Abstract
Primate societies are characterized by bonded social relationships of a kind that are rare in other mammal taxa. These bonded relationships, which provide the basis for coalitions, are underpinned by an endorphin mechanism mediated by social grooming. However, bonded relationships of this kind impose constraints on the size of social groups that are possible. When ecological pressures have demanded larger groups, primates have had to evolve new mechanisms to facilitate bonding. This has involved increasing the size of vocal and visual communication repertoires, increasing the time devoted to social interaction and developing a capacity to manage two-tier social relationships (strong and weak ties). I consider the implications of these constraints for the evolution of human social communities and argue that laughter was an early evolutionary innovation that helped bridge the bonding gap between the group sizes characteristic of chimpanzees and australopithecines and those in later hominins.
Keywords: social complexity, social network, primates, social bonding, laughter
1. Introduction
Mammalian social evolution has involved a radiation of lineages that have developed a variety of social strategies for dealing with the natural threats they face. This is reflected in the fact that the rate of encephalization across mammalian taxa is directly related to the extent to which their modern descendent species are characterized by bonded social systems [1]. By bonded, we mean groups in which pairs of individuals have close, long-lasting relationships, marked in primates at least by persistent physical proximity and intense, focused social grooming that is often nearly exclusive to that dyad. The mammalian families with the highest encephalization rates (notably the Primates, the Tylopods (camel family), the Hippomorphs (horse family), the Odontocetes (dolphin family) and the Caniniformes (dog family)) are all characterized by high proportions of living species with bonded social systems [1]. However, among these, the anthropoid primates stand out as being both unusually encephalized and characterized by social systems that are almost all of the bonded type [1,2].
What seems to define these bonded social systems is the intensity of the relationships between pairs of individuals [3]. Although operationalizing what we mean by ‘bonded’ is not at all easy, primates have the advantage of using social grooming as a major component of their affiliative social interactions, and so it is relatively easy to identify these relationships in their case [3]. These bonded relationships exist to buffer the individuals concerned against the stresses of living in relatively large, spatially compact groups [4]. Primate groups are, of course, numerically modest by comparison with the herds formed by some ungulates, but it is this contrast between the rather unstructured, loose herds of, say, wildebeest and the demographically stable, cohesive, highly structured groups of baboons that is the issue here. In the loose herds that characterize many ungulates, relationships between individuals are typically of the moment, and characterized by direct responses to environmental conditions, so that individuals are relatively free to join and leave as circumstances dictate. By contrast, anthropoid primate groups (and those of some other mammals such as the elephants and equids) are relatively stable and cohesive, with compositions that remain constant through time, and they are often heavily structured in terms of patterns of dyadic interaction and collective defence [1,2]. It is this contrast that requires explanation.
The bonded nature of primate social groups raises important issues about the structure and origins of multi-level social systems, and the multi-level selection processes that seem to underpin these. In one sense, this represents a sea-change in how we view the evolution of social behaviour and the selection processes that underpin this. Hitherto, our focus has largely been concerned with the fitness-guided behavioural decisions that individuals make (i.e. optimal foraging decisions, mate choice strategies and reproductive scheduling decisions). Here, other individuals are simply part of the context that defines the costs and benefits that have to be evaluated. In multi-level social systems, however, other individuals are not only part of the context, they are also part of the solution and the individual has to play a more complex game in trading off short- and long-term benefits in order to maximize fitness. Section 2 sketches out such a framework for the kinds of bonded social systems that we find in primates. Section 3 argues that these effects impose constraints on social group size, in part because the bonding mechanism used to create and service social cohesion in these kinds of groups (mainly social grooming) imposes an upper limit on the size of groups that can be maintained as stable, coherent entities. Primates have frequently had to break through these constraints in order to allow them to increase social group size when ecological circumstances have demanded this. I shall argue that this was a particular problem during the course of hominin evolution because there have been repeated pressures to increase community size far beyond those observed in other primates. I will suggest that one early way in which this was achieved was the introduction of a novel bonding process (laughter as a form of chorusing) that exploits the same underlying mechanism as social grooming.
2. Primate sociality and the costs of group-living
There seems little doubt about the fact that the principal selection pressure driving social group size in primates has been predation risk [5–7]. However, given that ungulates also group in response to predation without having bonded societies, the reason why primates opted to add a further complication to this process in the form of bonded groups remains unclear, not least because this kind of bondedness ultimately limits the size of social groups that can be held together as a coherent entity. One possibility is that kinship (which is invariably one of the defining features of bonded social systems) provides a more powerful basis for mutual defence against external threats, including defence of resources as well as a defence against predation: kin are more likely to take risks to come to each other's aid [8,9]. An alternative (and not necessarily mutually exclusive) possibility is that kin-based micro-coalitions are necessary to defuse the direct costs of living in groups [10]. Since living in groups of any kind creates stresses that would normally result in the group disbanding, species that live in stable social groups have to circumvent this problem if they are to prevent group size collapsing.
These costs derive from two distinct sources. The indirect costs are those generated by time-budgeting issues [11]. In essence, an individual needs to harvest a given area each day to acquire the nutrients it needs. As a result, the more the animals there are in the group, the proportionately bigger area the group has to cover each day to ensure that everyone meets their nutritional requirements, and hence the longer the day journey and the more the time that has to be devoted to travelling. The direct costs arise from the effect that squabbles and displacements have on the endocrine system, and include not only the physiological drain on the body from heightened cortisol titres but also, in the case of females, destabilized menstrual endocrinology resulting in reduced fertility [4,12–15].
For animals such as primates that live in bonded social groups, these costs need to be reduced to an acceptable level if the group is to maintain structural cohesion through time. There is, in practice, little that can be done about the indirect costs, other than reduce group size [16,17]. However, that would mean the inevitable loss of the benefits derived from grouping (namely, the reduction in predation risk). Such strategies are thus usually only possible when predation risk is low or can be dealt with by other means (e.g. increasing body size [18]). The direct costs, on the other hand, are often more tractable. Primates deal with these by forming coalitions through social grooming that have the effect of buffering the individual against most of the direct stresses of group-living by keeping other members of the group just far enough away that they do not impinge directly on the individual—but without driving them away completely [4]. In effect, primates seem to deal with the direct costs by means of a multi-level form of organization in which low-level coalitions buffer individuals against these costs so as to allow them to maintain cohesive groups. (It is this that then allows species such as spider monkeys and chimpanzees, and the gelada and hamadryas baboons, to have fission–fusion social systems, in addition.)
