Abstract
The complex interrelationships among individuals within social environments can exert selection pressures on social skills: those behaviours and cognitive processes that allow animals to manipulate and out-reproduce others. Social complexity can also have a developmental effect on social skills by providing individuals with opportunities to hone their skills by dealing with the challenges posed in within-group interactions. We examined how social skills develop in captive, adult male brown-headed cowbirds (Molothrus ater) that were exposed to differing levels of ‘social complexity’ across a 2-year experiment. After each year, subjects housed in groups with dynamic social structure (where many individuals entered and exited the groups during the year) outcompeted birds who had been housed in static groups. Exposure to dynamic structure subsequently led to substantial changes to the social networks of the home conditions during the breeding season. Static groups were characterized by a predictable relationship between singing and reproductive success that was stable across years. In dynamic conditions, however, males showed significant variability in their dominance status, their courting and even in their mating success. Reproductive success of males varied dramatically across years and was responsive to social learning in adulthood, and socially dynamic environments ‘trained’ individuals to be better competitors, even at an age when the development of many traits important for breeding (like song quality) had ended.
Keywords: social complexity, birdsong, social learning, development, reproductive success, cowbird
1. Introduction
Group living produces a multitude of challenges for the social animal. Members of group-living species routinely must remember and maintain relationships, communicate, navigate dominance hierarchies and compete for resources, such as mates. Those individuals who are better able attend to, learn from and remember social information should be better able to manipulate and outcompete others. This social complexity hypothesis (also known in various forms as the Machiavellian intelligence, social intelligence or the social brain hypothesis [1–3]) has focused on evolutionary function: social complexity selects for skills (traits, behaviour and cognitive processes) that allow individuals to outcompete others in their group to gain higher levels of reproductive success. The social complexity hypothesis has provided a powerful explanation for the evolution of a variety of traits across a diversity of animals, including insects, birds and primates (including humans) [1–8].
We propose that there is an underappreciated ontogenetic analogue to the social complexity hypothesis that is rarely investigated, yet can have important implications for the evolution of social behaviour. Social animals interact with numerous individuals during their lifetimes and each interaction provides the developing individual with opportunities for social learning and thus opportunities to hone the skills necessary to succeed in the social environment. More complex social environments can provide more diverse social experiences with more opportunities for learning social strategies. These experiences can be of paramount importance to develop effective breeding behaviour. From a functional perspective, therefore, it is the ability to manage social information and use it effectively that is intimately linked to fitness, and thus it may be the case that selection acts on social learning abilities early in life, such as tendencies to affiliate with, attend to and learn from others [9–11], provided that these abilities in some way promote survival or reproductive success.
To investigate the relationship between social development and the evolution of social skills, then, we require experiments that test the heritability of social intelligence. To do so, it is necessary to measure how social environments are structured and how they vary, how traits respond to those environments and the consequent reproductive outcomes of individuals living in those environments. Such an approach presents multiple challenges to experimental design: ‘social complexity’ is a problematic variable to conceptualize and to manipulate, and skills for capitalizing on social complexity are comparably difficult to isolate and measure. Work in the field has provided some support for a correlation between exposure to social complexity and reproductive success [12–15], but there remains a lack of experimental evidence for the direct link between group-level social complexity and individual fitness, a link necessary for long-term evolutionary change.
The history of work taking a developmental approach to understanding communication among gregarious songbirds offered the foundation to test the relationship between social skills and reproductive success. Birdsong studies have revealed that males' mature song, the signal integral to communication and reproductive success, represents the history of males' social experiences through life. This is because early experiences are critical for shaping the type and the quality of the song the male produces in adulthood [16–22]. Similarly, the social skills that a male uses during breeding, such as the ability to compete with other males, or court receptive females effectively and achieve reproductive success, could similarly be a product of social experiences earlier in life. In taking a developmental approach, recent work in brown-headed cowbirds has provided one of the few experimental demonstrations that exposure to different types of social networks altered the communicative and social skills of the group members, and moreover, variation in social complexity affected subjects' reproductive success. This work is reviewed briefly below.
The brown-headed cowbird is a gregarious songbird that has been the subject of numerous studies of social learning and development. Being obligate brood parasites, cowbirds' early social experiences differ from most other songbirds. Because cowbirds do not raise their own young and instead lay eggs in the nests of other bird species, young cowbirds experience a very different early learning environment from other songbirds. This is a time in life for most songbirds where social experiences in the nest are important for song learning and imprinting. Cowbirds, in contrast, do not have the opportunity to experience species-typical visual or auditory information from their foster parents or nest-mates. Despite this alien early social environment, cowbirds are able to develop a species-typical song and appropriate social and breeding behaviours. Across their range in nature, cowbirds display pronounced variability in group structure, and vocal and social behaviour [23–25]. Several studies have investigated whether the variation in social structure might provide differing opportunities for social learning and thus might have led to the observed variation in social behaviour.
Freeberg [26–28] was one of the first to examine how captive juvenile cowbirds learned their adult vocal repertoires from their social environment. He used birds from different macrogeographical home ranges that have distinctly different dialects of song. Freeberg asked whether birds of one ‘culture’ could learn aspects of the communicative system (song and mating preferences) of a different ‘culture’. He housed juvenile cowbirds with adult birds either from the same macrogeographical location or from the distant location. After 1 year of exposure in these conditions, the juvenile birds learned the dialect of the birds with whom they were housed, they mated assortatively within dialect, and these vocal dialects could be culturally transmitted to another generation [27]. One notable point from Freeberg's work was that the experiments were conducted in large outdoor semi-natural environments. Freeberg had effectively modified the social networks of the subjects and then left the birds to self-select the information most important for their vocal development.
