Abstract
Signal complexity has been linked to social complexity in vocal, but not chemical, communication. To address this gap, we examined the chemical complexity of male and female glandular secretions in eight species of Eulemur. In this diverse clade of macrosmatic primates, species differ by social or mating system and dominance structure. We applied principal component and linear discriminate analyses to data obtained by gas chromatography/mass spectrometry. Beyond the significant effects on chemical signals of gland type, sex, season and species, we found effects of social variables and phylogeny. Notably, female odours were more chemically complex in multimale–multifemale species than pair-bonded species, whereas male odours were more chemically complex in codominant species than female-dominant species. Also, the traditional sexual dimorphism, whereby male signal complexity exceeds that of females, was present in codominant species, but reversed in female-dominant species. Lastly, a positive relationship between the species' pairwise chemical distances and their pairwise phylogenetic distances supported a gradual, but relatively fast mode of signal evolution. We suggest that the comparative method can be a powerful tool in olfactory research, revealing species differences relevant to the understanding of current signal utility and evolutionary processes. In particular, social complexity in lemurs may have selected for olfactory complexity.
Keywords: female masculinization, olfactory communication, reproductive advertisement, sex differences, species signatures, strepsirrhine primate
1. Introduction
Within taxonomic groups, the richest elaboration of communicative signals has been associated with the most complex societies, suggesting that group living has driven the evolution of communication systems [1]. Tests of the association between social complexity and signal complexity, however, derive almost exclusively from the study of vocal communication (e.g. primates [2]; sciurid rodents [3]). Therefore, we have a limited understanding of whether sociality selects for complexity in other types of signals. Although chemosignalling is a primary mode of communication in mammalian taxa [4–6] that conveys a wealth of individual information (e.g. sex [6–8], reproductive state [5,8], identity [8,9], genetic quality [10,11] and health [12]) and social information (e.g. species [13,14], territory [15,16], group membership [17], dominance status [18] and kinship [10,19,20]), we know of no formal test between an animal's type of social organization and the complexity of its chemical signals. Here, we relate the chemical signals of eight, closely related species of strepsirrhine primates to various traits (e.g. morphological elaboration, type of gland, sex, reproductive season and pairwise phylogenetic or geographical distance), as well as to their social or mating system (i.e. pair-bonded versus multimale–multifemale or MM–MF groups) and their social dominance structure (i.e. female-dominant versus sexually egalitarian or codominant species).
Traditionally, researchers studying the role of chemosignalling in mammals have taken a behavioural approach, examining either the scent marking in signallers or the responses generated in receivers [4,5]; however, more widespread incorporation of analytical chemistry has allowed more recent expansion into characterizing the structure of olfactory signals [21,22]. The latter has provided a deeper understanding of the information content of signals (as revealed by the presence, absence or relative ratios of compounds) and has produced a growing literature on signal variability, typically within a species [8,18,23,24]. Comparative studies of mammalian chemosignals, however, are still conspicuously lacking.
The dearth of comparative olfactory studies is likely due to the logistical challenges of dealing with semiochemical data. By comparison with vocal signals that emanate from a single source, olfactory signals can derive from a multitude of sources (e.g. faeces, urine, sweat, skin and various glands) that vary significantly between species [25]. Studies of chemical communication are typically focused on a particular kind of odourant, thereby reflecting only a fraction of the available chemical information. Moreover, the relevant information expressed from any one source can be encoded within a range of highly volatile, low-molecular-weight compounds to non-volatile, heavy or proteinaceous compounds that would require a combination of tools and techniques for detection and quantification. Lastly, even for a given methodology, the complex signals produced by mammals often contain a large proportion of unknown compounds that, without relevant mass spectrometry data, cannot be matched or compared across studies [26,27]. These constraints considerably limit the possibilities of conducting comparative studies by synthesizing available literature. Consequently, most of the existing comparative studies are focused either on only a few species [28] or on only a few compounds (as is the case for certain insect pheromones [29]).