As always in biology, solutions to one problem always incur costs of their own. One of these is the fact that creating bonded groups in this way inevitably places constraints on the size of group that can be maintained as a coherent entity. This is not true for unbonded social systems because in this case group size can respond more flexibly to contextual variations in the costs and benefits of grouping (hence allowing very large herds to be sustained, at least temporarily). This constraint on group size arises in part from the fact that bonded social systems seem naturally to give rise to in-group/out-group effects (bonded relationships inevitably differentiate between those with whom you have explicit relationships and those with whom you do not). In addition, bonded social systems are implicit social contracts: to be a member of a bonded group, each individual has to sacrifice some of its immediate selfish interests in order to allow everyone else to share in the benefits provided by the group. If they do not get this balance right, it will have the effect of driving group members away, resulting in the loss of the benefits derived from grouping. In other words, this is a multi-level selection process that involves balancing the fitness benefits that accrue through pursuing immediate selfish interests against those that accrue through group-level effects generated by cooperation. The latter are usually delayed, thereby creating a conflict between incompatible short- and long-term benefits and the behavioural trajectories that generate them.
In effect, primates live in societies that are implicit social contracts. However, such societies are inevitably susceptible to freeriders—those who take the benefits provided by the social contract, but do so without paying their share of the costs, in particular those costs related to holding back from exploiting all the short-term benefits that have to be foregone if the contract is to work [19,20]. Freeriders inevitably destabilize the social contract because they risk imposing an unacceptably high burden on the other individuals. Once the costs outweigh the benefits for these individuals, they will withdraw from the social contract, and the contract will collapse (usually resulting in group fission).
Insofar as we understand the primate social world at all, social bonding seems to operate through a dual process mechanism that involves a psychopharmacological component (mediated by endorphins) and a cognitive component (whereby relationships of trust and reciprocity are built up) [4]. The psychopharmacological component is derived from social grooming, which is known to trigger β-endorphin activation in the central nervous system [21]. Although endorphins function principally as neurotransmitters, they are explicitly involved in the pain control system and one of their principal functional effects is analgesia [22,23]. This is experienced (in humans, at least) as a mild opiate ‘high’, but its effect is probably to relax the animal [24,25] and so allow it to continue interacting with another individual long enough to build a cognitive relationship of trust and obligation. There is now an extensive, albeit rather obscure, literature on the social role of endorphins (for reviews, see [26–28]). Although a great deal has been made of the role of the oxytocin/vasopression axis in relation to pairbonding and social behaviour [29,30], there is a longstanding view that this mechanism is too fragile and short-lived to be effective in managing long-lasting social bonds such as those associated with primates' uniquely bonded social systems [27,28,31]. Some more robust mechanism is needed over and above the regular mammalian oxytocin mechanism to maintain (or service) relationships of this kind, and the suggestion has been that the endorphin system was co-opted for this purpose [27,28].
All this notwithstanding, maintaining cohesion in social groups depends not just on cognitive functions determined by brain size and the investment in social grooming; it also depends on the moment-to-moment processes of interaction and the fine-tuned signalling that this requires. Animals need to be able to signal their intentions, and those being signalled need to be able to interpret these signals correctly. It is therefore no surprise to find that both visual (i.e. facial) and vocal signalling capacities correlate with social group size in anthropoid primates. Dobson's [32] index of facial mobility (based on the use of Ekman's [33] facial action coding system (FACS) muscle activity scoring system) is a measure of visual signalling complexity, and it exhibits a clear relationship with social group size across primate species (figure 1a). Similarly, McComb & Semple [34] demonstrated that primate vocal repertoire size also correlates with social group size across primate species (figure 1b). Taxa that typically live in larger social groups have larger vocal and visual signal repertoires. Although they do not affect the overall relationship, there are some notable outliers. Saguinus seems to have more vocal signals at the expense of fewer facial signals for its group size, perhaps reflecting the signalling difficulties such a small animal faces in dense Amazonian forest. In contrast, Miopithecus has a vocal repertoire (12 calls) that is a third the size that would be predicted for its group size (the regression line would predict approx. 30 calls): although it is possible that its repertoire has been underestimated, it is noteworthy that this species in particular is renowned for its unusually large, rather chaotic social groups [38,39]. These outliers notwithstanding, the broad picture is that species seem to be adjusting the complexity of their communication in a dose–response fashion with increasing social group size. To the extent that larger group size means more complex relationships—and absolutely more dyadic and triadic relationships—this suggests that one solution to the problem of social cohesion is to allow more complex interactions to take place, mediated by more complex signalling. However, we might reasonably ask whether there is a natural limit to this as a solution. If so, we might expect signalling complexity to rise to a natural asymptote beyond which it cannot be increased. If group size is to increase beyond the size that corresponds to this limit, then something else has to come into play to breach the glass ceiling. In fact, the original analyses for both datasets used double-log plots, implying some kind of nonlinear relationship. Figure 1 plots both datasets in raw form: both look suspiciously as though repertoire complexity is asymptotic, with their upper limit at group sizes of 15–20 individuals.
Figure 1.
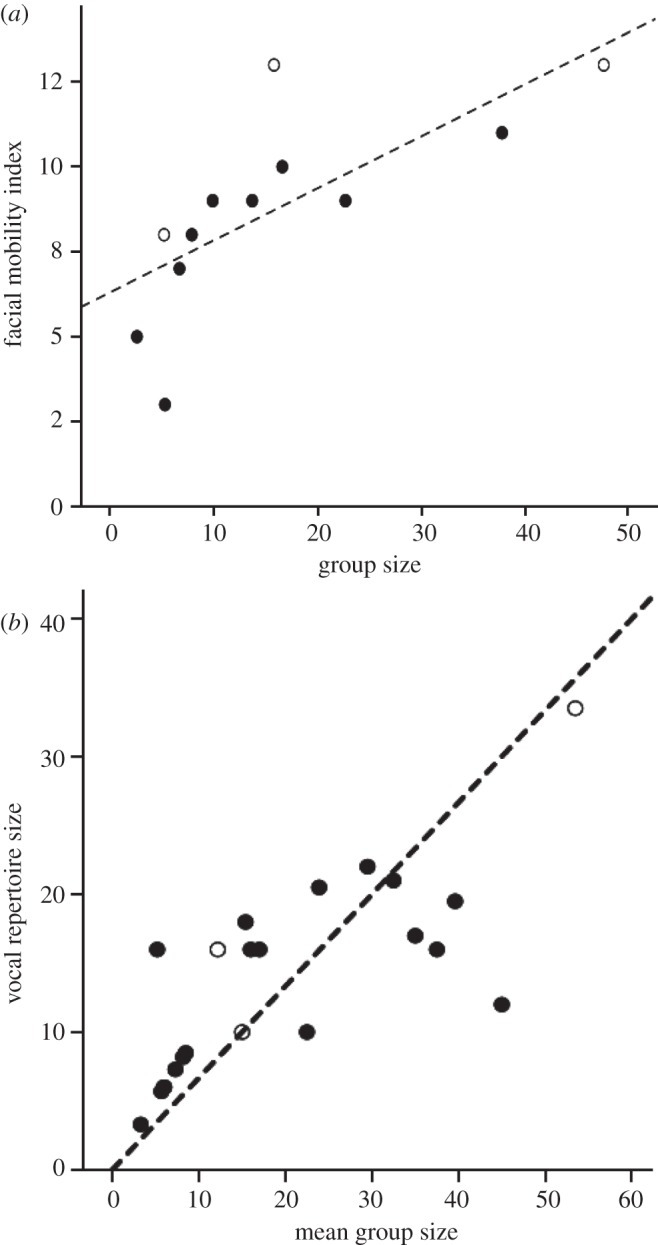
(a) Dobson's facial mobility index plotted against group size, for apes (open symbols) and monkeys (solid symbols). The outliers are: gorilla (top left, open symbol), Saguinus oedipus (lowest solid symbol). Source: adapted from Dobson [32]. (b) Vocal repertoire size plotted against mean group size for monkey (solid symbols) and ape (open symbols) genera. The outliers are: Saguinus (upper left), Miopithecus (lower right). Repertoire size from McComb & Semple [34]; group sizes from Dunbar [35], except for Mandrillus and Miopithecus [34], Theropithecus [36] and Pongo [37]. In both cases, the correlations are significant (facial mobility: r = 0.726, n = 12, p = 0.007; vocal repertoire: r = 0.746, n = 22, p < 0.001, respectively). Note that, in both cases, the originals plotted the data as double-log plots, with the regression analyses controlled for phylogeny.
