Abstract
Primates are intensely social and exhibit extreme variation in social structure, making them particularly well suited for uncovering evolutionary connections between sociality and vocal complexity. Although comparative studies find a correlation between social and vocal complexity, the function of large vocal repertoires in more complex societies remains unclear. We compared the vocal complexity found in primates to both mammals in general and human language in particular and found that non-human primates are not unusual in the complexity of their vocal repertoires. To better understand the function of vocal complexity within primates, we compared two closely related primates (chacma baboons and geladas) that differ in their ecology and social structures. A key difference is that gelada males form long-term bonds with the 2–12 females in their harem-like reproductive unit, while chacma males primarily form temporary consortships with females. We identified homologous and non-homologous calls and related the use of the derived non-homologous calls to specific social situations. We found that the socially complex (but ecologically simple) geladas have larger vocal repertoires. Derived vocalizations of geladas were primarily used by leader males in affiliative interactions with ‘their’ females. The derived calls were frequently used following fights within the unit suggesting that maintaining cross-sex bonds within a reproductive unit contributed to this instance of evolved vocal complexity. Thus, our comparison highlights the utility of using closely related species to better understand the function of vocal complexity.
Keywords: derived vocalizations, group size, homologous vocalizations, social complexity, vocal complexity, vocal repertoire
1. Introduction
The complexity of vocal communication varies enormously across species, from humans with an endless repertoire of sound combinations, to species of mongoose that produce only three different sounds [1]. As we continue to document the diversity that exists in nature, we are increasingly able to use comparative studies to identify the selective pressures responsible for increasing vocal complexity. One of the most salient findings that has emerged is that high levels of sociality are found in combination with a high degree of vocal complexity [2–5]. For example, ground-dwelling sciurid species with socially complex groups (i.e. many age/sex classes) produce more acoustically distinct alarm calls than species with fewer age/sex classes [2]. Although non-primate taxa can be excellent study subjects for investigating the evolution of vocal complexity in general (e.g. rodents: [6]; bats [5]) primates, as our closest relatives, can provide insight into the evolution of the most complex vocal system—our own [7,8]. Moreover, primates exhibit extreme variation in social structure, making them particularly well suited for uncovering evolutionary connections between sociality and vocal complexity.
Facets of primates' sociality distinguish them from most other mammals. First, primates exhibit an unusual degree of sociality that some have proposed has resulted in a kind of ‘Machiavellian intelligence’ [9,10] in that individuals are capable of forming coalitions [11], deceiving others [12] and maintaining strong, long-term social bonds with both kin and non-kin [13–15]. Second, primates are unusual among mammals in that the size of their groups is positively associated with some aspects of brain size [10,13]. One intriguing explanation for this relationship is that primates require sophisticated cognitive abilities for keeping track of and maintaining complex networks of social relationships [9,16,17]—particularly considering recent findings that social networks actually enhance individuals' fitness [18,19]. Offering further support, the strong positive relationship between group size, time spent grooming and diversity of vocal repertoire in primates [20] suggest that more vocalizations may indeed be necessary for navigating the complex network of social relationships in primate societies.
In this review, we first focus on the evolution of vocal complexity in primates, and then propose a novel approach for studying the function of vocal complexity. Although a species' repertoire size provides one useful comparative metric, it is a composite measure with no information about the function of the individual calls that comprise it. Here, we propose that the function of vocal complexity can be understood by comparing vocalizations among closely related species with differing repertoire sizes to identify species-specific derived calls. In such cases, the function of greater vocal complexity matches the function of the derived calls. As an example of how this approach can provide insights about the evolution of complexity, we compare the vocalizations of geladas (Theropithecus gelada) and chacma baboons (Papio ursinus)—two closely related Old World monkeys with overlapping vocal repertoires but very different ecological and social structures.
2. Vocal repertoires of primates and other mammals
(a). Repertoire size as a measure of complexity
Mammalian vocal communication is typically described as being made up of discrete, functional units, or ‘calls’ [21–23]. Based on these functional units, vocal complexity is quantified in terms of (i) number of discrete vocalizations in the repertoire (repertoire size) [2,20,24,25], or less commonly, (ii) degree of individuality within discrete calls [5,6,26]. Other ways of assessing vocal complexity include quantifying syllable complexity, amount of information contained within a call [5], or the number of calls within a specific category of vocalizations (e.g. alarm calls [2]).
Several mammalian species produce call variants, or graded calls, which vary slightly in acoustic properties [27–31], such as fundamental frequency [28], ‘pitch’ [29] or duration [24] and, as a result, have different meanings to receivers [29,32–34]. For species with small, fixed vocal repertoires, these subtle alterations may help to extend the flexibility of an otherwise limited repertoire [7]. However, identifying graded calls requires detailed acoustic and behavioural analyses and data of comparable detail are rarely available for multiple species. Therefore, as has become the convention in vocal studies [2,20,24,25], we refer to repertoire size as the number of discrete calls that animals in a population or species produce.
(b). Vocalization types
Vocalizations are produced in many different contexts. Some are produced in response to external stimuli such as predators and food. We call these ‘allospecific’ vocalizations (table 1) and include alarm calls and food calls. Alarm calls can communicate the degree of risk involved [2,24], indicate predator type (i.e. aerial or terrestrial) [53,54], or combine information on risk and predator type [51]. Primates, in particular, are known to produce alarm vocalizations specific to predator type, eliciting appropriate responses in receivers [55–57]. Notably, the complexity of primate alarm calls is generally attributed to a complex physical (rather than social) environment [58]. If different predators have different modes of hunting, primate prey should have evolved different predator responses to each. By contrast, the complexity of alarm calls in a small social carnivore, the meerkat (Suricata suricatta), has been attributed to the need for social coordination [4]. Relative to a sympatric-living herbivore species like Cape ground squirrels (Xerus inauris), meerkats travel farther from underground shelters in their open habitat to find living prey, and they depend on ‘sentinels’ to emit referential alarm calls that vary acoustically based on predator type [4]. This strategy allows individual meerkats to decrease time spent being vigilant and increase foraging efficiency.
Table 1.
Vocal repertoire size for exemplar species from Primata, Rodentia, and Carnivora broken down into six categories: Allospecific (alarm calls and food calls), long distance (separation calls, intergroup spacing calls), contact (short-range soft calls), competitive (threat and display calls), distress calls (fear calls during agonism) and other (contexts unknown or made in several different contexts). Sources from Primata are drawn from the repertoire analysis made by McComb & Semple [20], excluding captive studies. Total repertoire sizes in this paper are slightly different because we did not count sequences of discrete call units as separate calls if the units were produced singly. Sounds that are not strictly ‘vocalizations’, such as sneezes, coughs and teeth chattering, are excluded from the table. For comparison, we focus on exemplar species from Rodentia and Carnivora because of similarities in social and vocal behaviour.
| species name | allospecific | long distance | contact | competitive | distress | other | total size | citations |
|---|---|---|---|---|---|---|---|---|
| Order Primata | ||||||||
| Alouatta palliata | — | 1 | — | 8 | 2 | 1 | 12 | [35] |
| Arctocebus calabarensis | — | 1 | — | 1 | 1 | — | 3 | [36] |
| Callicebus moloch | 1 | 3 | 1 | 1 | 3 | 1 | 10 | [37] |
| Callimico goeldii | 4 | 7 | 3 | 6 | 4 | 1 | 25 | [38] |
| Cebus olivaceus | — | — | 4 | 4 | 2 | 1 | 11 | [39] |
| Cercocebus torquatus | 4 | 1 | 3 | 5 | 1 | — | 14 | [40] |
| Cercopithecus aethiops | 5 | — | 3 | 5 | 3 | 3 | 19 | [41] |
| Euoticus elegantulus | 2 | 1 | 2 | — | 1 | — | 6 | [36] |
| Galago alleni | 1 | — | 2 | 1 | 1 | — | 5 | [36] |
| G. demidovii | 1 | 1 | 3 | 1 | 1 | 1 | 8 | [36] |
| Macaca fascicularis | 2 | 2 | 1 | 1 | 4 | 5 | 15 | [42] |
| M. radiata | 1 | 1 | 3 | 7 | 4 | 3 | 19 | [43] |
| M. silenus | 1 | 2 | 3 | 5 | 2 | 1 | 14 | [43] |
| Mandrillus sphinx | 1 | 3 | 1 | — | 4 | — | 9 | [44] |
| Pan paniscus | — | — | 4 | — | 1 | 4 | 9 | [45] |
| P. troglodytes | 3 | 1 | 8 | 4 | 8 | 3 | 27 | [46] |
| Perodicticus potto | — | 1 | 2 | 1 | 1 | 5 | [36] | |
| Pongo pygmaeus | 1 | 1 | 1 | 2 | 2 | 1 | 8 | [47] |
| Presbytis entellus | 3 | 1 | 2 | 3 | 3 | 2 | 14 | [43] |
| P. johnii | 2 | 1 | 3 | 4 | 3 | 1 | 14 | [43] |
| Procolobus badius | 2 | — | — | 5 | 2 | 2 | 11 | [48] |
| Order Rodentia | ||||||||
| Notomys alexis | — | — | 1 | 1 | 1 | 2 | 5 | [23] |
| N. cervinus | — | — | — | 1 | 1 | 2 | 4 | [23] |
| N. mitchellii | — | — | 1 | 1 | 1 | 2 | 5 | [23] |
| N. fuscus | — | — | 1 | 1 | 1 | 2 | 5 | [23] |
| Octodon degus | 2 | — | 4 | 5 | 2 | — | 13 | [49] |
| Order Carnivora | ||||||||
| Lycaon pictus | 3 | 2 | 7 | 5 | 2 | 6 | 25 | [50] |
| Suricata suricatta | 11 | — | 7 | 2 | 2 | 3 | 25 | [51,52] |
| Cynictis penicillata | 3 | — | 2 | 2 | — | 1 | 8 | [24] |
| Speothos venaticus | — | 1 | 3 | 2 | 1 | — | 7 | [27] |
| Cerdocyon thous | 2 | 1 | 2 | 1 | — | — | 6 | [27] |
| Chrysocyon brachyurus | 1 | 2 | 2 | 2 | 1 | — | 8 | [27] |
The other allospecific vocalizations, food calls, are less variable than alarm calls. Only a handful of studies have demonstrated that variation in calls is related to the quantity or quality of the food source [59]; but most studies report a lack of variation [60]. Although alarm and food calls differ substantially in degree of complexity, they share two features: (i) they are elicited by non-conspecifics, and (ii) they are the only two contexts where proto-syntax (i.e. the combination of call elements to form new meanings) has been reported, specifically in primates (food calls [61]; alarm calls [62]).