To address an evolutionary or ecological hypothesis in a comparative framework thus requires de novo data in which odourant samples collected from several species and potentially from several sources are processed and analysed using the same methodology. This ‘de novo’ approach has been used successfully to compare semiochemicals from several species of plants [30], insects [31–35] and even mammals [14,36,37]. To our knowledge, however, in only one of these studies have researchers also used a comparative framework to show that socioecological factors might modulate chemical signals [14]. Interestingly, among the various urine-marking primates studied, the most socially complex species expressed the greatest diversity of urinary semiochemicals [14]. Likewise, the most socially complex of all the strepsirrhine primates, the ring-tailed lemur (Lemur catta), may express the greatest complexity of glandular semiochemicals [8,20]; unfortunately, it is the only member of its genus and has species-specific glands, thereby limiting its value for comparative studies.
Using chemical data obtained by gas chromatography and mass spectrometry (GC/MS), we determined whether volatile chemicals reflect glandular, sexual, seasonal and species ‘signatures’ or pairwise phylogenetic distance, as well as aspects of primate sociality, including social or mating system and type of dominance structure. For this study, we selected eight species from the genus Eulemur. These animals live in relatively small, family or social groups (thereby precluding a study of social complexity defined by group size), in which mates are either pair-bonded and relatively faithful or live in MM–MF groups and engage in more promiscuous mating [38–40]. Uniquely, although female dominance characterizes most of Lemuridae, the Eulemur clade contains the only exceptional species (i.e. Eulemur fulvus spp.) in which the sexes are codominant (i.e. lack obvious rank relations between the sexes) [41]. As strepsirrhine primates, they all possess a functional vomeronasal organ and rely heavily on chemical communication [42]. Moreover, both sexes share comparable glandular fields in their genital and perianal regions [42], permitting intersexual comparisons of their olfactory cues. As anogenital marking in Eulemur occurs in various contexts, with conspecifics being attracted both to the visual act of marking and to the deposited scent [43–46], early behavioural studies established that the secretions expressed from these glandular areas are used in social communication [43,45,47]; nevertheless, there has been no study of the chemical composition of these secretions. In a series of hypothesis-driven questions, we begin our study with an exploration of the morphological correlates and information content of these signals, and end by relating novel chemical information to the species' social and phylogenetic characteristics.
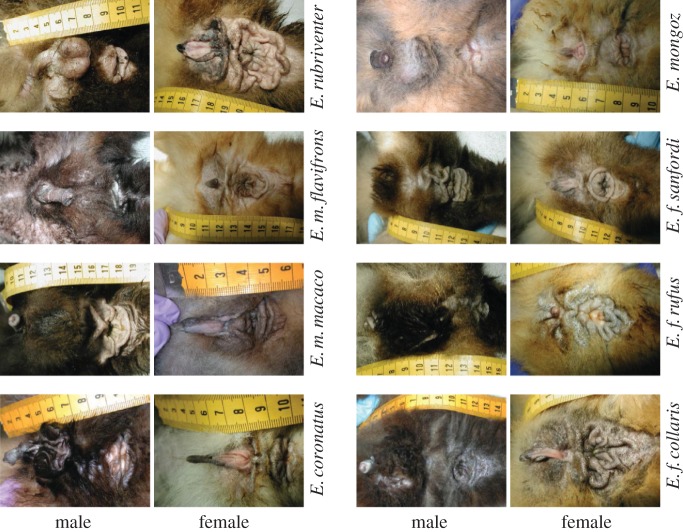
The anogenital region of the members of the Eulemur clade shows remarkable elaboration, particularly of the perianal glands (figure 1) that begs explanation. Our first hypothesis is that glandular morphology is functionally linked to its secretory attributes. Specifically, we predict that greater glandular elaboration might entail more varied chemical information, such that across Eulemur species, the degree of morphological complexity of the anogenital area might correlate positively with the richness (or abundance) of its chemical secretions. Additionally, glandular morphology might vary between the sexes or with the facets of social complexity. We also predict that the genital and perianal regions should produce distinctive secretions, so as to avoid signal redundancy. In male ring-tailed lemurs, for example, secretions produced in three separate glands (brachial, antebrachial and scrotal) are chemically and functionally distinct [8]. The genital and perianal glandular fields in Eulemur, despite their physical proximity and the potential mixing of their signals, should likewise contribute unique chemical components to their respective cues.