Thus, while signalling systems certainly do allow animals to adjust to the changing complexity of their social environments, there are hints in these data that tinkering with the signalling system is only good up to a certain point. Something more sophisticated is needed if a species is to lift group size beyond these limits. One possibility might be the way primates use coalitionary alliances to buffer themselves against the costs of living in larger groups. Since these are serviced by social grooming, it is perhaps no surprise that time devoted to social grooming increases more or less linearly with group size [40,41]. This does not, however, mean that they groom with more social partners. While there is a general correlation between grooming clique size (i.e. the number of core grooming partners) and group size across species (figure 2a), within species, the relationship between number of grooming partners and group size typically follows a humped distribution (figure 2b). Initially, as group size increases, individuals widen their social network, but after a certain point they contract it again to concentrate their grooming on fewer and fewer social partners, despite the fact that they are actually devoting more time to grooming. In effect, they appear to be concentrating their available social capital on the core set of individuals who will really matter when the stresses of group living reach critical levels. A nice example of this tendency to focus on core social partners when circumstances become stressful is provided by lactating gelada females: they gradually abandon interactions with casual acquaintances as the nutritional demands of their infants place increasing pressure on their time budgets through extra feeding time requirements [45]. These mothers seemed to be concentrating what time they can afford to devote to social interaction on those social partners who will be of most value in buffering them against harassment and conflict both with other members of their unit and with the members of neighbouring units ([14,46]; see also [47]). Similarly, in baboons, several studies have shown that female social and reproductive success is dependent on the number of core friends they have [48,49].
Figure 2.
(a) Mean grooming clique size plotted against group size for individual primate genera. A clique is defined as the number of social partners with which an animal devotes at least 10% of its available social time to grooming (see Lehmann & Dunbar [42]). Open circles, prosimians; solid triangles, New World monkeys; open squares, Old World monkeys; solid circle, apes. Adapted from Dunbar [4], based on Kudo & Dunbar [43]; (b) Number of principal grooming partners for adult gelada females plotted against number of females in the unit. Adapted from Dunbar & Schultz [44].
What this seems to suggest is that, when the stresses start to bite, the animals are anxious to ensure that their core relationships will work when they really need them. This seems to reflect the fact that the intensity, or quality, of the relationship depends on the time invested in it [50], something that we have also documented in humans [51]. In primates at least, coalitions of this kind are established ahead of the circumstances in which they are needed, and this is one of the features that sets primate relationships apart from those of other mammalian taxa [52]. The importance of this is illustrated in figure 3, which plots the likelihood that a female will go to the support of a member of her unit when that individual is under threat by members of another unit against the time the two have spent grooming each other. Note that these data also suggest that, at least for this species (gelada), there is an asymptotic value at about 10 per cent of available social time beyond which further investment in grooming does not yield commensurate benefits in support. (These conflicts between females of neighbouring units are generally squabbles rather than outright fights and are most often resolved simply by one party moving out of the other's social space; however, in those cases where neither party is willing to back off, the conflict escalates and other individuals are drawn in to support their own unit-member [14].)
Figure 3.
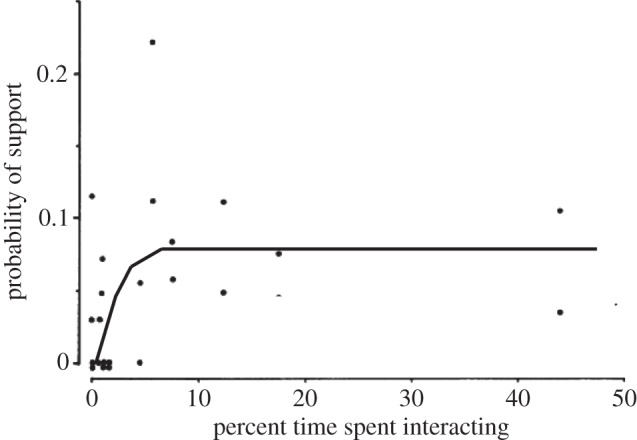
Probability of one adult female gelada supporting another adult female of her unit in conflicts with neighbouring units as a function of how much time they spent grooming with each other. Adapted from Dunbar [14].
This tendency to concentrate on core relationships is evident from an analysis of network density and connectedness for female cercopithecoid primates (i.e. species of the baboon-macaque-guenon group, all of whom live in multi-female groups of 20–50 individuals) [42,53]. This species-level analysis shows that, as the number of reproductive females in the group increases, the female social networks of larger-brained species become increasingly less inter-connected (figure 4). In other words, as group size increases, females seem to withdraw into their enclaves and focus their social attention on their principal allies.
Figure 4.
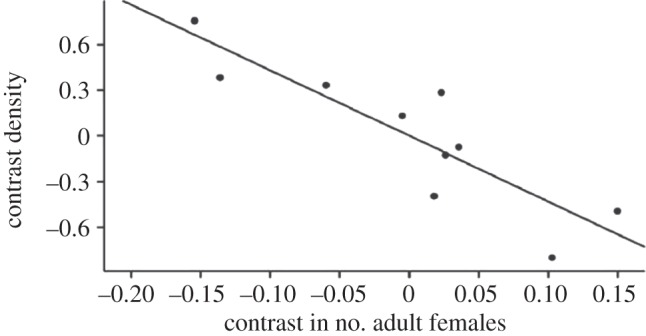
Network density among adult females plotted against number of females in the social group, for cercopithecoid primates (baboons, macaques and guenons). The data control for phylogenetic relatedness between species. Adapted from Lehmann & Dunbar [42].
The paradoxical nature of this effect is indicated by the fact that terrestrial monkeys (those that typically live in the largest social groups) have relatively smaller grooming cliques for group size than arboreal species (that live in smaller groups; figure 5). Because terrestrial species live in more predator-risky environments, they have to live in larger groups; but this imposes far greater stresses on them; so they need their alliances to work more effectively to minimize those stresses and allow them to maintain larger groups. However, by concentrating their social effort on a few key allies rather than distributing it around the wider social group, they inevitably have rather weak relationships with most of the other individuals in the group and the group is less cohesive and so more likely to fragment.