White et al. [29] extended this work to determine whether the dialect differences that Freeberg [27] was able to remove though social learning could instead be generated through social learning. White et al. [29] biased the social environments that juvenile male cowbirds experienced during their first year of life by changing the demographics of flocks. In one condition, juvenile males had access to females and adult males (juvenile–adult or JA condition); in the other condition, juveniles had access only to females (juveniles-only, or J condition). The experiment began as a song-learning experiment: JA juveniles had tutors that provided examples of effective, mature cowbird song, whereas the only tutors the J juveniles had were each other. Sure enough, by the birds' first breeding season there were substantial differences across condition in repertoire size, song structure and song attractiveness. What was more surprising, however, was that there were a variety of effects on aspects of the juvenile males' social behaviour apart from song structure. While JA juveniles developed social behaviour typical of wild cowbirds (competitive, aggressive singing bouts with other males, pair-bonding with females and monogamous mating patterns [30–32]), J juveniles engaged in atypical social interactions: they were not aggressive, they did not engage in many social singing interactions with males or with females, they did not pair-bond or mate-guard females and they mated promiscuously. Thus, a minor manipulation to the demographics of flocks revealed the pronounced flexibility in the vocal and social repertoires of juvenile male cowbirds. It also revealed the importance of the social environment in structuring the development of typical cowbird behaviour.
In a follow-up study, White et al. [33] moved the adult male tutors who were living with the JA juveniles into a new aviary with adult males who had spent the year in similar conditions without juvenile males (adults-only, or A condition males). When housed together, the JA adult males outcompeted and had higher levels of reproductive success than the A adult males. Thus, something about being housed with juvenile males honed the JA adults' reproductive skills. In this case, the effect could not have been owing to a change in the quality of the adult males' songs. Once crystallized, an adult male song no longer has the pronounced variability characteristic of juvenile song, and across-year tests of song attractiveness in adulthood typically have shown very little variability ([33,34] and N. Snyder-Mackler & D. J. White 2012, unpublished observations). Thus, manipulation to the social network of adult males affected their reproductive success through avenues other than changing song attractiveness. White et al. [33] hypothesized that juvenile males provided the adult males with a more complex social environment. The juveniles engaged in more diverse patterns of behaviour, occasionally courting females who were pair-bonded with adult males, occasionally challenging adult males to fights, and having highly variable actions and reactions across the year. These dynamic interactions were not characteristic of all-adult flocks. In adult flocks, birds established a stable dominance hierarchy early and remained in it for the duration of the time in the group.
The next step was to test this ‘developmental social complexity hypothesis’. We conducted a 2-year experiment to determine whether social challenges produced cognitive and behavioural changes in adult male cowbirds [35]. In the first year, we randomly assigned adult males to two conditions of flocks. The different social compositions were designed to give one condition greater access to social challenges. Past work has revealed that increased subgroup size relates to social complexity [36,37]; thus we created conditions that allowed some birds to interact with many different males and females over a series of immigrations and emigrations to the group (dynamic conditions: DC) and other conditions where birds remained in groups that did not change over the year (static conditions: SC). All groups retained the same overall size, density and sex-ratio at all times. DC birds were swapped in small subgroups, first in the middle of winter and then approximately every 8 days during the spring prior to the breeding season (at the beginning of May). With each manipulation, males in the DC flocks had to re-evaluate dominance relationships with new males. They also gained and lost opportunities to sing to females. At the start of the breeding season, we stopped manipulations in the DC flocks and returned birds to their home aviaries. For the first month of the breeding season, we observed the birds' courtship and communication in their home aviaries with the resident females. In this phase, we saw few distinct differences between conditions in singing patterns or copulation success. We then commenced the second phase of the experiment—a ‘mating-competency tournament’ designed to test individuals' abilities to court females quickly and effectively in new social conditions that mimicked the competitive demands of wild breeding flocks [23–25]. The mating-competency tournament revealed dramatic differences among males from the original conditions. The DC males outcompeted SC males, both in number of copulations and in the speed with which they reached criterion for success and exited the tournament (this was a measure, used in past work, based on pair-bonding, which effectively predicts copulation success; figure 1, year 1).
Figure 1.
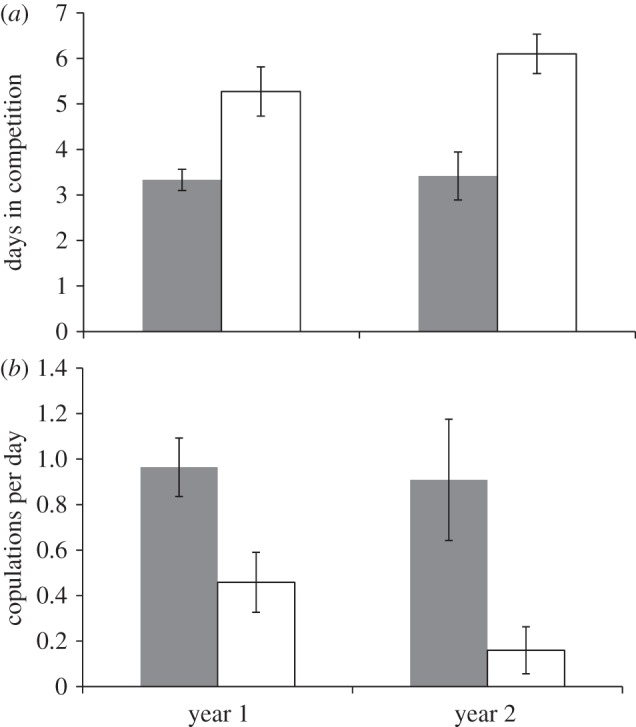
Patterns of behaviour from the mating competency tournaments for males in DC and SC conditions in each of the 2 years. (a) Number of days taken to reach the criterion for success (minimum 1, maximum 7) in the tournament. (b) Copulations achieved per day in the competition. Data are discussed in more detail in the published work [35,38]. Grey bars, dynamic; white bars, static.