Figure 1.
Representative pictures, in craniocaudal orientation from left to right, of male and female anogenital regions in eight Eulemur species.
Our second hypothesis is that anogenital secretions play a role in sexual and/or reproductive advertisement. Accordingly, we predict that the chemical composition of Eulemur secretions should differ between the sexes, and across the breeding and non-breeding seasons, as they do in ring-tailed lemurs [8].
Third, we hypothesize that social complexity drives chemical complexity. Accordingly, because the frequency and variety of social interactions are greater in larger, MM–MF groups than in smaller, pair-bonded families, we predict that chemical profiles should be richer in MM–MF species than in pair-bonded species. Likewise, because female dominance in ring-tailed lemurs is associated with greater complexity of genital semiochemicals in females than in males [20], we also predict that chemical signals in female Eulemur should be richer in female-dominant species than in codominant species. By contrast, in keeping with the traditional sexual dimorphisms of mammals, we predict that chemical signals in male Eulemur should be richer in codominant species than in female-dominant species. That is, given the expected relationship between dominance status and signal complexity, the more elevated social ranking of males in codominant species compared with female-dominant species should lead to greater complexity of male genital semiochemicals in codominant species than in female-dominant species.
Lastly, as interspecific mating is deleterious and hybridization is known to occur in captive Eulemur, there should be selection for distinctive chemical signals as an isolating mechanism [48,49]. Accordingly, our last hypothesis is that anogenital secretions play a role in species recognition or discrimination to support species isolation. Consistent with our previous findings for urinary signals in strepsirrhines [14], we expect that species will have distinctive chemical profiles or ‘signatures’. We also predict that closely related species will have more commonalities in their chemical profiles than will distantly related species, supporting a gradual mode of evolution [14,28]. Even if the phylogenetic distance between species does not relate to their chemical distance (and instead reflects a saltational mode of evolution), we would expect there to be a means of reducing species interference. For instance, those species that are more closely distributed geographically might produce more distinctive chemical profiles than species that are more geographically distant.
2. Material and methods
(a). Subjects
Our subjects were 48 adult animals (25 males and 23 females), aged 3–27 (mean ± s.e.m.: 16.65 ± 0.91) years, representing eight species of Eulemur (table 1), all members of the captive colony at the Duke Lemur Center (DLC) in Durham, NC, USA. The two E. macaco taxa (Eulemur macaco macaco and Eulemur macaco flavifrons) and the three E. fulvus taxa (Eulemur fulvus sanfordi, Eulemur fulvus rufus and Eulemur fulvus collaris) recently have been accorded species status [56]. Therefore, despite the subspecies notations, we refer to the eight Eulemur taxa as different species. Eulemur comprises species that are of conservation concern; when data are available, they show that species range from ‘near threatened’ to ‘critically endangered’ [57].
Table 1.
Phylogenetic relationships, social organization, morphological complexity of the anogenital area, and mean number of compounds (or chemical richness) in the eight species of Eulemur studied.
