doi:10.1093/cercor/bhh199) [DOI] [PubMed] [Google Scholar]
- 34.Bonatti L. L., Pena M., Nespor M., Mehler J. 2005. Linguistic constraints on statistical computations. Psychol. Sci. 16, 451–459 10.1111/j.0956-7976.2005.01556.x (doi:10.1111/j.0956-7976.2005.01556.x) [DOI] [PubMed] [Google Scholar]
- 35.Cristià A., Seidl A., Gerken L. 2011. Learning classes of sounds in infancy. University of Pennsylvania Working Papers in Linguistics, 17, article 9. See http://repository.upenn.edu/pwpl/vol17/iss11/19 [Google Scholar]
- 36.Heinz J., Idsardi W. 2011. Sentence and word complexity. Science 333, 295–297 10.1126/science.1210358 (doi:10.1126/science.1210358) [DOI] [PubMed] [Google Scholar]
- 37.Christiansen M. H., MacDonald M. C. 2009. A usage-based approach to recursion in sentence processing. Lang. Learn. 59, 126–161 10.1111/j.1467-9922.2009.00538.x (doi:10.1111/j.1467-9922.2009.00538.x) [DOI] [Google Scholar]
- 38.Misyak J. B., Christiansen M. H., Tomblin J. B. 2009. Statistical learning of nonadjacencies predicts on-line processing of long-distance dependencies in natural language. Proc. Cogn. Sci. Soc. 2009, 177–182 [Google Scholar]
- 39.Jackendoff R. 2002. Foundations of language: brain, meaning, grammar, evolution. Oxford, UK: Oxford University Press; [DOI] [PubMed] [Google Scholar]
- 40.Forkstam C., Petersson K. M. 2005. Towards an explicit account of implicit learning. Curr. Opin. Neurol. 18, 435–441 10.1097/01.wco.0000171951.82995.c4 (doi:10.1097/01.wco.0000171951.82995.c4) [DOI] [PubMed] [Google Scholar]
- 41.Stadler M. A., Frensch P. A. E. 1998. Handbook of implicit learning. Thousand Oaks, CA: Sage Publications [Google Scholar]
- 42.Lieberman M. D., Chang G. Y., Chiao J., Bookheimer S. Y., Knowlton B. J. 2004. An event-related fMRI study of artificial grammar learning in a balanced chunk strength design. J. Cogn. Neurosci. 16, 427–438 10.1162/089892904322926764 (doi:10.1162/089892904322926764) [DOI] [PubMed] [Google Scholar]
- 43.Friederici A. D., Fiebach C. J., Schlesewsky M., Bornkessel I. D., von Cramon D. Y. 2006. Processing linguistic complexity and grammaticality in the left frontal cortex. Cereb. Cortex 16, 1709–1717 10.1093/cercor/bhj106 (doi:10.1093/cercor/bhj106) [DOI] [PubMed] [Google Scholar]
- 44.Makuuchi M., Bahlmann J., Anwander A., Friederici A. D. 2009. Segregating the core computational faculty of human language from working memory. Proc. Natl Acad. Sci. USA 106, 8362–8367 10.1073/pnas.0810928106 (doi:10.1073/pnas.0810928106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meltzer J. A., McArdle J. J., Schafer R. J., Braun A. R. 2010. Neural aspects of sentence comprehension: syntactic complexity, reversibility, and reanalysis. Cereb. Cortex 20, 1853–1864 10.1093/cercor/bhp249 (doi:10.1093/cercor/bhp249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pallier C., Devauchelle A.-D., Dehaene S. 2011. Cortical representation of the constituent structure of sentences. Proc. Natl Acad. Sci. USA 108, 2522–2527 10.1073/pnas.1018711108 (doi:10.1073/pnas.1018711108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiebach C. J., Schlesewsky M., Lohmann G., von Cramon D. Y., Friederici A. D. 2005. Revisiting the role of Broca's area in sentence processing: syntactic integration versus syntactic working memory. Hum. Brain Mapp. 24, 79–91 10.1002/hbm.20070 (doi:10.1002/hbm.20070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben-Shachar M., Palti D., Grodzinsky Y. 2004. Neural correlates of syntactic movement: converging evidence from two fMRI experiments. NeuroImage 21, 1320–1336 10.1016/j.neuroimage.2003.11.027 (doi:10.1016/j.neuroimage.2003.11.027) [DOI] [PubMed] [Google Scholar]
- 49.Sakai K. 2005. Language acquisition and brain development. Science 310, 815–819 10.1126/science.1113530 (doi:10.1126/science.1113530) [DOI] [PubMed] [Google Scholar]