The vast majority of mammalian vocalizations are emitted during social interactions with conspecifics, under conditions of varying motivational states (e.g. mating, aggression, fear). We call these ‘social’ vocalizations and divide them into two main classes—calls that function over a long distance (‘loud calls’) and calls produced in close-range social interactions (‘close-range calls’, table 1). Loud calls may function to attract or defend mating partners [63], defend a territory or food source through maintenance of intergroup spacing [42,64], or re-establish contact with group members that are out of sight (‘separation calls’) [38].
Close-range calls are produced in agonistic, neutral or affiliative contexts. Calls produced in agonistic contexts may function to assess or warn rivals, such as contest calls that advertise fitness [42] or threat calls that maintain a dominance hierarchy [46,65]. Harassed individuals, on the other hand, may produce distress calls, which probably function to appease the aggressor and attract coalition partners [50]. The specific function of some close-range calls made in strictly neutral or affiliative social situations has been more difficult to ascertain and little is known about them besides the contexts in which they are produced [7]. Copulation calls, for example, do not appear to have the same function across different species and may even serve no function in some instances [66,67]. Other close-range calls are ascribed to an affiliative function, often described as ‘contact calls’ (e.g. in raccoons (Procyon lotor) [65]; capuchins (Cebus capucinus) [68]). Contact calls can be produced in various ‘friendly’ contexts, such as during post-conflict reconciliation interactions [69] and prior to friendly behaviours like allogrooming [50,70,71]. These close-range contact calls are also produced in more ‘neutral’ behavioural states like foraging and resting, and therefore could be involved in the maintenance of group cohesion and inter-individual spacing [72].
Primate vocal repertoires are similar to those of other terrestrial mammals (table 1). Although primate repertoires may be slightly larger (on average), there is considerable overlap between primates and other taxa, both in total repertoire size and within each category of calls. Species with large repertoires relative to other species in their order generally produce a large proportion of calls in just one or two categories of calls (e.g. long distance and competitive calls—Callicebus moloch [38]; distress and contact calls—Pan troglodytes [46]; allospecific and contact calls—Suricata suricatta [51,52]). This suggests that specific needs related to one domain (e.g. competition or affiliation) might drive the development of large repertoires, rather than an overall increase in repertoire across all categories. Within primates, no clear taxonomic pattern has emerged with respect to repertoire size. Each family of primates (including great apes) contains species with large and small repertoires. Surprisingly, despite the social complexity of primates, there is no consistent trend for primates to have more social calls than other mammals, which suggests that simple comparisons of numbers of calls are of limited utility.
(c). Function of larger repertoires
One of the primary hypotheses put forward to explain large, complex vocal repertoires is that social complexity creates the need for more vocalizations [21,73–76]. Comparative studies have found a positive relationship between social complexity and communicative complexity, providing support for this hypothesis [2,3,5,20]. In sciurids, the alarm call repertoire size increases with the number of demographic ‘roles’ [2]. Additionally, in primates, an increase in total vocal repertoire size was associated with both larger groups and increases in time spent grooming—a measure of social cohesion [20]. These studies have been important for pinpointing aspects of sociality (i.e. large sociable groups, various demographic roles) that may drive the evolution of large repertoires. However, vocal repertoires of different species may be ‘large’ for different reasons (table 1), and more work is clearly needed to understand the selective pressures underlying expansions in repertoires.
3. Vocal complexity of humans and other primates
Relative to humans, non-human primates (henceforth referred to as ‘primates’) exhibit surprisingly simplistic vocal production [77,78]. (Note that a focus on vocal production ignores the more sophisticated language-like abilities that primates exhibit in terms of vocal perception [79]). According to the ‘source-filter-theory’ first developed to describe human speech [80,81], vocal production entails two components: the ‘source’ of a vocalization (i.e. lungs and the vocal folds) and the means by which a vocalization is shaped, or ‘filtered’, in the vocal cavities (i.e. vocal tract). Speech relies heavily on the control of ‘formants’ or vocal resonances (a product of vocal tract morphology) to produce distinct syllables and hence encode information [81]. Primates also produce formants but the formant structure (i.e. distance between sound frequency ‘peaks and valleys’ [81]) mainly encodes limited information such as individual identity [82,83] and body size [84–86]. Even more elaborate are humans' filtering tools, the descended larynx and tongue [81]. In most primates and other mammals, the tongue remains flat inside the mouth. By contrast, humans have remarkable control over the location and shape of the tongue [81,87], giving humans unmatched plasticity in sound invention [88]. This unique vocal plasticity allows us to imitate complex sounds and invent novel sounds, a feat shared with some birds and cetaceans [89,90] but not with other primates [77,78].
Despite having a limited ability to imitate and create new sounds, there are some features of primate vocal production that show similarities to human language. For instance, some primates exhibit vocal dialects—geographical variation in the acoustic structure of certain vocalizations [91–93]. Calls are recognizably homologous between different populations of the same species, but show acoustic distinctions related to variation in habitat and the duration of isolation, similar to patterns in human linguistic diversity (e.g. [94]). Additionally, primates such as chimpanzees (Pan troglodytes) [95,96], blue monkeys (Cercopithecus mitis) [97] and capuchins (Cebus apella) [98,99] produce or suppress vocalizations depending on the composition of the conspecific audience.
Primate communication also resembles the semantic content of human language. Several primates exhibit potentially ‘referential’ allospecific calls that are elicited by external stimuli (table 1). In some cases, the referential nature of these calls has been supported with playback experiments. For example, each vervet monkey (Chlorocebus pygerythrus) alarm call ‘refers’ to a different type of predator (leopards, eagles and snakes). Experimental playbacks in the wild indicate that these different alarm calls produce different predator-appropriate responses in the absence of a predator [56]. In further support of the functionally referential nature of primate vocalizations, habituation–dishabituation experiments on Diana monkeys (Cercopithecus aethiops) demonstrated that playbacks of leopard alarm calls or leopard growls resulted in predator-appropriate responses. These results suggest that Diana monkey responses are based on the underlying referent (the predator) rather than any differences in the calls' acoustic properties [100].
Despite some language-like properties of primate communication, humans exhibit unrivaled flexibility in mixing and matching different sounds to create new meanings through syntax [77,78,101]. Very few mammalian species use combinations of calls, and even those that do are unlikely to use these combinations to generate new meanings. There are only a few known cases where primates combine calls in ways that change the meaning of the call elements (red-capped mangabeys (Cercocebus torquatus) [102]; Campbell's monkeys (Cercopithecus campbelli) [61]; Diana monkeys (Cercopithecus diana) [57]). Importantly, these semantic combinations of sounds only comprise a few specific elements and are highly constrained [102,103]. Non-human primate vocal ‘productivity’ [104] is therefore far simpler than human communication and may, at best, be labelled as ‘proto-syntax’—a term that refers to rule-governed, rather than random, combinations of discrete sounds that lack the sophistication of human grammar [61].
4. Function of derived vocalizations
Although previous studies have been pivotal for identifying aspects of sociality that drive vocal complexity, we still know relatively little about how large vocal repertoires function in complex societies. One reason for this is that comparisons of repertoire size alone fail to identify the specific calls that may have evolved in association with social complexity. With no knowledge about which calls are derived, we can say nothing about how those calls function. Another reason is that comparisons of group size alone fail to identify the specific features about group life that require an increase in vocal complexity. Thus, several questions remain unanswered: first, what specific aspects of sociality create a need for vocal complexity? Is it the number of relationships, the nature of relationships or something else? Second, can we identify the derived components of the vocal repertoire that relate to the demands of increased sociality? That is, if more social species have more calls, how are they using these ‘extra’ calls?
To help answer these questions, we propose a systematic investigation of closely related species that make detailed comparisons of the functions of ‘homologous’ (shared between species) and ‘derived’ (unique to a species) vocalizations. Note that, although a vocalization may be unique to a species because it was present in the common ancestor and lost in the other species, we call them derived calls for simplicity, although the direction of the change (gain or loss) remains a hypothesis that can be examined by comparison to an outgroup. Previous studies have used comparisons among closely related species to understand vocal evolution [71] although not with the goal of understanding vocal complexity per se. In the primate literature, several researchers have made comparisons of vocal behaviour between related species [36,43,105,106]. These studies often include general similarities and differences of call categories [36], acoustic structure [105] and/or contextual use [43]. In one case [43], there was a clear attempt to identify homologous and unique calls in two species of macaque (bonnet macaque (Macaca radiata) and lion-tailed macaque (M. silenus)) and two species of langurs (Nilgiri langur, Presbytis johnii) and common langur (P. entellus)); however, much of the ensuing analyses focused on the differential use of the homologous calls rather than explaining the function of unique calls. The only study to date to focus on unique calls [44] compared the vocal behaviour of the forest-dwelling mandrill (Mandrillus sphinx) to published accounts of savannah-living baboons (Papio spp.) and geladas (Theropithecus gelada). Kudo reported that mandrills produced two unique long-distance contact calls instead of the various short-range calls made by baboons and geladas. Kudo proposed that this difference was likely due to ecological pressures, as low-amplitude vocalizations do not travel well in a forested environment where visual contact is also limited [44].
Identifying homologous and derived vocalizations is critical for identifying the specific social or ecological factors that may account for complex vocal repertoires. Here, we use a comparison of the vocal complexity in geladas with that in chacma baboons to demonstrate how this homologous-derived vocalization strategy may be implemented. By analysing calls from both species (all obtained from wild populations under natural conditions), we control for variability in how calls are classified which may drive some of the variation in overall repertoire size found in meta-analyses.