The first-year results of our experiment revealed that every one of the DC males was able to court females effectively in the tournament, while SC males were significantly less successful. In the next year of the experiment, we recreated the conditions and manipulations from year 1 using the same birds; some were exposed to the same conditions as the year before, and some were switched (figure 2) [38]. In this iteration of the experiment, we searched for the behavioural mechanisms that created the main effect in the mating-competency tournament. By using the same birds with some in reversed conditions, we were able to examine whether the resultant effects were plastic enough to change across years and breeding seasons. Once again, the overall effect in the mating-competency tournament was pronounced, and DC birds outperformed SC birds in terms of copulation success and days to reach criterion for success (figure 1, year 2). In year 2, we were able to determine that there was not one characteristic of the DC males that was enhanced (such as song quality, more singing to females or more male aggression) that could account for the results. Instead, the results indicated that DC and SC birds differed in how they applied their behaviour to the competitive challenges posed by the tournament. DC birds of all dominance ranks devoted a greater proportion of their female-directed song to a single target female, a strategy that allowed them to take advantage of high dominance rank when they were able to achieve it, and to transcend low rank when others dominated them. DC birds also wasted comparatively little time singing to familiar competitors, a behaviour that correlated with slower, less-effective courtship. Those few SC birds that were successful, in contrast, were successful based on relatively ‘fixed’ traits, succeeding when they happened to land in a group where they had the most potent song or were most aggressive. When other birds sang better or dominated them, SC birds were unable to change strategies to succeed.
Figure 2.
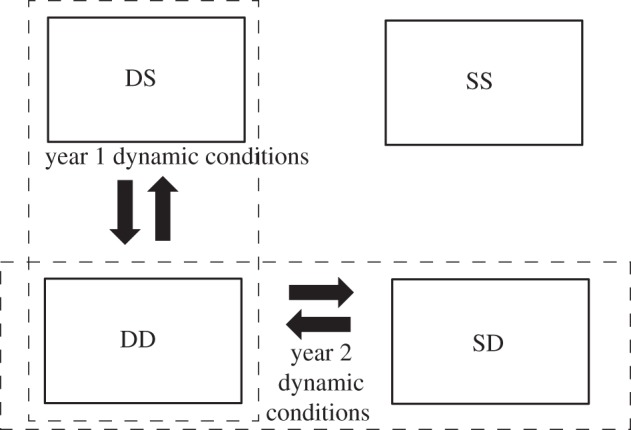
Illustration of the aviary conditions in the 2-year experiment. Males and females were rotated in groups of 2 or 3 during the winter and spring between the DD (dynamic/dynamic) condition and the DS (dynamic/static) condition in year 1 (the dynamic conditions for year 1) and between the DD and SD (static/dynamic) condition in year 2 (the dynamic conditions for year 2). All birds were returned to their home conditions and all manipulations ceased at the beginning of the breeding season (May 5) for both years. A total of 12–13 females were present in each aviary in each year (different females in year 1 and year 2). SS, static/static condition.
Because we used the same males across years, half of which were exposed to the same conditions as the year before, the other half being switched, we had a set of data that allowed us to study the changes (or lack thereof) in behaviour within males across the 2 years. Thus, here we examine what effects exposure to dynamic social conditions had on subsequent breeding behaviour in four groups of birds: those birds that were exposed to static conditions in year 1 and static again in year 2 (SS birds), those who were exposed to dynamic conditions in both years (DD birds) and those who were switched from 1 year to the next (stable in year 1, dynamic in year 2: SD birds, and dynamic in year 1 and stable in year 2, DS birds). We used as outcome measures the datasets from the first half of the breeding seasons from years 1 and 2. These datasets provide a wealth of information about the singing patterns, social behaviour and copulation success for all the males while they were all living in comparable groups with the exact same males from 1 year to the next. As such, the analysis provides a rare window into the consistency of mating and social behaviour across time within and across social conditions.
2. Methods
(a). Subjects and housing
Subjects were 32 adult male cowbirds, ranging in age from 901 to 1757 days at the start of the experiment. Approximately half of the birds were hand-reared and the rest were wild caught on the grounds of the Morris Arboretum in Montgomery County, PA, USA. During the winter and spring, male birds were evenly divided among four mixed-sex flocks containing 12–14 females in 18.3 × 6.1 × 4 m outdoor aviaries. Aviaries had trees, grass, shrubs, a feeding station, indoor shelters and ad libitum access to vitamin-treated water and a mixture of seed and a modified Bronx zoo diet for omnivorous birds. All birds wore coloured leg bands to permit individual identification. Five males died and two were injured over the course of the 2 years. We removed these birds from analysis (each aviary lost two birds except for one group—DS—that lost 1).
(b). Procedures for data collection
Data were collected in 15 min blocks by four observers daily across the 2 years of manipulations and testing, ranging from 1 to 4 h per day. Data were transcribed using automated speech recognition [39]. Observers spoke into wireless microphones (Telex WT 150 & Telex FMR 150; Telex Communications Ltd, Burnsville, MN, USA) and data were collected by IBM ViaVoice voice-recognition software (Millennium Pro Edition) running on PC-compatible computers. All data were subsequently imported into a 4th-Dimension database programmed to summarize data and detect errors (4th Dimension v. 6.5.1; ACI Inc., San Jose, CA, USA). Across the 2 years, we collected 137 501 datapoints on singing and social interactions (see §2c).
(c). Data collection
We collected data that past field and laboratory research had indicated were important for effective social, vocal and reproductive functioning [29,30,40,41]. In 15 min time blocks, we occasion-sampled males for vocalizations, noting each male that vocalized along with information on the social context surrounding the vocalization. Song was scored as ‘undirected’ (if the singing male was not oriented towards a male or female receiver), ‘female-directed’ or ‘male-directed’ (if the singer was oriented at a 0–45° angle to a female or a male, respectively, no farther than approximately 60 cm away—a distance beyond which notes of the song attenuate dramatically) For more detail on data collection procedures, see previously published work [33,35,39]. We used male singing interactions as a proxy measure for male dominance. For each male, we took the amount of male-directed song he sang and compared that with the amount of song that other males sang to him. This ‘singing ratio’ provides a measure of relative dominance rank within groups because males use song to dominate other males, and more dominant males will suppress the vocal output of more subordinate males by repeatedly singing to them. In addition to singing interactions, we also recorded any observed copulations during the two breeding season phases.