- 50.Hoen M., Golembiowski M., Guyot E., Deprez V., Caplan D., Dominey P. F. 2003. Training with cognitive sequences improves syntactic comprehension in agrammatic aphasics. NeuroReport 14, 495–499 10.1097/00001756-200303030-00040 (doi:10.1097/00001756-200303030-00040) [DOI] [PubMed] [Google Scholar]
- 51.Pothos E. M., Wood R. L. 2009. Separate influences in learning: evidence from artificial grammar learning with traumatic brain injury patients. Brain Res. 1275, 67–72 10.1016/j.brainres.2009.04.019 (doi:10.1016/j.brainres.2009.04.019) [DOI] [PubMed] [Google Scholar]
- 52.Floeel A., de Vries M. H., Scholz J., Breitenstein C., Johansen-Berg H. 2009. White matter integrity in the vicinity of Broca's area predicts grammar learning success. NeuroImage 47, 1974–1981 10.1016/j.neuroimage.2009.05.046 (doi:10.1016/j.neuroimage.2009.05.046) [DOI] [PubMed] [Google Scholar]
- 53.Engel A., Keller P. E. 2011. The perception of musical spontaneity in improvised and imitated jazz performances. Front. Psychol. 2, 83. 10.3389/fpsyg.2011.00083 (doi:10.3389/fpsyg.2011.00083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mutschler I., Schulze-Bonhage A., Glauche V., Demandt E., Speck O., Ball T. 2007. A rapid sound-action association effect in human insular cortex. PLoS ONE 2, e259. 10.1371/journal.pone.0000259 (doi:10.1371/journal.pone.0000259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schubotz R. I., von Cramon D. Y. 2004. Sequences of abstract nonbiological stimuli share ventral premotor cortex with action observation and imagery. J. Neurosci. 24, 5467–5474 10.1523/JNEUROSCI.1169-04.2004 (doi:10.1523/JNEUROSCI.1169-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seger C. A., Cincotta C. M. 2006. Dynamics of frontal, striatal, and hippocampal systems during rule learning. Cereb. Cortex 16, 1546–1555 10.1093/cercor/bhj092 (doi:10.1093/cercor/bhj092) [DOI] [PubMed] [Google Scholar]
- 57.Nelissen K., Borra E., Gerbella M., Rozzi S., Luppino G., Vanduffel W., Rizzolatti G., Orban G. A. 2011. Action observation circuits in the macaque monkey cortex. J. Neurosci. 31, 3743–3756 10.1523/JNEUROSCI.4803-10.2011 (doi:10.1523/JNEUROSCI.4803-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nelissen K., Luppino G., Vanduffel W., Rizzolatti G., Orban G. A. 2005. Observing others: multiple action representation in the frontal lobe. Science 310, 332–336 10.1126/science.1115593 (doi:10.1126/science.1115593) [DOI] [PubMed] [Google Scholar]
- 59.Miller E. K., Cohen J. D. 2001. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 10.1146/annurev.neuro.24.1.167 (doi:10.1146/annurev.neuro.24.1.167) [DOI] [PubMed] [Google Scholar]
- 60.Newell A. 1994. Unified theories of cognition. Cambridge, MA: Harvard University Press [Google Scholar]
- 61.Badre D. 2008. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn. Sci. 12, 193–200 10.1016/j.tics.2008.02.004 (doi:10.1016/j.tics.2008.02.004) [DOI] [PubMed] [Google Scholar]
- 62.Koechlin E., Ody C., Kouneiher F. 2003. The architecture of cognitive control in the human prefrontal cortex. Science 302, 1181–1185 10.1126/science.1088545 (doi:10.1126/science.1088545) [DOI] [PubMed] [Google Scholar]
- 63.Botvinick M. M. 2007. Multilevel structure in behaviour and in the brain: a model of Fuster's hierarchy. Phil. Trans. R. Soc. B 362, 1615–1626 10.1098/rstb.2007.2056 (doi:10.1098/rstb.2007.2056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jäger G., Rogers J. 2012. Formal language theory: refining the Chomsky hierarchy. Phil. Trans. R. Soc. B 367, 1956–1970 10.1098/rstb.2012.0077 (doi:10.1098/rstb.2012.0077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koechlin E., Jubault T. 2006. Broca's area and the hierarchical organization of human behavior. Neuron 50, 963–974 10.1016/j.neuron.2006.05.017 (doi:10.1016/j.neuron.2006.05.017) [DOI] [PubMed] [Google Scholar]