5. Geladas and baboons—a case study
Early researchers were struck by the intricate vocal behaviour of geladas as well as their unusually complex social groups [107–109]. Although some have proposed a causal connection between these factors [107], little progress has been made towards understanding why geladas, the only extant Theropithecus species, have elaborate vocal communication compared with other primates. Thus, a comparison between the vocal behaviour of geladas and Papio baboons serves two purposes. First, these two taxa split relatively recently (about 4 Myr ago), and Theropithecus and Papio are probably sister genera [110]. To human observers, they appear to produce similar calls in similar contexts (e.g. affiliative grunts, threat grunts and alarm calls). It is therefore relatively straightforward to identify homologous calls, and simultaneously, to pinpoint the unique call types that result in differences in vocal repertoire size. We can then assess how these unique calls are used to highlight the selective pressures that may have favoured greater vocal complexity.
Second, the differences in the social system and ecology of geladas and baboons make the comparison particularly useful for testing contrasting predictions about the evolution of behavioural differences [111]. Both species live in matrilineal groups in which males disperse [108,112,113]. Geladas aggregate into a multi-level, fission–fusion society (forming groups as large as 1100 individuals) [114,115] and within this group they only recognize and primarily interact with a small subset of the individuals within ‘harem-like’ reproductive units of 2–15 individuals [108,114–117]. In these reproductive units, ‘leader’ males must maintain social relationships with several females, and it is thought that maintaining close social bonds with his unit females may serve to decrease the likelihood that he will be out-competed by a non-unit, ‘bachelor’ male [118,119]. In contrast to their complex social system, gelada diets are simple and specialized, with grass as the primary food item [120–122].
Unlike geladas, many baboons (e.g. chacma baboons (Papio ursinus)) have a single-level, multimale–multifemale society with no discrete reproductive units (20–120 animals, [123–125]). Baboons maintain differentiated relationships based on kinship and dominance with all members of their group, but cross-sex relationships consist mainly of temporary consortships [123–126]. In terms of ecology, baboons are extremely complex; they live in a range of habitat types and consume anything from fruits and seeds to insects and vertebrates [127–130].
Given that geladas and baboons differ in their sociality and ecology, we predict corresponding differences in the call types comprising their vocal repertoires. For instance, geladas—specifically males—may produce more types of calls that are used in affiliative situations. On the other hand, baboons may use proportionally more allospecific calls to communicate about general features of the environment such as food items. To test our predictions, we compared the vocal behaviour of geladas with one representative of the Papio genus—the chacma baboon [44]—to identify derived call types. While we recognize that vocalizations from a single population may obscure variation within the genus, both the literature and our experience with multiple types of Papio baboons suggests that repertoire variation within Papio is minimal [44] and that the types of vocalizations used by chacma baboons are very similar to even the socially-divergent Papio species, P. hamadryas [44,131]. Furthermore, our descriptions of gelada vocalizations closely match those from captive geladas [132] suggesting that such vocalizations are not unique to one population. For any derived vocalizations, we then conducted intra-specific analyses to determine their possible functions.
(a). Study subjects
Data for this study come from 14 units within three different bands in one community of wild geladas (about 1200 individuals) living in the Sankaber area of the Simien Mountains National Park, Ethiopia (2008–2010) [113,114] and a single group of chacma baboons (group C) living in the Moremi Game Reserve in the Okavango Delta of Botswana (2001–2002). The gelada units comprised one leader male, 0–3 follower males, and 1–11 females and their immature offspring. The gelada habitat consisted of high-elevation open grassland and adjacent escarpments (sleeping sites). The chacma baboon group ranged from 82 to 91 individuals, including 9–11 adult males, 29–31 adult females, and their immature offspring. The baboon habitat was patchy scrub forest interspersed with seasonally flooded grasslands.
(b). Comparison of gelada and chacma vocal repertoires
(i). Data analysis
We opportunistically recorded vocalizations from 81 adult geladas (males = 36; Feb 2008–Apr 2010) and 32 adult chacma baboons (males = 11; Apr 2001–May 2002) with a Sennheiser ME66 directional microphone connected to a digital stereo recorder (Marantz PMD 660 Digital Recorder for geladas; Sony VW-D6 Professional Walkman for chacma baboons). The call types and contexts of all vocalizations were described at the time of recording. Our analyses focus on common calls that occurred repeatedly during focal sampling and we do not attempt to describe all vocalizations produced in each species. The inter-observer reliability (between assignments made in the field and assignments that were blind to previous designations and based on isolated calls in the absence of contextual information) of a subset of these calls (five exemplars/call type/species/sex class) was 96 per cent. We used Avisoft (v. 5.1.12, R. Specht, Berlin) to generate spectrograms with a fast Fourier transformation size of 1024 points. Focusing on spectrograms with high signal-to-noise ratio, we categorized call types by ear, visual inspection of the spectrograms and the contexts in which they occurred (chacma females = 50 calls; chacma males = 32 calls; gelada females = 72 calls; gelada males = 92 calls). There were an equal number of calls per individual (within species/sex class) for each call type (n = 1–3 call replicates per individual, 6–12 total calls per call type). We optimized the frequency range of different call types (11 or 22 kHz) where appropriate (time resolution of 2.667–2.903 ms and a 100% frame).
We used Avisoft to quantify eight temporal and spectrum-based acoustic parameters in the spectrograms: duration, mean bandwidth, frequency under which 25 per cent of the call's energy lies (start, maximum and mean), number of harmonic peaks under 20 dB (maximum and mean), maximum peak frequency. Then, to determine the probability of correctly assigning each vocalization to a pre-categorized call type, we performed stepwise discriminant function analyses (DFAs) with a subsequent leave-on-out cross-validation procedure for each of the four species/sex classes separately [133]. We used multivariate analyses of variance (MANOVAs) to verify the significance of the final DFA parameters. Finally, we identified homologous calls between species based on both acoustic and contextual similarity.
(ii). Results
Male and female geladas and chacma baboons produced a range of allospecific and social calls used in both affiliative contexts (e.g. grooming and copulation) and non-affiliative contexts (e.g. challenge displays and dominance interactions), with geladas producing a greater number of call types (table 2). Of the 14 call types we identified, eight were found in both geladas and chacmas and six were unique to geladas. The derived gelada calls occurred primarily in short-range affiliative contexts (table 2). Extant literature and our own observations indicated that most of the homologous call types are produced in a similar morphological way—a vocalized exhale—while geladas produce both inhaled and exhaled versions of calls that are acoustically distinct (we only separate inhaled and exhaled grunts here because they are the most common, but they also produce inhaled ‘moans’ and ‘wobbles’, table 2).
Table 2.
Descriptions of call types used by geladas and chacma baboons in short-range and in long-distance situations, including the way in which they are physically produced and the contexts in which they occur. CF, chacma female; CM, chacma male; GF, gelada female; GM, gelada male. Asterisks denote vocalizations that were not used in discriminant function analyses due to low sample size.
| call type | mode of production | context |
|---|---|---|
| I. Shared vocalizations in chacma baboons and geladas | ||
| affiliative grunt (CF, CM, GF, GM) | exhale | a soft tonal contact call used during approaching, grooming, and infant-handling, as well as while moving and foraging [30,109,132,134,135] |
| copulation call (CF, CM*, GF, GM) | exhale | loud grunts given before and during mating [132] |
| fear bark (CF, CM*, GF) | exhale with retracted lips | a ‘cough-like’ vocalization [136] given by subordinate individuals to high-ranking animals [132] |
| threat grunt (CF, CM, GF, GM) | exhale | a staccato-like vocalization uttered by the dominant individual in an aggressive encounter [132,137,138] |
| alarm call (CF*, CM*, GF) | exhale | noisy, harsh calls used in response to predators and other environmental threats [109,123] |
| display call or ‘wahoo’ (CM, GF*, GM) | inhale and exhale | loud calls typically uttered during competitive displays [132,136]; chacma and gelada males, in particular, make a ‘roar’ that often comes before these wahoo calls |
| lost call (CM*,CF*,GF*,GM*) | long exhale | a noisy vocalization that rises in pitch towards the end of the call and associated with separation from the group or particular individuals |
| scream (CF, GF, GM) | long exhale with retracted lips | a noisy drawn-out defensive call, usually given by subordinates when attacked by a higher-ranking individual [109,132,134] |
| II. Derived gelada vocalizations | ||
| inhaled grunt (GF*, GM) | vocal inhale | vocalized inhales often part of an affiliative grunt calling bout [135]; sometimes, inhaled grunts can have an audibly ‘nasal’ sound, produced by the withdrawn lip obscuring nasal passages [135] |
| moan (GF*, GM) | long exhale (sometimes inhaled) | long drawn-out affiliative grunt, often given by leader males to their unit's females ([109,132,139]; this study) |
| wobble (GF*, GM) | vocal inhale or exhale with lip or tongue-flicking | soft, undulating calls usually given by males to their unit females, often following ‘anxiety-producing’ situations (this study) |
| yawn (GF*, GM) | inhale | a vocalized yawn given in social contexts, often involving grooming sessions and also after mating or in competitive situations ([140]; this study) |
| pre-copulation call (GF) | short exhale | calls given by oestrous females while presenting their genitals to males |
| how barks (GM*) | exhale | high-pitched barks/whinnies given by non-leader males giving chase to other males in competitive displays |
We performed further analyses on 12 vocalization types, of which only seven were found in chacmas (figures 1 and 2). Other call types were excluded from further DFA analyses because they were rarely produced without overlapping vocalizations, and hence, there were too few high-quality recordings to analyse (gelada female: display calls, moans, inhaled grunts, wobbles and yawns; gelada male: how barks, nasal inhaled grunts and alarm calls; chacma female: alarm calls; chacma male: fear grunt, alarm calls and copulation calls). We were able to discriminate between all call types for each age-sex class, using DFAs; based on eight acoustic parameters, we classified call types at a higher rate (range: 67.4–93.8%; leave-one-out classification range: 50–90.6%) than expected by random classification (range: 10–33.3%). A MANOVA test carried out for each of the four species/sex classes showed that pre-categorized call types were significantly different from each other based on variation in at least four of the chosen acoustic parameters (p < 0.003).