(d). Conditions and manipulations
The manipulation schedule was the same for both years and is described in detail elsewhere [35,38]. Briefly, in spring of year 1, we randomly assigned four equal-sized flocks to two conditions (two DC and two SC flocks). The two SC flocks retained the same membership throughout the winter, spring and breeding season. The two DC flocks were subject to frequent changes in membership during the winter and spring, but not the breeding season. Specifically, we removed and introduced small subgroups of females and then males from both DC flocks every 3–8 days (see White et al. [35] for a list of all manipulations). The DC flocks were maintained at the same overall numbers throughout the manipulations.
When the breeding season began, we stopped all manipulations and returned all DC birds to their home aviaries. All birds remained in their home aviaries for 27 days while we collected data on reproductive behaviour. At the end of this period, we began our tournament. In year 2, males were housed with the same males as in year 1. They were placed in new aviaries with new females. We then randomly selected one of each of the DC and SC flocks from year 1 to remain in the same condition (thus becoming DD and SS groups across the 2 years) and the other two flocks to change condition (becoming SD and DS flocks). Once these conditions were in place, the year 2 procedure was the same as year 1 (figure 2).
(e). Data analysis
We focused our analysis on the 2 years of data collected during the first half of the breeding season where all birds were housed in their home conditions. Analyses of tournament behaviour and within-manipulation behaviour are published elsewhere [35,38]. Thus here we compare breeding season performance where all birds are held in identical conditions; the only difference among birds was their history of experiences in static or dynamic conditions in the months leading up to the breeding season. We compared changes in the individuals' social and singing behaviour across the 2 years. We then examined the characteristics of the home groups during the breeding season. We used multiple regression models to determine what social behaviour best predicted copulation success in each of the groups. We then compared the relative standing of each male within his home group from year to year using rank correlations.
3. Results
(a). Individual characteristics of males
On the basis of all the measures we tested, we could find no singing characteristic of the males that systematically distinguished the DC from the SC males. That is, the males in the DC did not do something more than the SC birds. The only variable we found to be close was that SC birds sang a higher proportion (± 1 s.e.m.) of their directed song to males compared with females (0.71 ± 0.03) compared with DC males (0.55 ± 0.05; Student's independent t-test, t23 = 2.592, p < 0.02). This difference was only a trend in year 2 (SC males: 0.75, DC males: 0.62; t23 = 1.79, p > 0.08) and certainly even this comparison would fail to reach significance if the inflated family-wise error risk associated with conducting numerous tests were controlled.
We also examined changes in the behaviour of those individuals who switched conditions (SD and DS males). There were no systematic within-male changes in singing patterns that related to condition. We tested: total amount of song sung, amount of undirected song, male- and female-directed song sung, dominance (singing ratio), proportion of directed song sung to males versus females and copulation success. No within-male test reached significance. The closest case was the amount of male-directed song sung, which tended to be higher when males were in the SC condition (paired t-test: t12 = 2.03, p < 0.06). Copulation success was not significantly higher in the DC over the SC (DC average: 2.13 ± 0.59 copulations per male; SC average: 1.94 ± 0.54 copulations; t12 = 0.21, p > 0.84).
Overall, the behaviour of the males did not in itself reveal any particular characteristic that was enhanced by exposure to social complexity. When we looked at overall characteristics of the groups, however, substantial differences across the conditions emerged.
(b). Characteristics of groups
We used multiple regression models to test variables that predicted copulation success (copulations observed per 15 min song census block) in the two conditions for each year. We used singing patterns as predictors including amount of: undirected song, male-directed song and female-directed song. These comprise all of the circumstances under which males sing. Past experiments across numerous groups have typically shown that female-directed song is the primary predictor of males’ copulation success [42].
Models were effective at predicting copulation success in the stable groups in both years (2007: R2 = 0.64, F3,10 = 5.92, p < 0.02; 2008: R2 = 0.57, F3,10 = 4.33, p < 0.05). In both years, only female-directed song proved to be a significant predictor of copulation success in the SC conditions (2007: B = 0.78, p < 0.02; 2008: B = 0.87, p < 0.01).
Models, however, were not effective at predicting copulation success in either of the DC groups (2007: R2 = 0.09, F3,12 = 0.41, p > 0.75; 2008: R2 = 0.10, F3,10 = 0.354, p > 0.78).
Thus, the two conditions differed in the characteristics that led to reproductive success, with the SC conditions having one strategy that led to success (singing high amounts of song to females) and the DC conditions being highly variable, with males gaining reproductive success through means other than just singing more to females.
We compared the consistency of the behaviour of the males from year 1 to year 2 by examining rank correlations of the males’ behaviours within their groups. SS males were highly consistent in the aspects of behaviour we tested. Those included amounts of female-directed song produced, dominance ranking (as measured by singing ratio) and copulation success (figure 3). For the SC birds, all correlations were higher than 0.82 and all were significant (p < 0.05). This was not surprising, as past work on males housed in the same conditions across years shows high degrees of consistency ([34] and N. Snyder-Mackler & D. J. White 2012, unpublished observations). What was surprising, however, was that no other group showed similar year-to-year consistency in their behaviour. None of the earlier-mentioned song-use-based variables was significantly correlated between years in any of the other three groups (Spearman R ranged from −0.18 to 0.55, all n.s., figure 3). The most striking lack of consistency came in copulation success. In past work, testing males from one year to another has always revealed a highly consistent ranking in copulation success ([34] and N. Snyder-Mackler & D. J. White 2012, unpublished observations). Here, we found no significant relationship in the three groups, with an average correlation from one year to the next of 0.17 (figure 3).