- 66.Sahin N. T., Pinker S., Cash S. S., Schomer D., Halgren E. 2009. Sequential processing of lexical, grammatical, and phonological information within Broca's area. Science 326, 445–449 10.1126/science.1174481 (doi:10.1126/science.1174481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gough P. M., Nobre A. C., Devlin J. T. 2005. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J. Neurosci. 25, 8010–8016 10.1523/JNEUROSCI.2307-05.2005 (doi:10.1523/JNEUROSCI.2307-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Snijders T. M., Vosse T., Kempen G., Van Berkum J. A., Petersson K. M., Hagoort P. 2009. Retrieval and unification in sentence comprehension: an fMRI study using word-category ambiguity. Cereb. Cortex 19, 1493–1503 10.1093/cercor/bhn187 (doi:10.1093/cercor/bhn187) [DOI] [PubMed] [Google Scholar]
- 69.Willems R. M., Özyürek A., Hagoort P. 2007. When language meets action: the neural integration of gesture and speech. Cereb. Cortex 17, 2322–2333 10.1093/cercor/bhl141 (doi:10.1093/cercor/bhl141) [DOI] [PubMed] [Google Scholar]
- 70.Thompson-Schill S. L., D'Esposito M., Aguirre G. K., Farah M. J. 1997. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc. Natl Acad. Sci. USA 94, 14 792–14 797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bunge S. A., Wendelken C., Badre D., Wagner A. D. 2005. Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cereb. Cortex 15, 239–249 10.1093/cercor/bhh126 (doi:10.1093/cercor/bhh126) [DOI] [PubMed] [Google Scholar]
- 72.Monti M., Osherson D., Martinez M., Parsons L. 2007. Functional neuroanatomy of deductive inference: a language-independent distributed network. NeuroImage 37, 1005–1016 10.1016/j.neuroimage.2007.04.069 (doi:10.1016/j.neuroimage.2007.04.069) [DOI] [PubMed] [Google Scholar]
- 73.Hindy N. C., Hamilton R., Houghtling A. S., Coslett H. B., Thompson-Schill S. L. 2009. Computer-mouse tracking reveals TMS disruptions of prefrontal function during semantic retrieval. J. Neurophysiol. 102, 3405–3413 10.1152/jn.00516.2009 (doi:10.1152/jn.00516.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thompson-Schill S. L., Jonides J., Marshuetz C., Smith E. E., Esposito M., Kan I. P., Knight R. T., Swick D. 2002. Effects of frontal lobe damage on interference effects in working memory. Cogn. Affect. Behav. Neurosci. 2, 109–120 10.3758/CABN.2.2.109 (doi:10.3758/CABN.2.2.109) [DOI] [PubMed] [Google Scholar]
- 75.Schnur T. T., Schwartz M. F., Kimberg D. Y., Hirshorn E., Coslett H. B., Thompson-Schill S. L. 2009. Localizing interference during naming: convergent neuroimaging and neuropsychological evidence for the function of Broca's area. Proc. Natl Acad. Sci. USA 94, 14 792–14 797 10.1073/pnas.0805874106 (doi:10.1073/pnas.0805874106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Monti M. M., Parsons L. M., Osherson D. N. 2009. The boundaries of language and thought in deductive inference. Proc. Natl Acad. Sci. USA 106, 12 554–12 559 10.1073/pnas.0902422106 (doi:10.1073/pnas.0902422106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buckner R. L. 2003. Functional–anatomic correlates of control processes in memory. J. Neurosci. 23, 3999–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gilbert S. J., Spengler S., Simons J. S., Steele J. D., Lawrie S. M., Frith C. D., Burgess P. W. 2006. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J. Cogn. Neurosci. 18, 932–948 10.1162/jocn.2006.18.6.932 (doi:10.1162/jocn.2006.18.6.932) [DOI] [PubMed] [Google Scholar]
- 79.Sakai K., Homae F., Hashimoto R. 2003. Sentence processing is uniquely human. Neurosci. Res. 46, 273–279 10.1016/S0168-0102(03)00122-6 (doi:10.1016/S0168-0102(03)00122-6) [DOI] [PubMed] [Google Scholar]