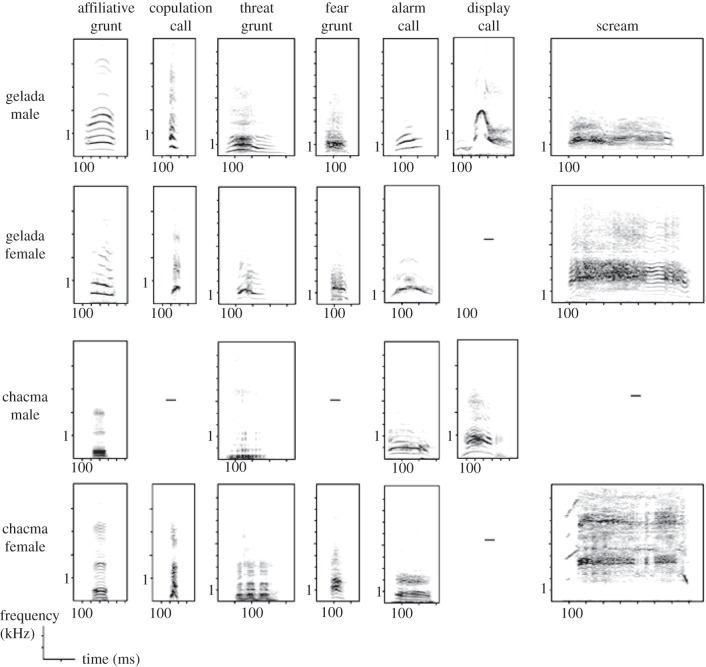
Figure 1.
Spectrograms of homologous calls shared by geladas and chacma baboons. Dashes represent calls that were not produced or produced at a very low rate.
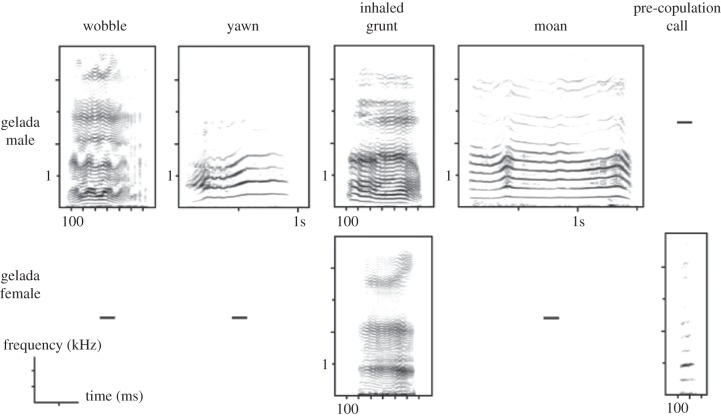
Figure 2.
Spectrograms of derived call types produced only by geladas. Dashes represent calls that were not produced or produced at a very low rate.
In sum, acoustic analysis shows that geladas share a number of vocalization types with chacma baboons. While chacma baboons did not appear to have any unique calls, the analysis allowed identification of at least five derived vocalization types in geladas: inhaled grunts, moans, pre-copulation calls, wobble calls and yawns. We then carried out intraspecific analysis to determine how these calls function in gelada society.
(c). Intraspecific analysis of derived gelada vocalizations
(i). Comparison of derived call use in gelada males and females
To determine the function of the derived gelada vocalizations identified above, we first examined potential sex differences in the use of these calls. By definition, pre-copulation calls were produced only by females in very straightforward contexts (i.e. produced prior to copulation). Thus, we focused here on the use of inhaled grunts, moans, wobbles and yawns. Behavioural data on adult male and female geladas were obtained between January 2009 and December 2010 during repeated 15-min focal follows of 53 females (mean ± s.d.: 6.55 ± 2.59 h per female; 348.50 h in total) and 13 leader males (6.60 ± 1.86 h per male, 85.75 h in total). During these focal follows we noted all vocalizations uttered by the focal animal, as well as all social behaviour (e.g. approaches and grooming interactions) involving the focal individual.
Next, we determined sex differences in the use of derived vocalizations by carrying out a general linear model (GLM) with sex and average reproductive unit size (over the entire study period) as fixed factors. We found that gelada males produced four of the derived calls (i.e. inhaled grunts, moans, wobbles and yawns) at a higher mean rate (14.13 calls per h) than did gelada females (0.39 calls per h) (F1,63 = 708.144, p < 0.001), and reproductive unit size did not come out as a significant covariate (F1,63 = 0.942, p = 0.336). Thus, males appeared to be the sex using derived vocalizations. We next explored whether these unique calls were used in contexts that are unique to males in gelada society.
(ii). Functionality of derived gelada male calls
First, we tested the hypothesis that derived social calls are used by males to maintain social relationships with the unit females by examining vocal behaviour in the context of conflict resolution. Using all adult female focal data, we identified every fight (both as actor and receiver) in which the focal female was involved. These fights (n = 107 events) were characterized by loud screams from the focal female (n = 48 events), or direct, physical attacks from the focal female that included biting and slapping (n = 59 events). We deliberately excluded any inconspicuous agonistic interactions (such as soft threat calls or visual threats) that may have gone unnoticed by other group members. For each fight event we counted all derived vocalizations directed at the focal animal by males in the 2 min preceding the event and the 2 min following the event and compared these values with binomial tests of proportions.
Second, we tested the hypothesis that derived social calls were used by males in association with the presence of non-unit, ‘bachelor’ males that pose a threat to the leader males (all leader males are eventually ousted by bachelor males). We used all adult leader male focal data for which the location of bachelors was stable throughout the entire 15-min focal sample. In other words, bachelor groups were either close to the focal male (within 20 m: n = 16 focals), far (more than 20 m; n = 24 focals), or out of sight (n = 26 focals). We carried out two GLMs with male identity as a random factor, bachelor distance as a fixed factor, and the rate of derived calls as the dependent variable (first model: close versus far; second model: close versus out of sight).
We found evidence that males used non-homologous derived calls to maintain cross-sex social relationships with females in his units. Specifically, we found that males directed derived non-homologous calls at females after fights happened (14 occurrences), and they never used them before a fight (binomial test of proportions: 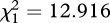 , p < 0.001). On the other hand, we did not find any evidence that males used the derived calls in response to the presence of bachelors. Leader males did not produce derived calls at a high rate when bachelor groups were close (3.23 calls per h) compared with when they were far away (2.04 calls per h) (F1,8 = 0.394, p = 0.548). Similarly, leader males did not produce derived calls at a higher rate when bachelor groups were close compared to when they were out of sight (2.51 calls per h) (F1,11 = 0.078, p = 0.785).
, p < 0.001). On the other hand, we did not find any evidence that males used the derived calls in response to the presence of bachelors. Leader males did not produce derived calls at a high rate when bachelor groups were close (3.23 calls per h) compared with when they were far away (2.04 calls per h) (F1,8 = 0.394, p = 0.548). Similarly, leader males did not produce derived calls at a higher rate when bachelor groups were close compared to when they were out of sight (2.51 calls per h) (F1,11 = 0.078, p = 0.785).
(d). General discussion
Geladas have an elaborate, almost ‘choral’ vocal repertoire [109] and live in a complex society with social groups of varying sizes, making geladas an important model system for addressing hypotheses about vocal evolution. Identifying homologous vocalizations shared with Papio baboons allowed us to study the function of derived gelada vocalizations. It did not appear that interacting with many individuals [20,141] was necessarily an important factor in the use of derived calls, as their production was not correlated with the size of the reproductive unit. Rather, the need to maintain long-term bonds within the unit seemed most important; leader males used these derived vocalizations after fights broke out within their units. Thus, the gelada-specific vocalizations may have evolved as an adaptation to simultaneously maintaining relationships both with and among multiple females—leader males that are better able to ‘keep the peace’ of their reproductive units may, in turn, have higher reproductive success [118]. It remains to be determined why the cross-sex bonds seen in geladas seem more tightly linked to vocal complexity than the within-sex bonds found in both species.
Our results suggest that future studies should examine whether hamadryas baboons (Papio anubis), a Papio species that also has a ‘harem-like’ structure [142], have any evidence of greater elaboration of affiliative call use by males. This comparison is particularly important for uncovering how vocalizations relate to specific aspects of long-term bonds because hamadryas males form long-term bonds with females but the relationship is more coercive than in geladas and there does not appear to be a need to ‘keep the peace’. In geladas, investigations of how females respond to derived vocalizations and the subsequent benefits to leader male fitness is an exciting direction for future research. It may be the case, for instance, that these derived vocalizations reduce female anxiety, similarly to the proposed anxiolytic effects of grooming [143–145].
One puzzling aspect of our findings is that the derived calls used by males are all used in similar contexts. Further work is needed to tease apart any potential differences between the derived gelada calls but this redundancy suggests an additional hypothesis. Perhaps the extremely large groups of geladas (herds can number up to 1000) and high rates of vocalizations (mean ± s.d.: chacmas: 8.84 ± 4.49 calls per h, geladas: 16.95 ± 8.51 calls per h) create ‘vocal clutter’ that the geladas have overcome by diversifying their most common call types—affiliative vocalizations. Thus the need to maintain bonds within a noisy backdrop of conspecific vocalizations may favour greater vocal complexity, possibly explaining some of the group size effects seen in other studies [20].
6. Conclusions
Comparisons of repertoire size and components suggest that primates are broadly similar to other mammals, despite primates having greater social complexity. However, our comparison of baboons and geladas highlights the utility of making detailed comparisons among closely related species to understand vocal evolution. We were able to examine the function of recently evolved calls in detail and examine the specific social implications of increased repertoires by focusing on specific call types, addressing sexual differences, and using behavioural measures to describe social complexity. We found that the larger vocal repertoire of geladas is linked to the maintenance of cross-sex bonds within the reproductive unit. Broadly focused theoretical and comparative analyses [2,3,5,20] are vital to drive the investigation of communicative complexity. We argue that there is also a need for more focused analyses among carefully chosen taxa using directly comparable measures in the study of vocal complexity.