Figure 3.
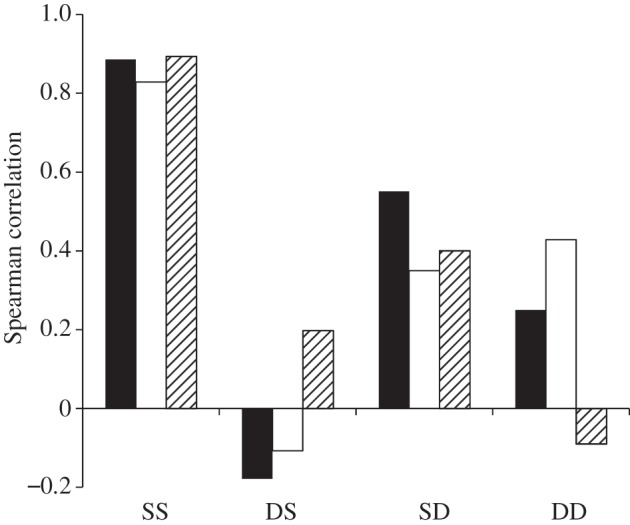
Year 1-to-year 2 rank correlations for behaviour among males within groups. SS, static year 1/static year 2 condition; DS, dynamic/static condition; SD, static/dynamic condition; DD, dynamic/dynamic condition. Female-directed song is the ranking of amount of song sung to females during each breeding season. Dominance was measured by male-directed singing ratio (amount of males directed song sung : song received from other males) during each breeding season. Copulation success is based on number of copulations observed during each breeding season. Black bars, female-directed song; white bars, dominance (singing ratio); striped bars, copulation success.
4. Discussion
The manipulation we conducted in the winters of both years—exposing DC birds to a rotating roster of new individuals, while holding SC birds in stable groups—generated broad differences in the social structures of the home flocks during the subsequent spring breeding seasons. Our past analyses [35,38] were able to detect only the effects of our manipulations on the birds when they were tested in the mating-competency tournament. Here, investigating subjects across years revealed differences in their home conditions. The two static groups in the breeding season were characterized by stable, predictable relationships between social behaviour and reproductive success: those males who sang high amounts of song to females achieved high levels of reproductive success. In contrast, the dynamic conditions had unpredictable relationships between social variables and reproductive success, suggesting that males managed to copulate using a variety of different social strategies.
In addition, the males who experienced static flocks in both years (SS birds) showed high levels of consistency in their behaviour and in their reproductive success across years. In contrast, all males that had at least one experience in a dynamic condition experienced different levels of dominance with other males, different levels of singing to females and even different levels of reproductive success relative to others in their group from year 1 to year 2. This lack of consistency in behaviour was surprising given that the breeding season social conditions contained the exact same competitors across the two years.
The failure to find any specific characteristic that was enhanced as a result of experience in dynamic conditions is consistent with past analyses of the mating-competency tournaments [38,42]. In the tournaments, DC males outcompeted SC males, but again there was no single singing, or song-quality characteristic that distinguished the two sets of males. It is also consistent with analyses of behaviour during the manipulations themselves, which revealed that the only difference between the two conditions was in the amount of variability in group structure [35], with the DC birds experiencing high variability in singing behaviour during the manipulations.
‘Complexity’ is a critical component of the social intelligence hypothesis, but it is rarely operationally defined. We attempted to manipulate complexity by varying the size of the subgroups that subjects experienced over the year (sensu [37]). The outcomes of the manipulations suggest that the important aspect of social complexity that drove the effects on competitive and courtship competency in the adult males was exposure to more variable social networks. While males in both conditions had to compete with other males and court females to gain reproductive success, SC males gained social experiences that were limited in scope: males never had the opportunity to experience either the changes in their dominance status or the disruptions to established consort relationships that occur when new males enter a flock and existing ones depart. In dynamic conditions, however, males experienced multiple social roles with other males and females and thus gained more opportunities to learn different patterns of interaction. These enhanced social experiences provided DC males with more control over varying conditions and the ability to enter new social environments, assess the competitors and react to them quickly and effectively.
Even though increasing group size influenced social skills in cowbirds, it does not mean that group size need be the defining characteristic of social complexity in all species. It is quite possible that in other species, other types of social challenges exist that will relate to social skills and reproductive success. For example, in some species, individuals may benefit by attending to a select few individuals within groups and overall group size may be irrelevant [15]. Identifying the mechanisms underlying social interactions is necessary to conceptualizing complexity in any given case and is thus essential for understanding how selection may act on social skills [3,6,15,37].
Our work thus far has taken a male-centred approach, yet past work has shown that female song and partner preferences are influenced by social experiences in cowbirds [43–46] and in other species of songbirds [47,48]. Future experiments systematically manipulating female social dynamics and testing their mating behaviour will provide a more complete picture of the role social complexity plays on mating success.
The developmental perspective used in this experiment provides a new view of the relationship between social complexity and social skills. Skills involved in communicating and competing with others were consistently in flux even in mature individuals. These skills could be sharpened or could atrophy quickly depending on social experiences. Our ability to reverse the conditions across one year and subsequently reverse the subjects’ reproductive performance highlights the importance of social interactions throughout the lifespan. Engaging with new individuals and having varying social experiences were necessary for adult male cowbirds to remain effective at navigating groups and to increase their reproductive success.