Figure 5.
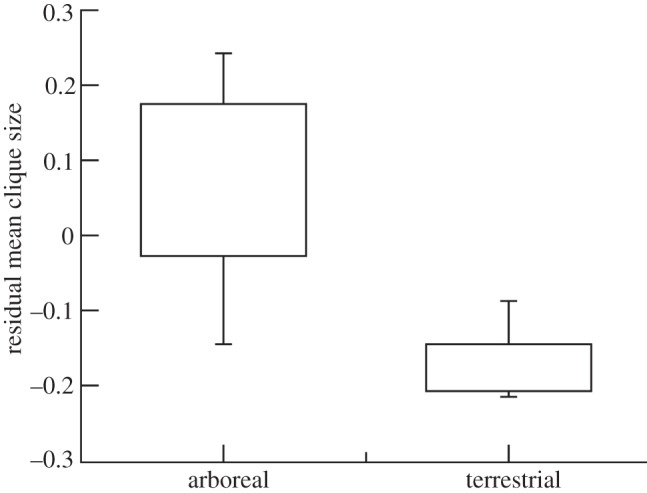
Mean (±50% and 95% ranges) residual grooming clique size (residualized against for group size) for arboreal versus terrestrial monkeys. Adapted from Kudo & Dunbar [43].
In effect, these animals have to manage two tiers of relationship (the grooming-based coalition and the group as a whole) simultaneously. In small social groups, like those of arboreal species that typically number 5–15 individuals, there is no practical difference between the grooming clique (the number of other individuals with whom an individual grooms) and the group as a whole, and so each individual only has to manage one kind of relationship (that based on grooming; figure 6). But species living in larger groups have to manage a more complex balance between maintaining the large group size needed for predator protection and investing in the small (but highly effective) coalitions that are needed to allow them to live in such large groups. In effect, they have two kinds of relationships to manage (strong links with coalition partners and weak links with others) and that has to be cognitively much more demanding. One indication that these species can manage more complex relationships is provided by the experimental evidence that baboons (perhaps the iconic species of large-brained, terrestrial primates that live in large social groups) are known to be able to process at least two dimensions to relationships at the same time (in this case, rank and kinship) [54].
Figure 6.
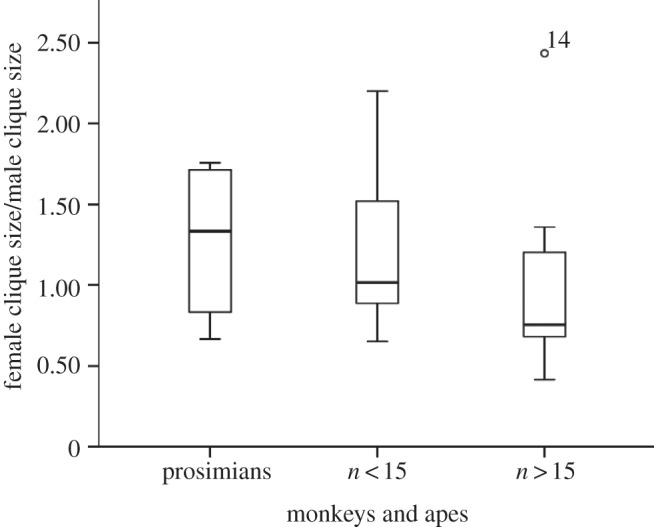
Ratio of female : male clique size in anthropoid monkeys. Adapted from Kudo & Dunbar [43].
An additional way in which species like baboons may be able to maintain the coherence of larger groups is to use males as weak links to bridge across between clusters of tightly bonded females [43]. Figure 6 plots the ratio of female to male grooming clique sizes (i.e. the number of other individuals groomed regularly) for prosimians and for anthropoid species that typically live in small (less than 15) and large (greater than 15) social groups. While there is considerable overlap, the relative size of males' social cliques gets progressively larger from prosimians to small- then large-group anthropoids. In fact, at least among the anthropoids, a pairwise comparison of small- and large-group species indicates that female clique sizes do not differ (F1,17 = 1.71, p = 0.209), but those for males in small groups are significantly smaller than those for males in large groups (F1,17 = 5.86, p = 0.027). It seems that in the large groups adult males act as bridges, or weak links, that help maintain the cohesion between clusters of females.
3. The transition to hominins: maintaining cohesion in super-large groups
Over the course of the last ca 6 million years since they branched off from the ancestral ape lineage, hominins (the lineage leading to modern humans) have undergone a progressive increase in group size, leading up to the community size of 150 that seems to be typical for contemporary humans [55–57]. The rate of increase has not, however, been linear: the estimates of community size for fossil populations (based on extrapolating from cranial volumes) suggest that group sizes remained comfortably within the upper limit for great apes (approx. 50) throughout the early australopithecine phase, exhibited a step shift to around approximately 70–80 with the appearance of Homo ergaster, stabilized for the better part of a million years, and then underwent a dramatic exponential increase after the appearance of archaic humans (Homo heidelbergensis) around 500 000 years ago (figure 7).
Figure 7.
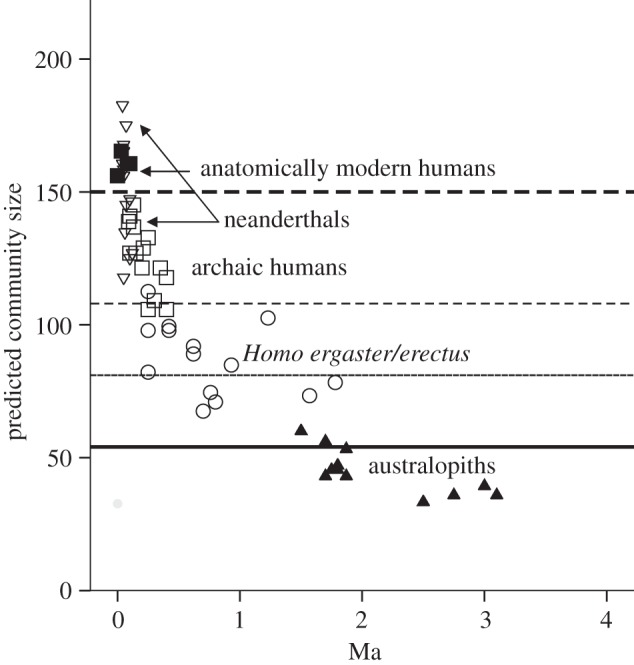
Estimated community size for individual hominin fossil populations, based on fossil cranial data from De Miguel & Heneberg [58] and equations from Dunbar [59]. A population is defined as all fossil crania from the same geographical site within a 50 000-year time period.