Acknowledgements
We are grateful to the Ethiopian Wildlife Conservation Department, the Amhara National Regional State Parks Development and Protection Authority, and the wardens and staff of the Simien Mountains National Park for granting us the permission to conduct this research in Ethiopia. We thank the Office of the President and the Department of Wildlife and National Parks of the Republic of Botswana for permission to conduct research in the Moremi Reserve. We would like to thank Esheti Jejaw, David Pappano, Eila Roberts, Noah Snyder-Mackler and Vanessa Wilson for their assistance with collecting behavioural data in the field. Tiffany Fritzler and Chelsea Miller helped us with the acoustic analysis and Ken Guire provided expert statistical advice. Research in Botswana was supported by NIH grant MH62249, an NRSA fellowship, the Leakey Foundation and the University of Pennsylvania. Research in Ethiopia was supported by the Wildlife Conservation Society (SSF grant no. 67250, the National Geographic Society (grant no. 8100–06), the Leakey Foundation, the National Science Foundation (grant no. BCS-0715179), and the University of Michigan. We thank Dr Robin Dunbar, Dr Jacinta Beehner and two anonymous reviewers for helpful comments on a previous version of this manuscript.
References
- 1.Baker C. 1988. Vocalization of captive water mongooses, Atilax paludinosus. Z. Saugetierkd 53, 83–91 [Google Scholar]
- 2.Blumstein D. T., Armitage K. B. 1997. Does sociality drive the evolution of communicative complexity? A comparative test with ground-dwelling sciurid alarm calls. Am. Nat. 150, 179–200 10.1086/286062 (doi:10.1086/286062) [DOI] [PubMed] [Google Scholar]
- 3.Freeberg T. M. 2006. Social complexity can drive vocal complexity: group size influences vocal information in Carolina chickadees. Psychol. Sci. 17, 557–561 10.1111/j.1467-9280.2006.01743.x (doi:10.1111/j.1467-9280.2006.01743.x) [DOI] [PubMed] [Google Scholar]
- 4.Furrer R. D., Manser M. B. 2009. The evolution of urgency-based and functionally referential alarm calls in ground-dwelling species. Am. Nat. 173, 400–410 10.1086/596541 (doi:10.1086/596541) [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson G. S. 2003. Social and vocal complexity in bats. In Animal social complexity: intelligence, culture, and individualized societies (eds de Waal F. B. M., Tyack P. L.), pp. 322–341 Cambridge, MA: Harvard University Press [Google Scholar]
- 6.Pollard K. A., Blumstein D. T. 2012. Evolving communicative complexity: insights from rodents and beyond. Phil. Trans. R. Soc. B 367, 1869–1878 10.1098/rstb.2011.0221 (doi:10.1098/rstb.2011.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedurek P., Slocombe K. E. 2011. Primate vocal communication: a useful tool for understanding human speech and language evolution? Hum. Biol. 83, 153–173 10.3378/027.083.0202 (doi:10.3378/027.083.0202) [DOI] [PubMed] [Google Scholar]
- 8.Pinker S., Bloom P. 1990. Natural-language and natural selection. Behav. Brain Sci. 13, 707–726 10.1017/S0140525X00081061 (doi:10.1017/S0140525X00081061) [DOI] [Google Scholar]
- 9.Byrne R. W., Whiten A. 1988. Machiavellian intelligence. Oxford, UK: Oxford University Press [Google Scholar]
- 10.Dunbar R. I. M. 1998. The social brain hypothesis. Evol. Anthropol. 6, 178–190 10.1002/(SICI)1520-6505(1998)6:5%3C178::AID-EVAN5%3E3.0.CO;2-8 (doi:10.1002/(SICI)1520-6505(1998)6:5<178::AID-EVAN5>3.0.CO;2-8) [DOI] [Google Scholar]
- 11.Whiten A., Byrne R. W. 1988. Tactical deception in primates. Behav. Brain Sci. 11, 233–273 10.1017/S0140525X00049682 (doi:10.1017/S0140525X00049682) [DOI] [Google Scholar]
- 12.Harcourt A., Stewart K. J. 1989. Functions of alliances in contests within wild gorilla groups. Behaviour 109, 176–190 10.1163/156853989X00213 (doi:10.1163/156853989X00213) [DOI] [Google Scholar]
- 13.Dunbar R. I. M., Shultz S. 2010. Bondedness and sociality. Behaviour 147, 775–803 10.1163/000579510X501151 (doi:10.1163/000579510X501151) [DOI] [Google Scholar]
- 14.Silk J. B. 2002. Using the ‘F’-word in primatology. Behaviour 139, 421–446 10.1163/156853902760102735 (doi:10.1163/156853902760102735) [DOI] [Google Scholar]
- 15.Smuts B. B. 1985. Sex and friendship in baboons. New York, NY: Aldine Publishing [Google Scholar]
- 16.Cheney D. L., Seyfarth R. M. 2007. Baboon metaphysics: the evolution of a social mind. Chicago, IL: University of Chicago Press [Google Scholar]
- 17.Dunbar R. I. M. 2009. The social brain hypothesis and its implications for social evolution. Ann. Hum. Biol. 36, 562–572 10.1080/03014460902960289 (doi:10.1080/03014460902960289) [DOI] [PubMed] [Google Scholar]
- 18.Silk J. B., Alberts S. C., Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231–1234 10.1126/science.1088580 (doi:10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 19.Silk J. B., Beehner J. C., Bergman T. J., Crockford C., Engh A. L., Moscovice L. R., Wittig R. M., Seyfarth R. M., Cheney D. L. 2010. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 20, 1359–1361 10.1016/j.cub.2010.05.067 (doi:10.1016/j.cub.2010.05.067) [DOI] [PubMed] [Google Scholar]
- 20.McComb K., Semple S. 2005. Coevolution of vocal communication and sociality in primates. Biol. Lett. 1, 381–385 10.1098/rsbl.2005.0366 (doi:10.1098/rsbl.2005.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauser M. D. 1996. The evolution of communication. Cambridge, MA: MIT Press [Google Scholar]
- 22.Mulligan B. E., Nellis D. W. 1975. Vocal repertoire of the mongoose Herpestes auropunctatus. Behaviour 55, 237–257 10.1163/156853975X00489 (doi:10.1163/156853975X00489) [DOI] [Google Scholar]
- 23.Watts C. H. S. 1975. Vocalizations of Australian hopping mice (Rodentia: Nutumys). J. Zool. Lond. 177, 247–263 10.1111/j.1469-7998.1975.tb05982.x (doi:10.1111/j.1469-7998.1975.tb05982.x) [DOI] [Google Scholar]
- 24.le Roux A., Cherry M. I., Manser M. B. 2009. The vocal repertoire in a solitary foraging carnivore, Cynictis penicillata, may reflect facultative sociality. Naturwissenschaften 96, 575–584 10.1007/s00114-008-0506-5 (doi:10.1007/s00114-008-0506-5) [DOI] [PubMed] [Google Scholar]
- 25.Wong J., Stewart P., Macdonald D. 1999. Vocal repertoire in the European badger (Meles meles): structure, context, and function. J. Mammal. 80, 570–588 10.2307/1383302 (doi:10.2307/1383302) [DOI] [Google Scholar]
- 26.Pollard K. A., Blumstein D. T. 2011. Social group size predicts the evolution of individuality. Curr. Biol. 21, 413–417 10.1016/j.cub.2011.01.051 (doi:10.1016/j.cub.2011.01.051) [DOI] [PubMed] [Google Scholar]
- 27.Brady C. A. 1981. The vocal repertoires of the bush dog (Speothos venaticus), crab-eating fox (Cerdocyon thous), and maned wolf (Chrysocyon brachyurus). Anim. Behav. 29, 649–669 10.1016/S0003-3472(81)80001-2 (doi:10.1016/S0003-3472(81)80001-2) [DOI] [Google Scholar]
- 28.Green S. 1975. Communication by a graded vocal system in Japanese monkeys. In Primate behavior, pp. 1–102 New York, NY: Academic Press [Google Scholar]
- 29.Leger D. W., Owings D. H., Gelfand D. L. 1980. Single-note vocalizations of California ground squirrels: graded signals and situation-specificity of predator and socially evoked calls. Z. Tierpsychol. 52, 227–246 10.1111/j.1439-0310.1980.tb00714.x (doi:10.1111/j.1439-0310.1980.tb00714.x) [DOI] [Google Scholar]
- 30.Meise K., Keller C., Cowlishaw G., Fischer J. 2011. Sources of acoustic variation: implications for production specificity and call categorization in chacma baboon (Papio ursinus) grunts. J. Acoust. Soc. Am. 129, 1631–1641 10.1121/1.3531944 (doi:10.1121/1.3531944) [DOI] [PubMed] [Google Scholar]
- 31.Peters G., Sliwa A. 1997. Acoustic communication in the aardwolf, Proteles cristatus (Carnivora: Hyaenidae). Z. Säugetierkd 62, 219–238 [Google Scholar]
- 32.Fischer J., Metz M., Seyfarth R. M., Cheney D. L. 2001. Baboon responses to graded bark variants. Anim. Behav. 61, 925–931 10.1006/anbe.2000.1687 (doi:10.1006/anbe.2000.1687) [DOI] [Google Scholar]
- 33.Harris M. A., Murie J. O., Duncan J. A. 1983. Responses of Columbian ground squirrels to playback of recorded calls. Z. Tierpsychol. 63, 318–330 10.1111/j.1439-0310.1983.tb00747.x (doi:10.1111/j.1439-0310.1983.tb00747.x) [DOI] [Google Scholar]
- 34.le Roux A., Jackson T. P., Cherry M. I. 2001. Does Brants' whistling rat (Parotomys brantsii) use an urgency-based alarm system in reaction to aerial and terrestrial predators? Behaviour 138, 757–773 10.1163/156853901752233398 (doi:10.1163/156853901752233398) [DOI] [Google Scholar]