| mean ± s.e.m. no. compounds |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| phylogenetic | social organization |
subjectsc |
anogenital complexityd |
genital secretion |
perianal secretion |
|||||||
| relationshipsa | species studied | female dominance | social systemb | M | F | M | F | M | F | M | F | references |
|
E. rubriventer | yes | pair-bonded | 3 | 3 | 1.3 | 4 | 16.3 ± 0.9 | 17 ± 2.1 | 16.3 ± 3.8 | 13 ± 3.2 | [39,40] |
| E. m. flavifrons | yes | MM–MF | 3 | 3 | 1 | 2.7 | 13.3 ± 1.9 | 16.7 ± 1.8 | 15 ± 2 | 16 ± 1.2 | [50] | |
| E. m. macaco | yes | MM–MF | 3 | 2 | 2.7 | 2.3 | 19 ± 4 | 23 ± 4 | 16 ± 2.7 | 21 | [51] | |
| E. coronatus | yes | MM–MF | 4 | 3 | 2 | 2.3 | 8.7 ± 1.8 | 17.7 ± 2 | 7.3 ± 1.9 | 17 ± 2.5 | [40] | |
| E. mongoz | yes | pair-bonded | 3 | 3 | 1 | 2.3 | 14.3 ± 0.3 | 17.7 ± 1.3 | 10 ± 1.5 | 13.3 ± 0.3 | [38] | |
| E. f. sanfordi | no | MM–MF | 3 | 3 | 3 | 2 | 22 ± 2.5 | 15 ± 2.7 | 21.7 ± 2.4 | 21.7 ± 2.9 | [52–54] | |
| E. f. rufus | no | MM–MF | 3 | 3 | 1 | 4 | 21.5 ± 4.5 | 17.3 ± 1.9 | 26.3 ± 1 | 17.5 ± 0.5 | [52,53] | |
| E. f. collaris | no | MM–MF | 3 | 3 | 1 | 4 | 19.7 ± 4.1 | 20 ± 2.1 | 19.7 ± 5.2 | 23 ± 2.7 | [54] | |
The DLC animals are housed socially, generally in large indoor/outdoor enclosures (23.2–951.3 m2, depending on group size), and are exposed to natural daylight and the local photoperiod. During the warmer months, some of the animals have access to larger, forested enclosures (1.5–27.2 acres), often with several species occupying the same habitat. The housing has been described previously [58]. Eulemur mongoz are fed folivore chow (Leaf-Eater Primate Diet, Mazuri), whereas the other Eulemur species are fed Old World monkey chow (Monkey Diet, LabDiet). The diets of all animals are supplemented with a mixture of fruits and vegetables. Those animals that semi-free-range can additionally supplement their normal diet with local vegetation and with insects foraged from the forest. The minimal effects of diet on chemical profiles are described elsewhere (C. Sacha, R. Canlas, W. W. Kesler III, M. Boulet, C. M. Drea & G. Dubay 2010, unpublished data). Lastly, all the animals had continuous access to water.
(b). Assessment of anogenital morphology
Using a digital camera (Nikon Coolpix 990), we photographed the scent glands of the subjects while they were anaesthetized for their regularly scheduled physical examinations. Representative photographs of the glandular areas of male and female Eulemur are presented in figure 1. To quantify the glandular elaboration for males and females of each species, we asked three researchers blind to the goals of our study to rate representative animals on a four-point scale (with ‘1’ for minimal elaboration, ‘2’ for modest elaboration, ‘3’ for significant elaboration and ‘4’ for extreme elaboration). We used these ratings to calculate a mean value within that four-point scale for each sex of each species (table 1).
(c). Odourant sample collection
We also collected scent samples from anaesthetized animals using a previously described methodology ([58]; see the electronic supplementary material). Typically, we collected two separate samples from the same animal, one each from the genital and the perianal area, but only if these areas appeared free of fresh urine and faeces. Thus, we did not always obtain both samples from the same animal on a given day. In such cases, we often obtained a replacement sample at a later date. The vast majority of our subjects were reproductively intact at the time of sampling, which occurred year round. For these subjects, we used historical records on mating and pregnancy to assign a reproductive season (i.e. breeding or non-breeding) to each animal's samples. Because of subject-pool limitations when dealing with endangered species, we also collected samples from animals that were ovariectomised (n = 1, for health reasons) or within the activity period of hormonal contraceptive treatments (n = 2). Because contraception with medroxyprogesterone acetate is known to affect chemical profiles in ring-tailed lemurs [59], we included data from these animals in only one set of within-subjects analyses (as noted in §2e(ii)).