Group sizes of around 50 represent the upper limit that can be bonded by the conventional primate mechanism of social grooming: this is because ecological constraints on the amount of time that can be devoted to social interaction (i.e. grooming) place an upper limit at about 20 per cent of total daytime on grooming time for living primates [40,41]. Because contemporary humans also observe this same time constraint (social time averages almost exactly 20% of waking time across a range of modern populations [61]), some mechanism must have been needed to break through this glass ceiling in order to allow groups larger than approximately 50 to be bonded. Otherwise, both of the phase shifts in group size that occurred during human evolution (that at around 2 Ma and that at around 0.5 Ma) would have placed impossible strains on hominins' capacities to balance their time budgets at the same time as maintaining social cohesion. Indeed, because of the habitats they were occupying, these strains had already become apparent among australopithecines long before the appearance of the genus Homo [62].
Since contemporary hunter–gatherers and chimpanzees operate a fission–fusion form of sociality, it is likely that all—or, at least, most—hominin species also lived in these kinds of societies. If so, then most of the indirect time-budget stresses would have been less intrusive, but the direct stresses from daily competition would still have had to be moderated by close bonding. In addition, fission–fusion social systems probably place an additional cognitive stress on the animals because of the need to be able to remember individuals who are not physically present on the day and factor their interests into decisions on how to behave [63]. Given that the grooming mechanism reaches the limits of its capacity at group sizes of approximately 50, something else must have been necessary to lift group (or, more correctly, community) size above the 50 limit. While a completely different mechanism for bonding relationships is always a possibility, a more obvious solution is to find a way of triggering the same endorphin mechanism on a larger scale. In this respect, the problem that sets the limit on grooming as a bonding mechanism is the fact that it is a one-on-one activity (and, indeed, still is with us). What might solve the problem is a way of triggering endorphin activation at a distance so that it can be induced in several individuals at the same time.
My suggestion is that laughter provided this mechanism [64,65]. Laughter seems a good candidate for this for four reasons. First, it is a behaviour that we share with the chimpanzees (and perhaps the other great apes) [60,66,67]. There are structural differences in the form of laughter that distinguish the human and chimpanzee versions: in humans, laughter involves a series of exhalations, whereas in chimpanzees involves a more complex series of exhalation–inhalation cycles [64] (see fig. 1 in Davila Ross et al. [60]). However, these are merely structural adjustments, since the basic form is the same and the laugh vocalization is used in exactly the same contexts (play) in both taxa [67]. Second, laughter has all the hallmarks of a relatively primitive chorusing vocalization: it is intensely social (laughter is 30 times more likely to occur in a social context than when alone) and is highly contagious [66,68]. In effect, it is a form of wordless chorusing. Third, laughter seems to play a particularly important role in facilitating social interactions: interactions with close friends in which laughter occurs are perceived as being more satisfying than those where laughter does not occur [69]. Fourth, and most importantly, laughter turns out to be an extremely potent mechanism for triggering endorphin activation (figure 8). In effect, it functions as a form of grooming-at-a-distance in which the need for physical contact to trigger the endorphin effect has now been replaced by a visual or vocal stimulus. It is important in this context not to confuse laughter with humour. We use humour to trigger laughter, but this presumably has only been the case since we evolved the language skills necessary for telling jokes, and language unquestionably evolved much later, perhaps only as recently as 200 kya [59]. Rather, there is a more basic form of laughter that is triggered simply by seeing others laugh (whether or not we understand the joke, and indeed whether or not there even is a joke, be this slapstick or verbal).
Figure 8.
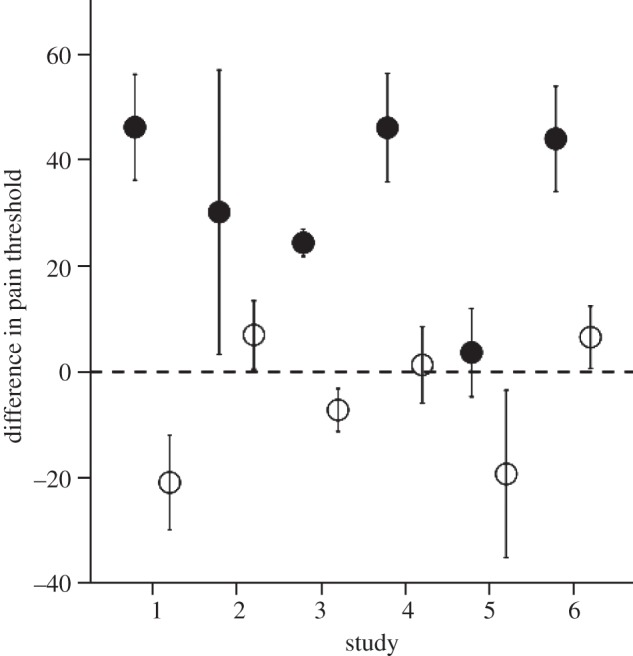
Mean (±s.e.) difference in pain threshold after watching either comedy (solid symbols) or neutral (open symbols) video in six separate experiments. Subjects took a pain test, watched video and then retook the pain test: the plotted value is the difference between post- and pre-intervention pain thresholds. In studies 1–5, subjects watched video clips; in study 6, subjects were actors or audience members at stand-up comedy or drama performances at the 2008 Edinburgh Fringe Festival. Adapted from Dunbar [68].
If laughter did provide the mechanism for bridging the bonding gap, then it raises two questions: (i) when did laughter become adapted in this way? and (ii) by how much did it raise the glass ceiling (assuming that it too has its own intrinsic limit on the number of individuals it can bond)? The first is the easier question to deal with. Figure 7 suggests that the most likely point of origin for laughter in its human form is right at the root of the Homo lineage, around 2 Ma. If it did not appear at this point, then something else that does the job as well would have had to, and we have no other plausible candidates. The second question is more difficult because there are no data on the limits of laughter as a bonding mechanism. However, one answer to this question might be to note that laughter only works as a form of chorusing in groups of individuals who are in close physical and auditory proximity. Although we can be stimulated to laugh just by hearing others laugh in large groups (e.g. in theatres), laughter does seem to be generated more readily and create greater intimacy in contexts where there is eye contact. It is one thing to laugh, but another to bond, and I suspect that, for it to act as a bonding mechanism, laughter requires face-to-face visual contact, probably at close quarters.
If this is so, then the limits are probably set by the size of natural conversation groups. These have a natural upper limit at four individuals in modern humans [70]. This is partly a function of speech detection across the circle occupied by the conversation group, and partly by cognitive limits on our ability to pay attention to several individuals simultaneously. If the conversation group represents the limit for close chorusing in the way suggested, then laughter might be deemed to be twice as efficient as grooming as a bonding mechanism: it allows the number of interacting individuals to increase from two (the limit on mutual grooming) to four (the limit for conversation groups). In effect, it allows a doubling of the interaction group.