- 35.Baldwin J. D., Baldwin J. I. 1976. Vocalizations of howler monkeys (Alouatta palliata) in southwestern Panama. Folia Primatol. 26, 81–108 10.1159/000155733 (doi:10.1159/000155733) [DOI] [PubMed] [Google Scholar]
- 36.Charles-Dominique P. 1977. Ecology and behaviour of nocturnal primates: prosimians of equatorial West Africa. New York, NY: Columbia University Press [Google Scholar]
- 37.Robinson J. G. 1979. An analysis of the organization of vocal communication in the titi monkey Callicebus moloch. Z. Tierpsychol. 49, 381–405 10.1111/j.1439-0310.1979.tb00300.x (doi:10.1111/j.1439-0310.1979.tb00300.x) [DOI] [PubMed] [Google Scholar]
- 38.Masataka N. 1982. A field study on the vocalizations of Goeldi's monkeys (Callimico goeldii). Primates 23, 206–219 10.1007/BF02381161 (doi:10.1007/BF02381161) [DOI] [Google Scholar]
- 39.Robinson J. G. 1984. Syntactic structures in the vocalizations of wedge-capped capuchin monkeys, Cebus olivaceus. Behaviour 90, 46–79 10.1163/156853984X00551 (doi:10.1163/156853984X00551) [DOI] [Google Scholar]
- 40.Range F., Fischer J. 2004. Vocal repertoire of sooty mangabeys (Cercocebus torquatus atys) in the Taï National Park. Ethology 110, 301–321 10.1111/j.1439-0310.2004.00973.x (doi:10.1111/j.1439-0310.2004.00973.x) [DOI] [Google Scholar]
- 41.Strushaker T. T. 1967. Auditory communication among vervet monkeys (Cercopithecus aethiops). In Social communication among primates (ed. Altmann S. A.) Chicago, IL: University of Chicago Press [Google Scholar]
- 42.Palombit R. A. 1992. A preliminary study of vocal communication in wild long-tailed macaques (Macaca fascicularis). I. Vocal repertoire and call emission. Int. J. Primatol. 13, 143–182 10.1007/BF02547839 (doi:10.1007/BF02547839) [DOI] [Google Scholar]
- 43.Hohmann G. 1991. Comparative analyses of age-specific and sex-specific patterns of vocal behavior in 4 species of Old-World monkeys. Folia Primatol. 56, 133–156 10.1159/000156538 (doi:10.1159/000156538) [DOI] [PubMed] [Google Scholar]
- 44.Kudo H. 1987. The study of vocal communication of wild mandrills in Cameroon in relation to their social structure. Primates 28, 289–308 10.1007/BF02381013 (doi:10.1007/BF02381013) [DOI] [Google Scholar]
- 45.Bermejo M., Omedes A. 1999. Preliminary vocal repertoire and vocal communication of wild bonobos (Pan paniscus) at Lilungu (Democratic Republic of Congo). Folia Primatol. 70, 328–357 10.1159/000021717 (doi:10.1159/000021717) [DOI] [PubMed] [Google Scholar]
- 46.Goodall J. 1986. The chimpanzees of Gombe. Cambridge, MA: Belknapp [Google Scholar]
- 47.MacKinnon J. 1974. The behaviour and ecology of wild orangutans (Pongo pygmaeus). Anim. Behav. 22, 3–74 10.1016/S0003-3472(74)80054-0 (doi:10.1016/S0003-3472(74)80054-0) [DOI] [Google Scholar]
- 48.Strushaker T. T. 1975. The red colobus monkey. Chicago, IL: University of Chicago Press [Google Scholar]
- 49.Long C. V. 2007. Vocalisations of the degu Octodon degus, a social caviomorph rodent. Bioacoustics 16, 223–244 [Google Scholar]
- 50.Robbins R. L. 2000. Vocal communication in free-ranging African wild dogs (Lycaon pictus). Behaviour 137, 1271–1298 10.1163/156853900501926 (doi:10.1163/156853900501926) [DOI] [Google Scholar]
- 51.Manser M. B. 2001. The acoustic structure of suricates' alarm calls varies with predator type and the level of response urgency. Proc. R. Soc. Lond. B 268, 2315–2324 10.1098/rspb.2001.1773 (doi:10.1098/rspb.2001.1773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manser M. B. 1998. The evolution of auditory communication in suricates, Suricata suricatta. PhD thesis, University of Cambridge, UK [Google Scholar]
- 53.Ackers S. H., Slobodchikoff C. N. 1999. Communication of stimulus size and shape in alarm calls of Gunnison's prairie dogs, Cynomys gunnisoni. Ethology 105, 149–162 10.1046/j.1439-0310.1999.00381.x (doi:10.1046/j.1439-0310.1999.00381.x) [DOI] [Google Scholar]
- 54.Greene G., Meagher T. 1998. Red squirrels, Tamiasciurus hudsonicus, produce predator-class specific alarm calls. Anim. Behav. 55, 511–518 10.1006/anbe.1997.0620 (doi:10.1006/anbe.1997.0620) [DOI] [PubMed] [Google Scholar]
- 55.Crockford C., Boesch C. 2003. Context-specific calls in wild chimpanzees, Pan troglodytes verus: analysis of barks. Anim. Behav. 66, 115–125 10.1006/anbe.2003.2166 (doi:10.1006/anbe.2003.2166) [DOI] [Google Scholar]
- 56.Seyfarth R. M., Cheney D. L., Marler P. 1980. Vervet monkey alarm calls: semantic communication in a free-ranging primate. Anim. Behav. 28, 1070–1094 10.1016/S0003-3472(80)80097-2 (doi:10.1016/S0003-3472(80)80097-2) [DOI] [Google Scholar]
- 57.Stephan C., Zuberbühler K. 2008. Predation increases acoustic complexity in primate alarm calls. Biol. Lett. 4, 641–644 10.1098/rsbl.2008.0488 (doi:10.1098/rsbl.2008.0488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macedonia J. M., Evans C. S. 1993. Variation among mammalian alarm call systems and the problem of meaning in animal signals. Ethology 93, 177–197 10.1111/j.1439-0310.1993.tb00988.x (doi:10.1111/j.1439-0310.1993.tb00988.x) [DOI] [Google Scholar]
- 59.Hauser M. D., Teixidor P., Field L., Flaherty R. 1993. Food-elicited calls in chimpanzees–effects of food quantity and divisability. Anim. Behav. 45, 817–819 10.1006/anbe.1993.1096 (doi:10.1006/anbe.1993.1096) [DOI] [Google Scholar]
- 60.Roush R. S., Snowdon C. T. 2000. Quality, quantity, distribution and audience effects on food calling in cotton-top tamarins. Ethology 106, 673–690 10.1046/j.1439-0310.2000.00581.x (doi:10.1046/j.1439-0310.2000.00581.x) [DOI] [Google Scholar]
- 61.Ouattara K., Lemasson A., Zuberbühler K. 2009. Campbell's monkeys concatenate vocalizations into context-specific call sequences. Proc. Natl Acad. Sci. USA 106, 22 026–22 031 10.1073/pnas.0908118106 (doi:10.1073/pnas.0908118106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnold K., Zuberbühler K. 2006. The alarm-calling system of adult male putty-nosed monkeys, Cercopithecus nictitans martini. Anim. Behav. 72, 643–653 10.1016/j.anbehav.2005.11.017 (doi:10.1016/j.anbehav.2005.11.017) [DOI] [Google Scholar]
- 63.Cap H., Deleporte P., Joachim J., Reby D. 2008. Male vocal behavior and phylogeny in deer. Cladistics 24, 917–931 10.1111/j.1096-0031.2008.00223.x (doi:10.1111/j.1096-0031.2008.00223.x) [DOI] [PubMed] [Google Scholar]
- 64.Knornschild M., Glockner V., von Helversen O. 2010. The vocal repertoire of two sympatric species of nectar-feeding bats (Glossophaga soricina and G. commissarisi). Acta Chiropterol. 12, 205–215 10.3161/150811010X504707 (doi:10.3161/150811010X504707) [DOI] [Google Scholar]
- 65.Sieber O. J. 1984. Vocal communication in raccoons (Procyon lotor). Behaviour 90, 80–113 10.1163/156853984X00560 (doi:10.1163/156853984X00560) [DOI] [Google Scholar]
- 66.Hamilton W. J., III, Arrowood P. C. 1978. Copulatory vocalizations of chacma baboons (Papio ursinus), gibbons (Hylobates hoolock), and humans. Science 200, 1405–1409 10.1126/science.663622 (doi:10.1126/science.663622) [DOI] [PubMed] [Google Scholar]
- 67.Henzi P. S. 1996. Copulation calls and paternity in chacma baboons. Anim. Behav. 51, 233–234 10.1006/anbe.1996.0021 (doi:10.1006/anbe.1996.0021) [DOI] [Google Scholar]
- 68.Gros-Louis J. 2002. Contexts and behavioral correlates of trill vocalizations in wild white-faced capuchin monkeys (Cebus capucinus). Am. J. Primatol. 57, 189–202 10.1002/ajp.10042 (doi:10.1002/ajp.10042) [DOI] [PubMed] [Google Scholar]
- 69.Silk J. B., Cheney D. L., Seyfarth R. M. 1996. The form and function of post-conflict interactions between female baboons. Anim. Behav. 52, 259–268 10.1006/anbe.1996.0171 (doi:10.1006/anbe.1996.0171) [DOI] [Google Scholar]
- 70.Palombit R. A., Cheney D. L., Seyfarth R. M. 1999. Male grunts as mediators of social interaction with females in wild chacma baboons (Papio cynocephalus ursinus). Behaviour 136, 221–242 10.1163/156853999501298 (doi:10.1163/156853999501298) [DOI] [Google Scholar]
- 71.Peters G., Tonkin-Leyhausen B. A. 1999. Evolution of acoustic communication signals of mammals: friendly close-range vocalizations in Felidae (Carnivora). J. Mamm. Evol. 6, 129–159 10.1023/A:1020620121416 (doi:10.1023/A:1020620121416) [DOI] [Google Scholar]