(d). Chemical analysis and compound identification
We used a similar GC/MS protocol to the one previously described for L. catta [8] with some modifications for Eulemur (see the electronic supplementary material). We analysed the chemical data using the GCMSsolution PostRun Analysis software (v. 2.50; Shimadzu Scientific Instruments). Detected peaks were individually verified. We retained only those peaks with an area greater than 0.5 per cent of the total chromatogram area. Similarities between mass spectra were the primary criteria for determining whether two peaks in two samples represented the same compound, followed by similarity in retention times. We also used available mass spectral libraries (National Institute of Standards and Technology, Wiley Registry) when feasible. Compounds present included alcohols, acids, aldehydes, ketones, hydrocarbons, sterols and fatty acid esters. Details on the analytical chemistry techniques used to verify compound identification are presented elsewhere (C. Sacha, R. Canlas, W. W. Kesler III, M. Boulet, C. M. Drea & G. Dubay 2010, unpublished data).
(e). Statistical analyses
(i). Testing correlates of glandular morphology
We determined whether morphological complexity correlated with mean chemical richness using Pearson's correlation and tested for sex differences in morphological complexity using paired t-tests (SPSS 14). We then tested whether morphological complexity differed in relation to social organization, using independent t-tests. In these analyses, we were unable to apply phylogenetic corrections owing to the small number of species available; however, it must be noted that all the species under study belong to the same genus. Lastly, we determined if species that were more closely related showed more similarity in their morphological complexity by creating a matrix of phylogenetic distances between pairs of species using a recent molecular phylogeny that includes our eight Eulemur species [55]. We also created a matrix of similarities in anogenital complexity between each pair of species using the average ratings presented in table 1. We used a Mantel test (PC-ORD 5.31) to compare these phylogenetic and morphological matrices.
(ii). Detecting glandular differences in chemical signals
We tested whether the chemical composition of the genital and perianal secretions were different using an approach detailed previously [8]. Briefly, we ran a principal component analysis (PCA) on the relative abundances of the chemical compounds detected, selected those principal components that explained at least 1 per cent of the variation, then used those principal components as covariates for a linear discriminant analysis (LDA), with gland type as the X category (JMP 8). For these (and other) analyses, we excluded odourant samples obtained from ovariectomised or contracepted females. Thus, the respective sample sizes for the genital and perianal secretions were n = 40 (from 22 males and 18 females) and n = 43 (from 24 males and 19 females) or n = 83 ‘intact’ samples.
Next, to determine whether the genital and perianal secretions were chemically distinctive within individual animals, we used data from the subset of subjects (n = 37) for which we collected paired secretions (n = 74) on the same day. These samples derived from the following 18 male (M) and 19 female (F) subjects, by species: Eulemur rubriventer: 1M/1F; E. m. flavifrons: 2M/3F; E. m. macaco: 2M/2F; E. coronatus: 2M/3F; E. mongoz: 3M/3F; E. f. sanfordi: 3M/2F; E. f. rufus: 2M/2F; and E. f. collaris: 3M/3F. Within this subject pool, one female E. m. macaco was ovariectomised, and the female E. rubriventer and one female E. f. sanfordi were within the activity period of contraceptive treatments. Nonetheless, we included pairs of samples from these three subjects because, for these analyses, we were interested in the comparison of the two secretion types within each individual, as opposed to across individuals.
As in previous studies [10,20], we calculated three diversity indices (richness, Shannon and Simpson) for each of the 74 paired samples (using PC-ORD 5.31). The richness index refers to the number of compounds in a sample, the Shannon index is influenced by the compounds that are of intermediate abundance and the Simpson index is most sensitive to the most abundant compounds [60]. Given that the number of chemical compounds considered in the analyses was the same across samples, the Shannon and Simpson indices take into account the number and evenness of the compounds in one sample. Consequently, two samples with a different set of compounds may result in similar diversity indices if their number and evenness are similar. We determined whether the values of these indices differed between the two types of secretions in the 37 individuals, using paired t-tests. We also determined whether the degree of diversity in one secretion related to the degree of diversity in the other secretion, using Pearson's correlations.