- 80.Amunts K., Lenzen M., Friederici A. D., Schleicher A., Morosan P., Palomero-Gallagher N., Zilles K. 2010. Broca's region: novel organizational principles and multiple receptor mapping. PLoS Biol. 8, e1000489. 10.1371/journal.pbio.1000489 (doi:10.1371/journal.pbio.1000489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hagoort P., Hald L., Bastiaansen M., Petersson K. M. 2004. Integration of word meaning and world knowledge in language comprehension. Science 304, 438–441 10.1126/science.1095455 (doi:10.1126/science.1095455) [DOI] [PubMed] [Google Scholar]
- 82.Tesink C. M. J. Y., Petersson K. M., van Berkum J. J. A., van den Brink D., Buitelaar J. K., Hagoort P. 2009. Unification of speaker and meaning in language comprehension: an fMRI study. J. Cogn. Neurosci. 21, 2085–2099 10.1162/jocn.2008.21161 (doi:10.1162/jocn.2008.21161) [DOI] [PubMed] [Google Scholar]
- 83.Amunts K., von Cramon D. Y. 2006. The anatomical segretation of the frontal cortex: what does it mean for function? Cortex 42, 525–528 10.1016/S0010-9452(08)70392-7 (doi:10.1016/S0010-9452(08)70392-7) [DOI] [PubMed] [Google Scholar]
- 84.Amunts K., Schleicher A., Bürgel U., Mohlberg H., Uylings H. B. M., Zilles K. 1999. Broca's region revisited: cytoarchitecture and intersubject variability. J. Comp. Neurol. 412, 319–341 (doi:10.1002/(SICI)1096-9861(19990920)412:2<319::AID-CNE10>3.0.CO;2-7) [DOI] [PubMed] [Google Scholar]
- 85.Hagoort P. 2005. On Broca, brain, and binding: a new framework. Trends Cogn. Sci. 9, 416–423 10.1016/j.tics.2005.07.004 (doi:10.1016/j.tics.2005.07.004) [DOI] [PubMed] [Google Scholar]
- 86.Mesulam M.-M. 2002. The human frontal lobes: transcending the default mode through contingent encoding. In Principles of frontal lobe function (eds Stuss D. T., Knight R. T.), pp. 8–31 Oxford, UK: Oxford University Press [Google Scholar]
- 87.Douglas R. J., Martin K. A. C. 2004. Neuronal circuits of the neocortex. Annu. Rev. Neurosci. 27, 419–451 10.1146/annurev.neuro.27.070203.144152 (doi:10.1146/annurev.neuro.27.070203.144152) [DOI] [PubMed] [Google Scholar]
- 88.Uhl F., Franzen P., Lindinger G., Lang W., Deecke L. 1991. On the functionality of the visually deprived occipital cortex in early blind persons. Neurosci. Lett. 124, 256–259 10.1016/0304-3940(91)90107-5 (doi:10.1016/0304-3940(91)90107-5) [DOI] [PubMed] [Google Scholar]
- 89.Paine R. W., Tani J. 2005. How hierarchical control self-organizes in artificial adaptive systems. Adapt. Behav. 13, 211–225 10.1177/105971230501300303 (doi:10.1177/105971230501300303) [DOI] [Google Scholar]
- 90.Anwander A., Tittgemeyer M., von Cramon D. Y., Friederici A. D., Knösche T. R. 2007. Connectivity-based parcellation of Broca's area. Cereb. Cortex 17, 816–825 10.1093/cercor/bhk034 (doi:10.1093/cercor/bhk034) [DOI] [PubMed] [Google Scholar]
- 91.Frey S., Campbell J. S., Pike G. B., Petrides M. 2008. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J. Neurosci. 28, 11 435–11 444 10.1523/JNEUROSCI.2388-08.2008 (doi:10.1523/JNEUROSCI.2388-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saur D., et al. 2008. Ventral and dorsal pathways for language. Proc. Natl Acad. Sci. USA 105, 18 035–18 040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keller S. S., Crow T., Foundas A., Amunts K., Roberts N. 2009. Broca's area: nomenclature, anatomy, typology and asymmetry. Brain Lang. 109, 29–48 10.1016/j.bandl.2008.11.005 (doi:10.1016/j.bandl.2008.11.005) [DOI] [PubMed] [Google Scholar]
- 94.Smaers J. B., Steele J., Case C. R., Cowper A., Amunts K., Zilles K. 2011. Primate prefrontal cortex evolution: human brains are the extreme of a lateralized ape trend. Brain Behav. Evol. 77, 67–78 10.1159/000323671 (doi:10.1159/000323671) [DOI] [PubMed] [Google Scholar]