- 72.Koda H., Shimooka Y., Sugiura H. 2008. Effects of caller activity and habitat visibility on contact call rate of wild Japanese macaques (Macaca fuscata). Am. J. Primatol. 70, 1055–1063 10.1002/ajp.20597 (doi:10.1002/ajp.20597) [DOI] [PubMed] [Google Scholar]
- 73.Marler P. 1977. The evolution of communication. In How animals communicate (ed. Sebeok T. A.), pp. 45–70 Bloomington, IN: Indiana University Press [Google Scholar]
- 74.Marler P., Mitani J. 1988. Vocal communication in primates and birds: parallels and contrasts. In Primate vocal communication (eds Todt D., Goedeking P., Symmes D.), pp. 3–14 Berlin, Germany: Springer [Google Scholar]
- 75.Philips M., Austad S. N. 1990. Animal communication and social evolution. In Interpretation and explanation in the study of animal behavior, Vol. 1. Interpretation, intentionality and communication (eds Bekoff M., Jamieson D.), pp. 254–268 Boulder, CO: Westview [Google Scholar]
- 76.Waser P. M. 1982. The evolution of male loud calls among mangabeys and baboons. In Primate communication (eds Snowdon C. T., Brown C. H., Petersen M. R.), pp. 117–143 Cambridge, UK: Cambridge University Press [Google Scholar]
- 77.Janik V. M., Slater P. J. B. 1997. Vocal learning in mammals. Adv. Stud. Behav. 26, 59–99 10.1016/S0065-3454(08)60377-0 (doi:10.1016/S0065-3454(08)60377-0) [DOI] [Google Scholar]
- 78.Zuberbühler K. 2003. Referential signaling in non-human primates: cognitive precursors and limitations for the evolution of language. Adv. Stud. Behav. 33, 265–307 10.1016/S0065-3454(03)33006-2 (doi:10.1016/S0065-3454(03)33006-2) [DOI] [Google Scholar]
- 79.Seyfarth R. M., Cheney D. L. 2010. Production, usage, and comprehension in animal vocalizations. Brain Lang. 115, 92–100 10.1016/j.bandl.2009.10.003 (doi:10.1016/j.bandl.2009.10.003) [DOI] [PubMed] [Google Scholar]
- 80.Fant G. 1960. Acoustic theory of speech production. With calculations based on X-ray studies of Russian articulations. 's-Gravenhage, The Netherlands: Mouton [Google Scholar]
- 81.Fitch W. T. 2000. The evolution of speech: a comparative review. Trends Cogn. Sci. 4, 258–267 10.1016/S1364-6613(00)01494-7 (doi:10.1016/S1364-6613(00)01494-7) [DOI] [PubMed] [Google Scholar]
- 82.Hauser M. D. 1992. Articulatory and social factors influence the acoustic structure of rhesus monkey vocalizations: a learned mode of production? J. Acoust. Soc. Am. 91, 2175–2179 10.1121/1.403676 (doi:10.1121/1.403676) [DOI] [PubMed] [Google Scholar]
- 83.Rendall D., Rodman P. S., Emond R. E. 1996. Vocal recognition of individuals and kin in free-ranging rhesus monkeys. Anim. Behav. 51, 1007–1015 10.1006/anbe.1996.0103 (doi:10.1006/anbe.1996.0103) [DOI] [Google Scholar]
- 84.Fitch W. T. 1997. Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J. Acoust. Soc. Am. 102, 1213–1222 10.1121/1.421048 (doi:10.1121/1.421048) [DOI] [PubMed] [Google Scholar]
- 85.Fitch W. T., Giedd J. 1999. Morphology and development of the human vocal tract: a study using magnetic resonance imaging. J. Acoust. Soc. Am. 106, 1511–1522 10.1121/1.427148 (doi:10.1121/1.427148) [DOI] [PubMed] [Google Scholar]
- 86.Fitch W. T., Kelley J. P. 2000. Perception of vocal tract resonances by whooping cranes Grus americana. Ethology 106, 559–574 10.1046/j.1439-0310.2000.00572.x (doi:10.1046/j.1439-0310.2000.00572.x) [DOI] [Google Scholar]
- 87.Lieberman P. H., Klatt D. H., Wilson W. H. 1969. Vocal tract limitations on the vowel repertoires of rhesus monkey and other nonhuman primates. Science 164, 1185–1187 10.1126/science.164.3884.1185 (doi:10.1126/science.164.3884.1185) [DOI] [PubMed] [Google Scholar]
- 88.Snowdon C. T. 2009. Plasticity of communication in nonhuman primates. Adv. Stud. Behav. 40, 239–276 10.1016/S0065-3454(09)40007-X (doi:10.1016/S0065-3454(09)40007-X) [DOI] [Google Scholar]
- 89.Pepperberg I. M. 1981. Functional vocalizations by an African grey parrot (Psittacus erithacus). Z. Tierpsychol. 55, 139–160 10.1111/j.1439-0310.1981.tb01265.x (doi:10.1111/j.1439-0310.1981.tb01265.x) [DOI] [Google Scholar]
- 90.Ralls K., Fiorelli P., Gish S. 1985. Vocalizations and vocal mimicry in captive harbor seals, Phoca vitulina. Can. J. Zool. 63, 1050–1056 10.1139/z85-157 (doi:10.1139/z85-157) [DOI] [Google Scholar]
- 91.Konrad R., Geissmann T. 2006. Vocal diversity and taxonomy of Nomascus in Cambodia. Int. J. Primatol. 27, 713–745 10.1007/s10764-006-9042-3 (doi:10.1007/s10764-006-9042-3) [DOI] [Google Scholar]
- 92.Méndez-Cárdenas M., Randrianambinina B., Rabesandratana A., Rasoloharijaona S., Zimmermann E. 2008. Geographic variation in loud calls of sportive lemurs (Lepilemur ssp.) and their implications for conservation. Am. J. Primatol. 70, 828–838 10.1002/ajp.20554 (doi:10.1002/ajp.20554) [DOI] [PubMed] [Google Scholar]
- 93.Mitani J. C., Hunley K. L., Murdoch M. E. 1999. Geographic variation in the calls of wild chimpanzees: a reassessment. Am. J. Primatol. 47, 133–151 (doi:10.1002/(SICI)1098-2345(1999)47:2<133::AID-AJP4>3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- 94.Moore J. L., Manne L., Brooks T., Burgess N. D., Davies R., Rahbek C., Williams P., Balmford A. 2002. The distribution of cultural and biological diversity in Africa. Proc. R. Soc. Lond. B 269, 1645–1653 10.1098/rspb.2002.2075 (doi:10.1098/rspb.2002.2075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Slocombe K. E., Zuberbühler K. 2007. Chimpanzees modify recruitment screams as a function of audience composition. Proc. Natl Acad. Sci. USA 104, 17 228–17 233 10.1073/pnas.0706741104 (doi:10.1073/pnas.0706741104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Slocombe K. E., Kaller T., Turman L., Townsend S. W., Papworth S., Squibbs P., Zuberbühler K. 2010. Production of food-associated calls in wild male chimpanzees is dependent on the composition of the audience. Behav. Ecol. Sociobiol. 64, 1959–1966 10.1007/s00265-010-1006-0 (doi:10.1007/s00265-010-1006-0) [DOI] [Google Scholar]
- 97.Papworth S., Böse A., Barker J., Schel A. M., Zuberbühler K. 2008. Male blue monkeys alarm call in response to danger experienced by others. Biol. Lett. 4, 472–475 10.1098/rsbl.2008.0299 (doi:10.1098/rsbl.2008.0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Di Bitetti M. S. 2005. Food-associated calls and audience effects in tufted capuchin monkeys, Cebus apella nigritus. Anim. Behav. 69, 911–919 10.1016/j.anbehav.2004.05.021 (doi:10.1016/j.anbehav.2004.05.021) [DOI] [Google Scholar]
- 99.Pollick A. S., Gouzoules H., de Waal F. B. M. 2005. Audience effects on food calls in captive brown capuchin monkeys, Cebus apella. Anim. Behav. 70, 1273–1281 10.1016/j.anbehav.2005.03.007 (doi:10.1016/j.anbehav.2005.03.007) [DOI] [Google Scholar]
- 100.Zuberbühler K., Cheney D. L., Seyfarth R. M. 1999. Conceptual semantics in a nonhuman primate. J. Comp. Psychol. 113, 33–42 10.1037/0735-7036.113.1.33 (doi:10.1037/0735-7036.113.1.33) [DOI] [Google Scholar]
- 101.Doupe A. J., Kuhl P. K. 1999. Birdsong and human speech: common themes and mechanisms. Annu. Rev. Neurosci. 22, 567–631 10.1146/annurev.neuro.22.1.567 (doi:10.1146/annurev.neuro.22.1.567) [DOI] [PubMed] [Google Scholar]
- 102.Bouchet H., Pellier A., Blois-Heulin C., Lemasson A. 2010. Sex differences in the vocal repertoire of adult red-capped mangabeys (Cercocebus torquatus): a multi-level acoustic analysis. Am. J. Primatol. 72, 360–375 10.1002/ajp.20791 (doi:10.1002/ajp.20791) [DOI] [PubMed] [Google Scholar]
- 103.Bohn K. M., Schmidt-French B., Schwartz C., Smotherman M., Pollak G. D. 2009. Versatility and stereotypy of free-tailed bat songs. PLoS ONE 4, 6746. 10.1371/journal.pone.0006746 (doi:10.1371/journal.pone.0006746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hockett C. F. 1960. The origin of speech. Sci. Am. 203, 88–96 10.1038/scientificamerican0960-88 (doi:10.1038/scientificamerican0960-88) [DOI] [PubMed] [Google Scholar]
- 105.Cleveland J., Snowdon C. T. 1982. The complex vocal repertoire of the adult cotton-top tamarin (Saguinus oedipus oedipus). Z. Tierpsychol. 58, 231–270 10.1111/j.1439-0310.1982.tb00320.x (doi:10.1111/j.1439-0310.1982.tb00320.x) [DOI] [Google Scholar]
- 106.MacLanahan E. B., Green K. M. 1977. The vocal repertoire and an analysis of the contexts of vocalizations in Leontopithecus rosalia. In The biology and conservation of the Callitrichidae (ed. Kleiman D. G.), pp. 251–269 Washington, DC: Smithsonian Institute [Google Scholar]
- 107.Aiello L. C., Dunbar R. I. M. 1993. Neocortex size, group size, and the evolution of language. Curr. Anthropol. 34, 184–193 10.1086/204160 (doi:10.1086/204160) [DOI] [Google Scholar]