The developmental approach also provides some insights into how selection may act on social and communicative skills. Our data suggest that selection is not favouring just one specialized skill, such as high aggression or a highly attractive song, but instead seems to favour a more general flexibility in behaviour allowing individuals to navigate inherently flexible social environments. Aspects of social environments—other members of the group—can recombine in unpredictable ways (e.g. change mating partnerships or competitive alliances; shift their intentions from neutrality to aggression, and from cooperation to competition; or alter their relevance through emigration or immigration). If these changes lead to variation in the behaviours associated with reproductive success (as seen here), then a single trait will never work effectively in all environments. For example, it may be that in some groups, female choice is the primary determinant of mating success for males, and in other groups male–male aggression is the most important (such variation as has been found in past groups [33]). Succeeding across these variable environments requires the ability to control one's behaviour in response to changes in the social context: to turn some behaviours on (or up) and others off (or down) to suit the demands of a given social configuration. This idea of behavioural control has met with some support in primates (e.g. capuchin monkeys [49]; chimpanzees and bonobos [50]; howler monkeys [51]) and complements work on the evolutionary importance of personality and behavioural syndromes where suites of behaviour show inflexibility across contexts [52–54].
For cowbirds, under natural conditions, the degree of social variability experienced by an individual would be governed partly by environment (the number, size and composition of cowbird flocks available for juvenile or migrant males to join) and partly by those aspects of individual phenotype that regulate social exposure (aggressiveness, tendencies to affiliate, attend to or interact with others). These latter traits would then be sites for natural selection to act, by favouring those individuals with the greatest tendency to seek out and capitalize upon social-learning opportunities. In this sense, the social environment ought to select for ever more ‘sociable’ individuals (and some support does exist in the literature for males displaying variation in these types of traits [55]).
Studying the same individuals longitudinally in varying circumstances allows for the measurement of the repeatability of behaviour. Repeatability has often been considered a proxy for heritability [56], but here consistency in singing and in reproductive success was maintained by the social environment. Care should be taken in studies of sexual selection where assumptions are made about how evolution may act on traits that are associated with reproductive success. Without being able to measure the repeatability of behaviour within subjects across conditions, how evolution might act on traits remains an open question. Classical comparative psychologists made this type of critique of ethology. They argued that underappreciated aspects of the environment can be reliable and stable structuring elements of behavioural repertoires [57,58]. Thus, aspects of behaviour could be inherited in the absence of heritability [59]. The same argument can be applied to underappreciated aspects of the social environment serving to organize and structure behaviour.
Care should also be taken in those studies of the evolution of social intelligence where a social and an asocial species are compared using a cognitive task to test the hypothesis that selection had provided the social species with enhanced cognitive abilities. The social subjects might indeed have the advantage in these types of tasks owing to their evolutionary history of social living selecting for cognitive skills. It is also possible, however, that the social subjects have the advantage because their cognitive skills have been enhanced as a result of the social interactions they experienced across development (that the asocial species never has the opportunity to gain).
Much is still to be learned about the mechanisms of effect between groups and individuals’ social skills, as well as how individuals influence the structure of groups. The cowbird work highlights that this interrelationship between the social environment and the social animal changes across development and has important consequences for reproductive success. As such, social environments play an important role in both the ontogeny and evolution of communication and breeding behaviour.
Acknowledgements
Work was supported under NSF IOS-1021801 to D.J.W. and NSF graduate fellowships to A.S.G. and N.S. Experiments were conducted under the approval of the University of Pennsylvania's animal use and care committee (no. 800439).
References
- 1.Byrne R., Whiten A. 1988. Machiavellian intelligence: social expertise and the evolution of intellect in monkeys, apes, and humans. New York, NY: Oxford University Press [Google Scholar]
- 2.Byrne R., Whiten A. 1997. Machiavellian intelligence II: extensions and evaluations. Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Dunbar R. I. M. 2007. Evolution in the social brain. Science 317, 1344–1347 10.1126/science.1145463 (doi:10.1126/science.1145463) [DOI] [PubMed] [Google Scholar]
- 4.Bugnyar T., Heinrich B. 2005. Ravens, Corvus corax, differentiate between knowledgeable and ignorant competitors. Proc. R. Soc. B 272, 1641–1646 10.1098/rspb.2005.3144 (doi:10.1098/rspb.2005.3144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton N. S., Dickinson A. 1998. Episodic-like memory during cache recovery by scrub jays. Nature 395, 272–274 10.1038/26216 (doi:10.1038/26216) [DOI] [PubMed] [Google Scholar]