A doubling of interaction group size between the chimpanzees and the hominin lineage should (according to figure 2a) almost double the size of the social group (or community). Since the mean observed community size for chimpanzees given by Dunbar [36] is 53.8, this would imply a limit on laughter-bonded communities of 53.8×2 ≈ 108 in hominins. This is plotted as the middle dashed line in figure 7, and turns out to be the upper limit for estimated community size in the H. ergaster/erectus lineage and the lower limit for archaic humans (Homo heidelbergensis). Presumably, this form of close chorusing would have arisen in stepwise fashion via an intermediate interaction group size of 3. If so, this would give a limiting community size of 53.8 × 1.5 ≈ 81 (thin solid line in figure 7), which is exactly the estimated community size in Homo ergaster populations when they first emerge at 2 Ma, marking the disjunction with the australopithecines.
In effect, laughter seems to have effectively bridged the bonding gap well enough to lift community sizes to around 100 (presumably as a function of the amount of time devoted to laughter and grooming in combination). To increase community beyond this new threshold, as archaic humans did from around 500 kya, would then have required an additional mechanism allowing an endorphin effect to be created on an even larger scale. Music (including singing and dancing), and after that the rituals of religion, may have successively provided the additional mechanisms [64,65,71].
4. Conclusion
I have suggested that the particular kinds of bonded social systems that primate evolved impose constraints on the animals' capacity to increase group size. This is because of the focused intensity of the dyadic relationships involved. The intensity of these social bonds seems to depend on the time invested in them, and thus the number of individuals that can be groomed to the criterion required to ensure an effective coalition is ultimately limited by the amount of time the animals can afford to devote to social grooming. In simple social systems, this limits the size of group that can be maintained through time as a coherent unit (probably to about 15 individuals). To increase group size beyond this requires the need to develop a two-tier form of sociality whereby grooming is confined to core alliance partners, and bridging relationships are used to link sets of coalitions into larger groupings. This seems to require a phase shift in terms of the cognitive basis for relationships.
Humans provide a more complex case in that they have been able to increase group (i.e. community) size well beyond the range found in the non-human primates. Nonetheless, they seemed to have faced the same problem, because the most social monkeys and apes had already exhausted what could be managed with the standard mechanism for social bonding (i.e. grooming and the endorphin activation that grooming triggers). To break through the glass ceiling imposed by the ecological limit on the time available for grooming, humans either had to reduce other time-budget requirements or had to change the behaviour used in bonding. The first was probably not feasible: modelling the time budgets of the australopithecines suggests that they were already operating at the very limits of what was possible [62]. I have suggested that, instead, later hominins opted to exploit new ways of triggering the endorphin mechanism. The first step in this sequence may have been laughter, a form of vocal communication that would already have been in use in the ancestral common ancestor with the great apes: hominins exploited and exaggerated this behaviour to provide a form of grooming-at-a-distance that could involve several individuals simultaneously. Estimates of how effective this might have been for bonding suggest that laughter might have conveniently bridged the bonding gap between the australopithecines (functioning as very conventional apes) and the appearance of archaic humans (for whom additional bonding mechanisms would then have been required).
References
- 1.Shultz S., Dunbar R. I. M. 2010. Encephalization is not a universal macroevolutionary phenomenon in mammals but is associated with sociality. Proc. Natl Acad. Sci. USA 107, 21 582–21 586 10.1073/pnas.1005246107 (doi:10.1073/pnas.1005246107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shultz S., Dunbar R. I. M. 2007. The evolution of the social brain: anthropoid primates contrast with other vertebrates. Proc. R. Soc. B 274, 2429–2436 10.1098/rspb.2007.0693 (doi:10.1098/rspb.2007.0693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunbar R. I. M., Shultz S. 2010. Bondedness and sociality. Behaviour 147, 775–803 10.1163/000579510X501151 (doi:10.1163/000579510X501151) [DOI] [Google Scholar]
- 4.Dunbar R. I. M. 2010. Brain and behaviour in primate evolution. In Mind the gap: tracing the origins of human universals (eds Kappeler P. H., Silk J.), pp. 315–330 Berlin, Germany: Springer [Google Scholar]
- 5.van Schaik C. P. 1983. Why are diurnal primates living in groups. Behaviour 87, 120–144 10.1163/156853983X00147 (doi:10.1163/156853983X00147) [DOI] [Google Scholar]
- 6.Dunbar R. I. M. 1988. Primate social systems. London, UK: Chapman & Hall [Google Scholar]
- 7.Shultz S., Dunbar R. I. M. 2006. Both social and ecological factors predict ungulate brain size. Proc. R. Soc. B 273, 207–215 10.1098/rspb.2005.3283 (doi:10.1098/rspb.2005.3283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrangham R. W. 1980. An ecological model of female-bonded primate groups. Behaviour 75, 262–300 10.1163/156853980X00447 (doi:10.1163/156853980X00447) [DOI] [Google Scholar]
- 9.van Schaik C. P. 1989. The ecology of social relationships among female primates. In Comparative socioecology: the behavioural ecology of humans and other mammals (eds Standen V., Foley R. A.), pp. 195–218 Oxford, UK: Blackwell [Google Scholar]
- 10.Dunbar R. I. M. 2010. Brain and behaviour in primate evolution. In Mind the gap: tracing the origins of human universals (eds Kappeler P. H., Silk J.), pp. 315–330 Hamburg, Germany: Springer [Google Scholar]
- 11.Dunbar R. I. M., Korstjens A. H., Lehmann J. 2008. Time as an ecological constraint. Biol. Rev. 84, 413–429 10.1111/j.1469-185X.2009.00080.x (doi:10.1111/j.1469-185X.2009.00080.x) [DOI] [PubMed] [Google Scholar]