- 108.Dunbar R. I. M., Dunbar P. 1975. Social dynamics of gelada baboons. Basel, Switzerland: Karger; [PubMed] [Google Scholar]
- 109.Richman B. 1987. Rhythm and melody in gelada vocal exchanges. Primates 28, 199–223 10.1007/BF02382570 (doi:10.1007/BF02382570) [DOI] [Google Scholar]
- 110.Page S. L., Chiu C., Goodman M. 1999. Molecular phylogeny of Old World monkeys (Cercopithecidae) as inferred from γ-globin DNA sequences. Mol. Phylogenet. Evol. 13, 348–359 10.1006/mpev.1999.0653 (doi:10.1006/mpev.1999.0653) [DOI] [PubMed] [Google Scholar]
- 111.Bergman T. J., Kitchen D. M. 2009. Comparing responses to novel objects in wild baboons (Papio ursinus) and geladas (Theropithecus gelada). Anim. Cogn. 12, 63–73 10.1007/s10071-008-0171-2 (doi:10.1007/s10071-008-0171-2) [DOI] [PubMed] [Google Scholar]
- 112.Beehner J. C., Bergman T. J., Cheney D. L., Seyfarth R. M., Whitten P. L. 2006. Testosterone predicts future dominance rank and mating activity among male chacma baboons. Behav. Ecol. Sociobiol. 59, 469–479 10.1007/s00265-005-0071-2 (doi:10.1007/s00265-005-0071-2) [DOI] [Google Scholar]
- 113.le Roux A., Beehner J. C., Bergman T. J. 2011. Female philopatry and dominance patterns in wild geladas. Am. J. Primatol. 73, 422–430 10.1002/ajp.20916 (doi:10.1002/ajp.20916) [DOI] [PubMed] [Google Scholar]
- 114.Snyder-Mackler N., Bergman T. J., Beehner J. C. In press. Defining higher levels in a gelada multilevel society. Int. J. Primatol. (doi:10.1007/s10764-012-9584-5) [Google Scholar]
- 115.Kawai M., Ohsawa H., Mori U., Dunbar R. 1983. Social organization of gelada baboons: social units and definitions. Primates 24, 13–24 10.1007/BF02381450 (doi:10.1007/BF02381450) [DOI] [Google Scholar]
- 116.Bergman T. J. 2010. Experimental evidence for limited vocal recognition in a wild primate: implications for the social complexity hypothesis. Proc. R. Soc. B 277, 3045–3053 10.1098/rspb.2010.0580 (doi:10.1098/rspb.2010.0580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.le Roux A., Bergman T. J. 2012. Indirect rival assessment in a social primate, Theropithecus gelada. Anim. Behav. 83, 249–255 10.1016/j.anbehav.2011.10.034 (doi:10.1016/j.anbehav.2011.10.034) [DOI] [Google Scholar]
- 118.Dunbar R. I. M. 1984. Reproductive decisions: an economic analysis of gelada baboon social strategies. Princeton, NJ: Princeton University Press [Google Scholar]
- 119.Mori U. 1979. Reproductive behaviour. In Ecological and sociological studies of gelada baboons (ed. Kawai M.), pp. 183–199 Tokyo: Kodansha [Google Scholar]
- 120.Dunbar R. I. M. 1977. Feeding ecology of gelada baboons: a preliminary report. In Primate ecology (ed. Clutton-Brock T. H.), pp. 250–273 London, UK: Academic Press [Google Scholar]
- 121.Dunbar R. I. M., Bose U. 1991. Adaptation to grass-eating in gelada baboons. Primates 32, 1–7 10.1007/BF02381596 (doi:10.1007/BF02381596) [DOI] [Google Scholar]
- 122.Iwamoto T. 1993. The ecology of Theropithecus gelada. In Theropithecus: the rise and fall of a primate genus (ed Jablonski N. G.), pp. 441–452 Cambridge, UK: Cambridge University Press [Google Scholar]
- 123.Fischer J., Hammerschmidt K., Cheney D. L., Seyfarth R. M. 2001. Acoustic features of female chacma baboon barks. Ethology 107, 33–54 [Google Scholar]
- 124.Owren M. J., Seyfarth R. M., Cheney D. L. 1997. The acoustic features of vowel-like grunt calls in chacma baboons (Papio cyncephalus ursinus): implications for production processes and functions. J. Acoust. Soc. Am. 101, 2951–2963 10.1121/1.418523 (doi:10.1121/1.418523) [DOI] [PubMed] [Google Scholar]
- 125.Rendall D., Seyfarth R. M., Cheney D. L., Owren M. J. 1999. The meaning and function of grunt variants in baboons. Anim. Behav. 57, 583–592 10.1006/anbe.1998.1031 (doi:10.1006/anbe.1998.1031) [DOI] [PubMed] [Google Scholar]
- 126.Fischer J., Hammerschmidt K., Cheney D. L., Seyfarth R. M. 2002. Acoustic features of male baboon loud calls: influences of context, age, and individuality. J. Acoust. Soc. Am. 111, 1465–1974 10.1121/1.1433807 (doi:10.1121/1.1433807) [DOI] [PubMed] [Google Scholar]
- 127.Altmann S. A. 1998. Foraging for survival: yearling baboons in Africa. Chicago, IL: University of Chicago Press [Google Scholar]
- 128.Altmann S. A., Altmann J. 1970. Baboon ecology. Chicago, IL: University of Chicago Press [Google Scholar]
- 129.Norton G. W., Rhine R. J., Wynn G. W., Wynn R. D. 1987. Baboon diet: a five-year study of stability and variability in the plant feeding and habitat of the yellow baboon (Papio cynocephalus) of Mikumi National Park, Tanzania. Folia Primatol. 48, 78–120 10.1159/000156287 (doi:10.1159/000156287) [DOI] [PubMed] [Google Scholar]
- 130.Rowell T. E. 1966. Forest living baboons in Uganda. J. Zool. 149, 344–364 10.1111/j.1469-7998.1966.tb04054.x (doi:10.1111/j.1469-7998.1966.tb04054.x) [DOI] [Google Scholar]
- 131.Hall K. R. L., DeVore I. 1965. Baboon social behavior. In Primate social behavior (eds DeVore I., Hall K. R. L.), pp. 53–110 New York, NY: Holt, Rinehart & Winston [Google Scholar]
- 132.Aich H., Moos-Heilen R., Zimmermann E. 1990. Vocalizations of adult gelada baboons (Theropithecus gelada): acoustic structure and behavioural context. Folia Primatol. 55, 109–132 10.1159/000156508 (doi:10.1159/000156508) [DOI] [PubMed] [Google Scholar]
- 133.Lehner P. N. 1996. Handbook of ethological methods. Cambridge, UK: Cambridge University Press [Google Scholar]
- 134.Cheney D. L., Seyfarth R. M., Silk J. B. 1995. The role of grunts in reconciling opponents and facilitating interactions among adult female baboons. Anim. Behav. 50, 249–257 10.1006/anbe.1995.0237 (doi:10.1006/anbe.1995.0237) [DOI] [Google Scholar]
- 135.Richman B. 1976. Some vocal distinctive features used by gelada monkeys. J. Acoust. Soc. Am. 60, 718–776 10.1121/1.381144 (doi:10.1121/1.381144) [DOI] [PubMed] [Google Scholar]
- 136.Fischer J., Kitchen D. M., Seyfarth R. M., Cheney D. L. 2004. Baboon loud calls advertise male quality: acoustic features and their relation to rank, age, and exhaustion. Behav. Ecol. Sociobiol. 56, 140–148 10.1007/s00265-003-0739-4 (doi:10.1007/s00265-003-0739-4) [DOI] [Google Scholar]
- 137.Kitchen D. M., Cheney D. L., Seyfarth R. M. 2005. Male chacma baboons (Papio hamadryas ursinus) discriminate loud call contests between rivals of different relative ranks. Anim. Cogn. 8, 1–6 10.1007/s10071-004-0222-2 (doi:10.1007/s10071-004-0222-2) [DOI] [PubMed] [Google Scholar]
- 138.Wittig R. M., Crockford C., Seyfarth R. M., Cheney D. L. 2007. Vocal alliances in chacma baboons (Papio hamadryas ursinus). Behav. Ecol. Sociobiol. 61, 899–909 10.1007/s00265-006-0319-5 (doi:10.1007/s00265-006-0319-5) [DOI] [Google Scholar]
- 139.Andrew R. J. 1976. Use of formants in the grunts of baboons and other nonhuman primates. Ann. NY Acad. Sci. 280, 673–693 10.1111/j.1749-6632.1976.tb25530.x (doi:10.1111/j.1749-6632.1976.tb25530.x) [DOI] [PubMed] [Google Scholar]
- 140.Palagi E., Leone A., Mancini G., Ferrari P. F. 2009. Contagious yawning in gelada baboons as a possible expression of empathy. Proc. Natl Acad. Sci. USA 106, 19 262–19 267 10.1073/pnas.0910891106 (doi:10.1073/pnas.0910891106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dunbar R. I. M. 1993. Coevolution of neocortical size, group size and language in humans. Behav. Brain Sci. 16, 681–693 10.1017/S0140525X00032325 (doi:10.1017/S0140525X00032325) [DOI] [Google Scholar]
- 142.Schreier A. L., Swedell L. 2009. The fourth level of social structure in a multi-level society: ecological and social functions of clans in hamadryas baboons. Am. J. Primatol. 71, 948–955 10.1002/ajp.20736 (doi:10.1002/ajp.20736) [DOI] [PubMed] [Google Scholar]
- 143.Aureli F., Preston S. D., de Waal F. 1999. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. J. Comp. Psychol. 113, 59–65 10.1037/0735-7036.113.1.59 (doi:10.1037/0735-7036.113.1.59) [DOI] [PubMed] [Google Scholar]
- 144.Dunbar R. I. M. 2010. The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neurosci. Biobehav. Rev. 34, 260–268 10.1016/j.neubiorev.2008.07.001 (doi:10.1016/j.neubiorev.2008.07.001) [DOI] [PubMed] [Google Scholar]
- 145.Keverne E. B., Martensz N. D., Tuite B. 1989. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology 14, 155–161 10.1016/0306-4530(89)90065-6 (doi:10.1016/0306-4530(89)90065-6) [DOI] [PubMed] [Google Scholar]