- 6.Emery N. J., Seed A. M., von Bayern A. M. P., Clayton N. S. 2007. Cognitive adaptations of social bonding in birds. Phil. Trans. R. Soc. Lond. B 362, 489–505 10.1098/rstb.2006.1991 (doi:10.1098/rstb.2006.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiten A. 2007. The evolution of animal ‘cultures’ and social intelligence. Phil. Trans. R. Soc. B 362, 603–620 10.1098/rstb.2006.1998 (doi:10.1098/rstb.2006.1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson C., McShea D. W. 2007. Individual versus social complexity, with particular reference to ant colonies. Biol. Rev. 76, 211–237 10.1017/S1464793101005656 (doi:10.1017/S1464793101005656) [DOI] [PubMed] [Google Scholar]
- 9.Laland K. N., Richerson P. J., Boyd R. 1996. Developing a theory of animal social learning. In Social learning in animals: the roots of culture (eds Heyes C. M., Jr, Galef B. G., Jr), pp. 129–154 San Diego, CA: Academic Press [Google Scholar]
- 10.Thornton A., Clutton-Brock T. H. 2011. Social learning and the development of individual and group behaviour in mammal societies. Phil. Trans. R. Soc. B 366, 978–987 10.1098/rstb.2010.0312 (doi:10.1098/rstb.2010.0312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Schaik C. P., Burkhart J. M. 2011. Social learning and evolution: the cultural intelligence hypothesis. Phil. Trans. R. Soc. B 366, 1008–1016 10.1098/rstb.2010.0304 (doi:10.1098/rstb.2010.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunbar R. I. M. 1992. Neocortex size as a constraint on group size in primates. J. Hum. Evol. 20, 469–493 10.1016/0047-2484(92)90081-J (doi:10.1016/0047-2484(92)90081-J) [DOI] [Google Scholar]
- 13.Silk J. B., Alberts S. C., Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231–1234 10.1126/science.1088580 (doi:10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 14.Cameron E. Z., Setsaas T. H., Linklater W. L. 2009. Social bonds between unrelated females increase reproductive success in feral horses. Proc. Natl Acad. Sci. USA 106, 13 850–13 853 10.1073/pnas.0900639106 (doi:10.1073/pnas.0900639106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silk J. B., Beehner J. C., Bergman T. J., Crockford C., Engh A. L., Moscovice L. R., Wittig R. M., Seyfarth R. M., Cheney D. L. 2009. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc. R. Soc. B 276, 3099–3104 10.1098/rspb.2009.0681 (doi:10.1098/rspb.2009.0681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baptista L. F., Petrinovich L. 1986. Song development in the white-crowned sparrow: social factors and sex differences. Anim. Behav. 34, 1359–1371 10.1016/S0003-3472(86)80207-X (doi:10.1016/S0003-3472(86)80207-X) [DOI] [Google Scholar]
- 17.Marler P. 1975. On strategies of behavioral development. In Function and evolution in behavior (eds Baerends G., Manning A., Beer C.), pp. 254–275 Oxford, UK: Clarendon Press [Google Scholar]
- 18.Nordby J. C., Campbell S. E., Burt J. M., Beecher M. D. 2000. Social influences during song development in the song sparrow: a laboratory experiment simulating field conditions. Anim. Behav. 59, 1187–1197 10.1006/anbe.1999.1412 (doi:10.1006/anbe.1999.1412) [DOI] [PubMed] [Google Scholar]
- 19.Nowicki S., Peters S., Podos J. 1998. Song learning, early nutrition and sexual selection in songbirds. Am. Zool. 38, 179–190 [Google Scholar]
- 20.Payne R. B., Payne L. L., Woods J. L., Sorenson M. D. 2000. Imprinting and the origin of parasite–host species associations in brood parasitic indigobirds, Vidua chalybeata. Anim. Behav. 59, 69–81 10.1006/anbe.1999.1283 (doi:10.1006/anbe.1999.1283) [DOI] [PubMed] [Google Scholar]
- 21.Smith W. J. 1977. The behavior of communicating. Cambridge, MA: Harvard University Press [Google Scholar]
- 22.Snowdon C. T., Hausberger M. (eds) 1997. Social influences on vocal development. Cambridge, UK: Cambridge University Press [Google Scholar]
- 23.Friedmann H. 1929. The cowbirds: a study in the biology of social parasitism. Springfield, IL: C. C. Thomas [Google Scholar]
- 24.Ortega C. P. 1998. Cowbirds and their hosts. Tucson, AZ: University of Arizona Press [Google Scholar]
- 25.Smith J. N. M., Cook T. L., Rothstein S. I., Robinson S. F., Sealy S. G. (eds) 2000. Ecology and management of cowbirds and their hosts. Austin, TX: University of Texas Press [Google Scholar]
- 26.Freeberg T. M. 1996. Assortative mating in captive cowbirds is predicted by social experience. Anim. Behav. 52, 1129–1142 10.1006/anbe.1996.0260 (doi:10.1006/anbe.1996.0260) [DOI] [Google Scholar]
- 27.Freeberg T. M. 1998. The cultural transmission of courtship patterns in cowbirds, Molothrus ater. Anim. Behav. 56, 1063–1073 10.1006/anbe.1998.0870 (doi:10.1006/anbe.1998.0870) [DOI] [PubMed] [Google Scholar]
- 28.Freeberg T. M., White D. J. 2006. Social traditions and the maintenance and loss of geographic variation in mating patterns of brown-headed cowbirds. Int. J. Comp. Psychol. 19, 206–222 [Google Scholar]
- 29.White D. J., King A. P., West M. J. 2002. Facultative development of courtship and communication in juvenile male cowbirds (Molothrus ater). Behav. Ecol. 13, 487–496 10.1093/beheco/13.4.487 (doi:10.1093/beheco/13.4.487) [DOI] [Google Scholar]
- 30.Rothstein S. I., Yokel D. A., Fleischer R. C. 1986. Social dominance, mating, and spacing systems, female fecundity and vocal dialects in captive and free-ranging brown-headed cowbirds. Curr. Ornithol. 3, 127–185 [Google Scholar]
- 31.Rothstein S. I., Yokel D. A., Fleischer R. C. 1988. The agonistic and sexual functions of vocalizations of male brown-headed cowbirds, Molothrus ater. Anim. Behav. 36, 73–86 10.1016/S0003-3472(88)80251-3 (doi:10.1016/S0003-3472(88)80251-3) [DOI] [Google Scholar]
- 32.Davies N. B. 2000. Cuckoos, cowbirds and other cheats. London, UK: T & AD Poyser [Google Scholar]