- 12.Abbott D. H., Keverne E. B., Moore G. F., Yodyinguad U. 1986. Social suppression of reproduction in subordinate talapoin monkeys, Miopithecus talapoin. In Primate ontogeny (eds Else J., Lee P. C.), pp. 329–341 Cambridge, UK: Cambridge University Press [Google Scholar]
- 13.Bowman L. A., Dilley S., Keverne E. B. 1978. Suppression of oestrogen-induced LH surges by social subordination in talapoin monkeys. Nature 275, 56–58 10.1038/275056a0 (doi:10.1038/275056a0) [DOI] [PubMed] [Google Scholar]
- 14.Dunbar R. I. M. 1980. Determinants and evolutionary consequences of dominance among female gelada baboons. Behav. Ecol. Sociobiol. 7, 253–265 10.1007/BF00300665 (doi:10.1007/BF00300665) [DOI] [Google Scholar]
- 15.Smuts B. B., Nicholson N. 1989. Dominance rank and reproduction in female baboons. Am. J. Primatol. 19, 229–246 10.1002/ajp.1350190405 (doi:10.1002/ajp.1350190405) [DOI] [PubMed] [Google Scholar]
- 16.Korstjens A. H., Verhoeckx I., Dunbar R. I. M. 2006. Time as a constraint on group size in spider monkey. Behav. Ecol. Sociobiol. 60, 683–694 10.1007/s00265-006-0212-2 (doi:10.1007/s00265-006-0212-2) [DOI] [Google Scholar]
- 17.Lehmann J., Korstjens A. H., Dunbar R. I. M. 2007. Fission–fusion social systems as a strategy for coping with ecological constraints: a primate case. Evol. Ecol. 21, 613–634 10.1007/s10682-006-9141-9 (doi:10.1007/s10682-006-9141-9) [DOI] [Google Scholar]
- 18.Lehmann J., Dunbar R. I. M. 2009. Implications of body mass and predation for ape social system and biogeographical distribution. Oikos 118, 379–390 10.1111/j.1600-0706.2008.16382.x (doi:10.1111/j.1600-0706.2008.16382.x) [DOI] [Google Scholar]
- 19.Enquist M., Leimar O. 1993. The evolution of cooperation in mobile organisms. Anim. Behav. 45, 747–757 10.1006/anbe.1993.1089 (doi:10.1006/anbe.1993.1089) [DOI] [Google Scholar]
- 20.Nettle D., Dunbar R. I. M. 1997. Social markers and the evolution of reciprocal exchange. Curr. Anthropol. 38, 93–99 10.1086/204588 (doi:10.1086/204588) [DOI] [Google Scholar]
- 21.Keverne E. B., Martensz N. D., Tuite B. 1989. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology 14, 155–161 10.1016/0306-4530(89)90065-6 (doi:10.1016/0306-4530(89)90065-6) [DOI] [PubMed] [Google Scholar]
- 22.Stephano G., et al. 2000. Endogenous morphine. Trends Neurosci. 23, 436–442 10.1016/S0166-2236(00)01611-8 (doi:10.1016/S0166-2236(00)01611-8) [DOI] [PubMed] [Google Scholar]
- 23.Zubieta J.-K., Smith Y.-R., Bueller J. A., Xu K., Kilbourn M. R., Jewett D. M., Meyer C. R., Koeppe R. A., Stohler C. S. 2001. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 293, 311–315 10.1126/science.1060952 (doi:10.1126/science.1060952) [DOI] [PubMed] [Google Scholar]
- 24.Goosen C. 1981. On the function of allogrooming in Old-World monkeys. In Primate behaviour and sociobiology (eds Chiarelli A. B., Corruccini R. S.), pp. 110–120 Berlin, Germany: Springer [Google Scholar]
- 25.Schino G., Scucchi S., Maestripieri D., Turillazzi P. G. 1988. Allogrooming as a tension-reduction mechanism: a behavioural approach. Am. J. Primat. 16: 43–50 10.1002/ajp.1350160106 (doi:10.1002/ajp.1350160106) [DOI] [PubMed] [Google Scholar]
- 26.Depue R. A., Morrone-Strupinsky J. V. 2005. A neurobehavioral model of affiliative bonding: implications for conceptualising a human trait of affiliation. Behav. Brain Sci. 28, 313–395 [DOI] [PubMed] [Google Scholar]
- 27.Dunbar R. I. M. 2011. The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neurosci. Biobehav. Rev. 34, 260–268 10.1016/j.neubiorev.2008.07.001 (doi:10.1016/j.neubiorev.2008.07.001) [DOI] [PubMed] [Google Scholar]
- 28.Machin A. J., Dunbar R. I. M. 2011. The brain opioid theory of social attachment: a review of the evidence. Behaviour 148, 985–1025 [Google Scholar]
- 29.Carter C. S., DeVries A. C., Getz L. L. 1995. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci. Biobehav. Rev. 16, 131–144 10.1016/S0149-7634(05)80176-9 (doi:10.1016/S0149-7634(05)80176-9) [DOI] [PubMed] [Google Scholar]
- 30.Young L. J., Wang Z. 2004. The neurobiology of pairbonding. Nat. Neurosci. 7, 1048–1054 10.1038/nn1327 (doi:10.1038/nn1327) [DOI] [PubMed] [Google Scholar]
- 31.Curley J. P., Keverne E. B. 2005. Genes, brains and mammalian social bonds. Trends Ecol. Evol. 20, 561–567 10.1016/j.tree.2005.05.018 (doi:10.1016/j.tree.2005.05.018) [DOI] [PubMed] [Google Scholar]
- 32.Dobson S. D. 2009. Socioecological correlates of facial mobility in nonhuman anthropoids. Am. J. Phys. Anthropol. 139, 413–420 10.1002/ajpa.21007 (doi:10.1002/ajpa.21007) [DOI] [PubMed] [Google Scholar]
- 33.Ekman P., Friesen W. V., Hager J. C. 2002. Facial action coding system: the manual on CD-ROM. Salt Lake City, UT: Research Nexus [Google Scholar]
- 34.McComb K., Semple S. 2005. Coevolution of vocal communication and sociality in primates. Biol. Lett. 1, 381–385 10.1098/rsbl.2005.0366 (doi:10.1098/rsbl.2005.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunbar R. I. M. 1992. Neocortex size as a constraint on group size in primates. J. Hum. Evol. 22, 469–493 10.1016/0047-2484(92)90081-J (doi:10.1016/0047-2484(92)90081-J) [DOI] [Google Scholar]
- 36.Dunbar R. I. M. 1995. Neocortex size and group size in primates: a test of the hypothesis. J. Hum. Evol. 28, 287–296 10.1006/jhev.1995.1021 (doi:10.1006/jhev.1995.1021) [DOI] [Google Scholar]
- 37.Dunbar R. I. M. 2011. Evolutionary basis of the social brain. In Oxford handbook of social neuroscience (eds Decety J., Cacioppo J.), pp. 28–38 Oxford, UK: Oxford University Press [Google Scholar]
- 38.Gautier-Hion A. 1970. L'organisation sociale d'une bande de talapoins (Miopithecus talapoin) dans le nord-ouest du Gabon. Folia Primatol. 12, 116–141 10.1159/000155285 (doi:10.1159/000155285) [DOI] [PubMed] [Google Scholar]
- 39.Rowell T. E. 1973. Social organization of wild talapoin monkeys. Am. J. Phys. Anthropol. 38, 593–597 10.1002/ajpa.1330380273 (doi:10.1002/ajpa.1330380273) [DOI] [PubMed] [Google Scholar]
- 40.Dunbar R. I. M. 1991. Functional significance of social grooming in primates. Folia Primatol. 57, 121–131 10.1159/000156574 (doi:10.1159/000156574) [DOI] [Google Scholar]
- 41.Lehmann J., Korstjens A. H., Dunbar R. I. M. 2007. Group size, grooming and social cohesion in primates. Anim. Behav. 74, 1617–1629 10.1016/j.anbehav.2006.10.025 (doi:10.1016/j.anbehav.2006.10.025) [DOI] [Google Scholar]