To determine whether the two samples obtained from the genital and perianal areas, respectively, of the same individual contained the same types of chemicals or different types of chemicals (i.e. reflected the same or different kinds of secretion), we performed a non-metric multidimensional scaling (NMS) analysis with the chemical data from the 74 samples. We ran the NMS in PC-ORD 5.31 using Sorensen (Bray–Curtis) distances [60]. For each subject, we calculated Euclidean distances between the genital and perianal values in the three-dimensional NMS space, checked normality of all the distances with the Kolmogorov–Smirnov test (normality was met) and then used a one-sample t-test with the null hypothesis being that the ‘chemical distances’ between the genital and perianal secretions of each individual were not significantly different from zero. Support of the null hypothesis would indicate that the genital and perianal secretions in each individual were chemically similar.
(iii). Detecting sex and seasonal differences in chemical signals
Using the 83 ‘intact’ samples, we next analysed the chemical components detected in the genital and perianal secretions separately. For each secretion type, we ran a separate PCA (as described in §2e(ii)) and selected those principal components that explained at least 1 per cent of the variation. Accordingly, for genital samples (n = 40), we selected 23 principal components (with eigenvalues greater than 1.42, that cumulatively explained 93.17% of the variation) and for perianal samples (n = 43), we selected 28 principal components (with eigenvalues greater than 1.31, that cumulatively explained 93.94% of the variation). We then used those principal components as covariates for two separate LDAs in which the X category was sex or season.
(iv). Linking social complexity to the richness of chemical signals
Again using the 83 ‘intact’ samples, we calculated the average chemical richness (i.e. number of compounds) for each sex and species (table 1). We then determined whether the average chemical richness differed as a function of a species' social system (i.e. MM–MF versus pair-bonded) or dominance structure (female-dominant versus codominant), using independent t-tests.
(v). Testing relationships between chemical distances and phylogenetic or geographical distances between species
We tested whether the chemical differences between species were related to their phylogenetic relationships by comparing a matrix of chemical distances between pairs of species to a matrix of phylogenetic distances between pairs of species. To create the chemical matrix we performed a NMS using Sorensen (Bray–Curtis) distances [60]. The NMS resulted in a three-dimensional space in which the distance between two samples represented their dissimilarity in chemical composition. We calculated the centroid or average location in that three-dimensional space for all the samples representing each species, and then calculated Euclidean distances between each pair of species' centroids. We used a Mantel test to compare the matrix of chemical distances to the matrix of phylogenetic distances. As proposed by Symonds [28,32], a positive correlation between chemical distance and phylogenetic distance would support a gradual mode of evolution for chemical signals.
We tested whether the chemical differences between Eulemur species were related to the geographical distances separating those species by comparing a matrix of chemical distances between pairs of species to a matrix of geographical distances between pairs of species. We constructed the geographical matrix using the distribution areas available in Mittermeier et al. [61]. For each pair of species, we measured the minimal distance (in km) between their distribution areas. We then used a Mantel test to compare the matrix of chemical distances (described above) to this matrix of geographical distances.
3. Results
(a). Sex reversal in glandular morphology
Contrary to our prediction, we found no correlation between the number of chemical compounds and glandular complexity (Pearson's correlation: p > 0.05 for separate analyses on genital and perianal secretions per sex). Noteworthy, however, was the unusual reversal of the sex difference in glandular morphology that one traditionally observes in mammals: the anogenital folds of females were significantly more elaborate (mean complexity rating = 2.96 ± 0.31) than those of males (1.63 ± 0.29; paired t-test: t7 = –2.44, p = 0.044; figure 1). Despite this impressive sex difference, morphological complexity did not differ as a function of a species' social system or dominance structure (independent t-tests: p > 0.05 for separate analyses on males and females). Lastly, there was also no relationship between the phylogenetic distance between species and any similarity in their morphological complexity (Mantel test: p > 0.05 for separate analyses on males and females). Therefore, whereas the external appearance of the glandular field suggests something unusual about the role of female scent marking in this clade, more detailed chemical information is required to examine the functional significance of scent signals.