- 33.White D. J., King A. P., West M. J. 2002. Plasticity in adult development: experience with young males enhances mating competence in adult male cowbirds, Molothrus ater. Behaviour 139, 713–728 10.1163/156853902320262781 (doi:10.1163/156853902320262781) [DOI] [Google Scholar]
- 34.Gros-Louis J., White D. J., King A. P., West M. J. 2006. Do juvenile males affect adult males’ reproductive success in brown-headed cowbirds (Molothrus ater)? Behaviour 143, 219–237 10.1163/156853906775900748 (doi:10.1163/156853906775900748) [DOI] [Google Scholar]
- 35.White D. J., Gersick A. S., Freed-Brown G., Snyder-Mackler N. 2010. The ontogeny of social skills: increasing social complexity enhances reproductive success in adult cowbirds. Anim. Behav. 79, 385–390 10.1016/j.anbehav.2009.11.014 (doi:10.1016/j.anbehav.2009.11.014) [DOI] [Google Scholar]
- 36.Freeberg T. M. 2006. Social complexity can drive vocal complexity. Psychol. Sci. 17, 557–561 10.1111/j.1467-9280.2006.01743.x (doi:10.1111/j.1467-9280.2006.01743.x) [DOI] [PubMed] [Google Scholar]
- 37.Kudo H., Dunbar R. I. M. 2001. Neocortex size and social network size in primates. Anim. Behav. 62, 711–722 10.1006/anbe.2001.1808 (doi:10.1006/anbe.2001.1808) [DOI] [Google Scholar]
- 38.Gersick A. S., Snyder-Mackler N., White D. J. In press Ontogeny of social skills: social complexity improves mating and competitive strategies in male brown-headed cowbirds. Anim. Behav. [Google Scholar]
- 39.White D. J., King A. P., Duncan S. D. 2002. Voice recognition technology as a tool for behaviour research. Behav. Res. Methods Instrum. Comput. 34, 1–5 10.3758/BF03195418 (doi:10.3758/BF03195418) [DOI] [PubMed] [Google Scholar]
- 40.Dufty A. M., Jr 1986. Singing and the establishment and maintenance of dominance hierarchies in captive brown-headed cowbirds. Behav. Ecol. Sociobiol. 19, 49–55 10.1007/BF00303842 (doi:10.1007/BF00303842) [DOI] [Google Scholar]
- 41.King A. P., White D. J., West M. J. 2003. Female proximity stimulates development of male competition in juvenile brown-headed cowbirds, Molothrus ater. Anim. Behav. 66, 817–828 10.1006/anbe.2003.2280 (doi:10.1006/anbe.2003.2280) [DOI] [Google Scholar]
- 42.White D. J., Gros-Louis J., West M. J., King A. P., Tuttle E. M. 2010. Effects of singing on copulation success and egg production in brown-headed cowbirds, Molothrus ater. Behav. Ecol. 21, 211–218 10.1093/beheco/arp178 (doi:10.1093/beheco/arp178) [DOI] [Google Scholar]
- 43.Freeberg T. M., Duncan S. D., Kast T. L., Enstrom D. A. 1999. Cultural influences on female mate choice: an experimental test in cowbirds, Molothrus ater. Anim. Behav. 57, 421–426 10.1006/anbe.1998.0988 (doi:10.1006/anbe.1998.0988) [DOI] [PubMed] [Google Scholar]
- 44.King A. P., West M. J., White D. J. 2003. Female cowbird song perception: evidence for plasticity of preference. Ethology 109, 1–13 10.1046/j.1439-0310.2003.00837.x (doi:10.1046/j.1439-0310.2003.00837.x) [DOI] [Google Scholar]
- 45.West M. J., King A. P., White D. J., Gros-Louis J., Freed-Brown G. 2006. The development of local song preferences in female cowbirds (Molothrus ater): flock living stimulates learning. Ethology 112, 1095–1107 10.1111/j.1439-0310.2006.01264.x (doi:10.1111/j.1439-0310.2006.01264.x) [DOI] [Google Scholar]
- 46.White D. J., Gros-Louis J., King A. P., West M. J. 2006. A method to measure the development of song preferences in female cowbirds, Molothrus ater. Anim. Behav. 72, 181–188 10.1016/j.anbehav.2006.01.008 (doi:10.1016/j.anbehav.2006.01.008) [DOI] [Google Scholar]
- 47.Riebel K. 2000. Early exposure leads to repeatable preferences for male song in female zebra finches. Proc. R. Soc. Lond. B 267, 2553–2558 10.1098/rspb.2000.1320 (doi:10.1098/rspb.2000.1320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauay C., Gerlach N. M., Adkins-Regan E., deVoogd T. J. 2004. Female zebra finches require early song exposure to prefer high-quality song as adults. Anim. Behav. 68, 1249–1255 10.1016/j.anbehav.2003.12.025 (doi:10.1016/j.anbehav.2003.12.025) [DOI] [Google Scholar]
- 49.Fragaszy D. M., Visalberghi E., Robinson J. G. 1990. Variability and adaptability in the genus Cebus. Folia Primatol. 54, 114–118 10.1159/000156434 (doi:10.1159/000156434) [DOI] [PubMed] [Google Scholar]
- 50.Boesch C., Hohmann G., Marshant L. F. 2002. Behavioral diversity in chimpanzees and bonobos. Cambridge, UK: Cambridge University Press [Google Scholar]
- 51.Jones C. B. 2000. Alouatta palliata politics: empirical and theoretical aspects of power. Primate Report 56, 3–21 [Google Scholar]
- 52.Pigliucci M. 2001. Phenotypic plasticity: beyond nature and nurture. Baltimore, MD: Johns Hopkins Press [Google Scholar]
- 53.Sih A., Bell A., Johnson J. C. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 54.Dingemanse N. J., Kazem A. J. N., Reale D., Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89 10.1016/j.tree.2009.07.013 (doi:10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 55.Smith V. A., King A. P., West M. J. 2002. The context of social learning: association patterns in a captive flock of brown-headed cowbirds. Anim. Behav. 63, 23–35 10.1006/anbe.2001.1886 (doi:10.1006/anbe.2001.1886) [DOI] [Google Scholar]
- 56.Lessells C. M., Boag P. T. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121 [Google Scholar]
- 57.Gottlieb G. 1992. Individual development and evolution: the genesis of novel behavior. New York, NY: Oxford University Press [Google Scholar]
- 58.Lehrman D. S. 1953. A critique of Konrad Lorenz's theory of instinctive behavior. Q. Rev. Biol. 28, 337–363 10.1086/399858 (doi:10.1086/399858) [DOI] [PubMed] [Google Scholar]
- 59.West M. J., King A. P., Arberg A. A. 1988. An inheritance of niches: the role of ecological legacies in ontogeny. In Develpmental psychobiology and behavioral ecology (ed. Blass E. M.), pp. 41–62 New York, NY: Plenum [Google Scholar]