- 42.Lehmann J., Dunbar R. I. M. 2009. Network cohesion, group size and neocortex size in female-bonded Old World primates. Proc. R. Soc. B 276, 4417–4422 10.1098/rspb.2009.1409 (doi:10.1098/rspb.2009.1409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kudo H., Dunbar R. I. M. 2001. Neocortex size and social network size in primates. Anim. Behav. 62, 711–722 10.1006/anbe.2001.1808 (doi:10.1006/anbe.2001.1808) [DOI] [Google Scholar]
- 44.Dunbar R. I. M., Shultz S. 2007. Evolution in the social brain. Science 317, 1344–1347 10.1126/science.1145463 (doi:10.1126/science.1145463) [DOI] [PubMed] [Google Scholar]
- 45.Dunbar R. I. M., Dunbar P. 1988. Maternal time budgets of gelada baboons. Anim. Behav. 36, 970–980 10.1016/S0003-3472(88)80055-1 (doi:10.1016/S0003-3472(88)80055-1) [DOI] [Google Scholar]
- 46.Dunbar R. I. M. 1989. Reproductive strategies of female gelada baboons. In Sociobiology of sexual and reproductive strategies (eds Rasa A., Vogel C., Voland E.), pp. 74–92 London, UK: Chapman & Hall [Google Scholar]
- 47.Wittig R. M., Crockford C., Lehmann J., Whitten P. L., Seyfarth R. M., Cheney D. L. 2008. Focused grooming networks and stress alleviation in wild female baboons. Horm. Behav. 54, 170–177 10.1016/j.yhbeh.2008.02.009 (doi:10.1016/j.yhbeh.2008.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silk J. B., Alberts S. C., Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1232–1234 10.1126/science.1088580 (doi:10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 49.Silk J. B., Beehner J. C., Bergman T. J., Crockford C., Engh A. L., Moscovice L. R., Wittig R. M., Seyfarth R. M., Cheney D. L. 2009. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc. R. Soc. B 276, 3099–3104 10.1098/rspb.2009.0681 (doi:10.1098/rspb.2009.0681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seyfarth R. M. 1977. A model of social grooming among adult female monkeys. J. Theor. Biol. 65, 671–698 10.1016/0022-5193(77)90015-7 (doi:10.1016/0022-5193(77)90015-7) [DOI] [PubMed] [Google Scholar]
- 51.Roberts S. B. G., Dunbar R. I. M. 2011. The costs of family and friends: an 18-month longitudinal study of relationship maintenance and decay. Evol. Hum. Behav. 32, 186–197 [Google Scholar]
- 52.Harcourt A. H. 1992. Coalitions and alliances: are primates more complex than non-primates? In Coalitions and alliances in humans and other animals (eds Harcourt A. H., de Waal F. B. M.). Oxford, UK: Oxford University Press [Google Scholar]
- 53.Lehmann J., Andrews K., Dunbar R. I. M. 2009. Social networks and social complexity in female-bonded primates. In Social brain, distributed mind (eds Dunbar R., Gamble C., Gowlett J.), pp. 57–83 Oxford, UK: Oxford University Press [Google Scholar]
- 54.Bergman T. J., Beehner J. C., Cheney D. L., Seyfarth R. M. 2003. Hierarchical classification by rank and kinship in baboons. Science 302, 1234–1236 10.1126/science.1087513 (doi:10.1126/science.1087513) [DOI] [PubMed] [Google Scholar]
- 55.Dunbar R. I. M. 1992. Coevolution of neocortex size, group size and language in humans. Behav. Brain Sci. 16, 681–735 10.1017/S0140525X00032325 (doi:10.1017/S0140525X00032325) [DOI] [Google Scholar]
- 56.Aiello L. C., Dunbar R. I. M. 1993. Neocortex size, group size and the evolution of language. Curr. Anthropol. 34, 184–193 10.1086/204160 (doi:10.1086/204160) [DOI] [Google Scholar]
- 57.Gowlett J. A. J., Gamble C., Dunbar R. I. M. In press. Human evolution and the archaeology of the social brain. Curr. Anthropol. [Google Scholar]
- 58.De Miguel C., Heneberg M. 2001. Variation in hominin brain size: how much is due to method? Homo 52, 3–58 10.1078/0018-442X-00019 (doi:10.1078/0018-442X-00019) [DOI] [PubMed] [Google Scholar]
- 59.Dunbar R. I. M. 2009. Why only humans have language. In The prehistory of language (eds Botha R., Knight C.), pp. 12–35 Oxford, UK: Oxford University Press [Google Scholar]
- 60.Davila Ross M., Owren M. J., Zimmermann E. 2009. Reconstructing the evolution of laughter in great apes and humans. Curr. Biol. 19, 1–6 10.1016/j.cub.2009.05.028 (doi:10.1016/j.cub.2009.05.028) [DOI] [PubMed] [Google Scholar]
- 61.Dunbar R. I. M. 1998. Theory of mind and the evolution of language. In Approaches to the evolution of language (eds Hurford J., Studdart-Kennedy M., Knight C.), pp. 92–110 Cambridge, UK: Cambridge University Press [Google Scholar]
- 62.Bettridge C. 2009. Exploring the Behavioural Ecology of Extinct Hominins. DPhil thesis, University of Oxford, Oxford, UK [Google Scholar]
- 63.Barrett L., Henzi S. P., Dunbar R. I. M. 2003. Primate cognition: from ‘what now?’ to ‘what if?’ Trends Cogn. Sci. 7, 494–497 10.1016/j.tics.2003.09.005 (doi:10.1016/j.tics.2003.09.005) [DOI] [PubMed] [Google Scholar]
- 64.Dunbar R. I. M. 2008. Mind the gap: or why humans aren't just great apes. Proc. Brit. Acad. 154, 403–423 [Google Scholar]
- 65.Dunbar R. I. M. 2009. Mind the bonding gap: constraints on the evolution of hominin societies. In Pattern and process in cultural evolution (ed. Shennan S.), pp. 223–234 Berkeley, CA: University of California Press [Google Scholar]
- 66.Provine R. 1996. Laughter: a scientific investigation. London, UK: Faber & Faber [Google Scholar]
- 67.Waller B., Dunbar R. I. M. 2005. Differential behavioural effects of ‘smiling’ and ‘laughing’ in chimpanzees (Pan troglodytes). Ethology 111, 129–142 10.1111/j.1439-0310.2004.01045.x (doi:10.1111/j.1439-0310.2004.01045.x) [DOI] [Google Scholar]
- 68.Dunbar R. I. M., et al. 2012. Social laughter is correlated with an elevated pain threshold. Proc. R. Soc. B 279, 1161–1167 10.1098/rspb.2011.1373 (doi:10.1098/rspb.2011.1373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vlahovic T., Roberts S. B. G., Dunbar R. I. M. In press Effects of duration and laughter on subjective happiness within different modes of communication. J. Comput. Mediat. Commun. [Google Scholar]
- 70.Dunbar R. I. M., Duncan N., Nettle D. 1995. Size and structure of freely forming conversational groups. Hum. Nat. 6, 67–78 10.1007/BF02734136 (doi:10.1007/BF02734136) [DOI] [PubMed] [Google Scholar]
- 71.Mithen S. 2005. The singing Neanderthals: the origins of music, language, mind and body. London, UK: Weidenfeld & Nicholson [Google Scholar]