(b). Glandular differences in the chemical composition of scent signals
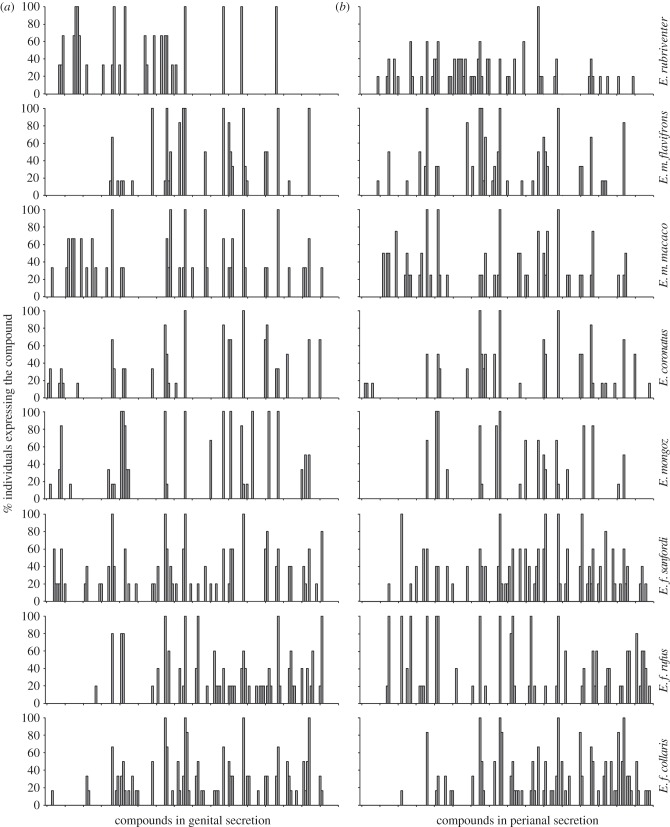
Across species, the total number of compounds we detected by GC/MS in both types of secretions was 158, with 113 compounds being expressed in the genital secretions and 129 compounds being expressed in the perianal secretions (figure 2). Of the total 158 compounds, 84 (53%) were present in both types of secretions, whereas the remaining 74 (47%) compounds were specific to one type of secretion. Using LDA on the subset of compounds present in all scent samples (n = 83), we significantly discriminated between genital and perianal secretions (Wilks' lambda = 0.47, p = 0.015; figure 3). Thus, as predicted, each glandular field expresses distinctive secretions that contribute a unique set of chemicals to the anogenital scent marks of Eulemur.
Figure 2.
Histograms of the percentage of individuals, by species of Eulemur, expressing each of 113 or 129 chemical compounds detected in (a) genital and (b) perianal secretions, respectively. The compounds on the x-axes are ordered by increasing retention times.
Figure 3.
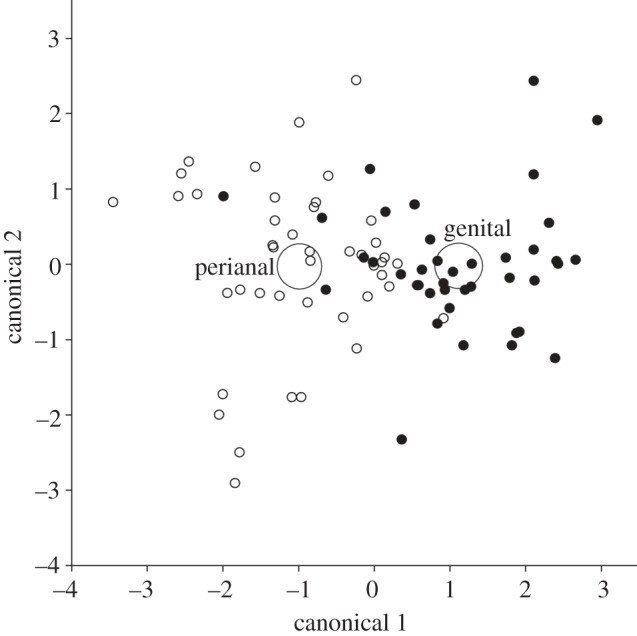
Patterns in the chemical composition of genital (filled circles) and perianal (open circles) secretions across Eulemur species. The two large circles correspond to the 95% confidence limits for the means of the genital and perianal secretions. The x- and y-axes are isometric.
To further explore this issue in individual lemurs, we used the subset of paired samples collected from the same animals on the same day. We found that, across species, the chemical diversity indices were similar for the two types of secretion (paired t-tests: richness index, t36 = −0.61, p = 0.55; Shannon index, t36 = 0.34, p = 0.73; Simpson index, t36 = 0.02, p = 0.99). We also found a positive correlation between the diversity indices of the genital and perianal secretions from the same individuals (Pearson's correlation: richness index, r = 0.55, n = 37, p < 0.0005; Shannon index, r = 0.6, n = 37, p < 0.0005; Simpson index, r = 0.71, n = 37, p < 0.0005). Thus, for any given species or individual, chemical investment in one secretion was associated with comparable investment in the other secretion. Moreover, the chemical distances between the genital and perianal secretions for each individual were significantly different from zero (one-sample t-test: t36 = 6.9, p < 0.0005), indicating that the two types of secretions had different chemical compositions. Thus, an animal's genital and perianal secretions were comparably diverse, but chemically distinct. We therefore treat the two types of secretions separately in all subsequent analyses.
(c). Reproductive signalling: sex and seasonal differences in the chemical composition of secretions
For both types of secretions, the mean numbers of compounds expressed by males and females are summarized in table 1. Although each species had compounds that were jointly expressed by both sexes and compound richness did not differ by sex (paired t-tests: p > 0.05 for separate analyses of the genital and perianal secretions), certain compounds were uniquely expressed by one sex only. These latter data are consistent with sex-specific signatures; however, we used PCA/LDA to further test for reproductive signalling.
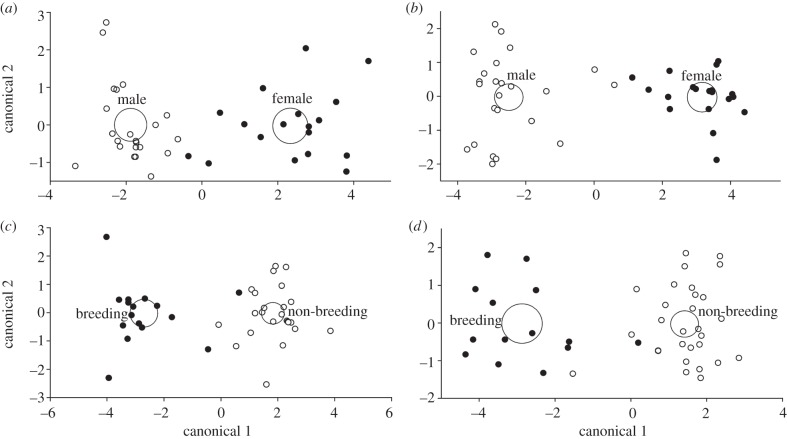
As predicted, we found significant separation of genital (Wilks' lambda = 0.18, p = 0.01; figure 4a) and perianal (Wilks' lambda = 0.11, p = 0.004; figure 4b) secretions based on sex. Likewise, we found significant separation of genital secretions based on season (Wilks' lambda = 0.16, p = 0.005; figure 4c), but statistically unreliable separation of perianal secretions based on season (Wilks' lambda = 0.19, p = 0.071, n.s.; figure 4d). Thus, Eulemur scent signals contain chemical information that could allow members to differentiate between the sex and broad reproductive state of conspecifics.
Figure 4.
(a,b) Sex (female, filled circles; male, open circles) and (c,d) seasonal (breeding, filled circles; non-breeding, open circles) differences in the chemical composition of (a,c) genital and (b,d) perianal secretions. Large circles represent the 95% confidence limits of the means. The x- and y-axes in each panel are isometric.
(d). Social complexity and the richness of chemical signals
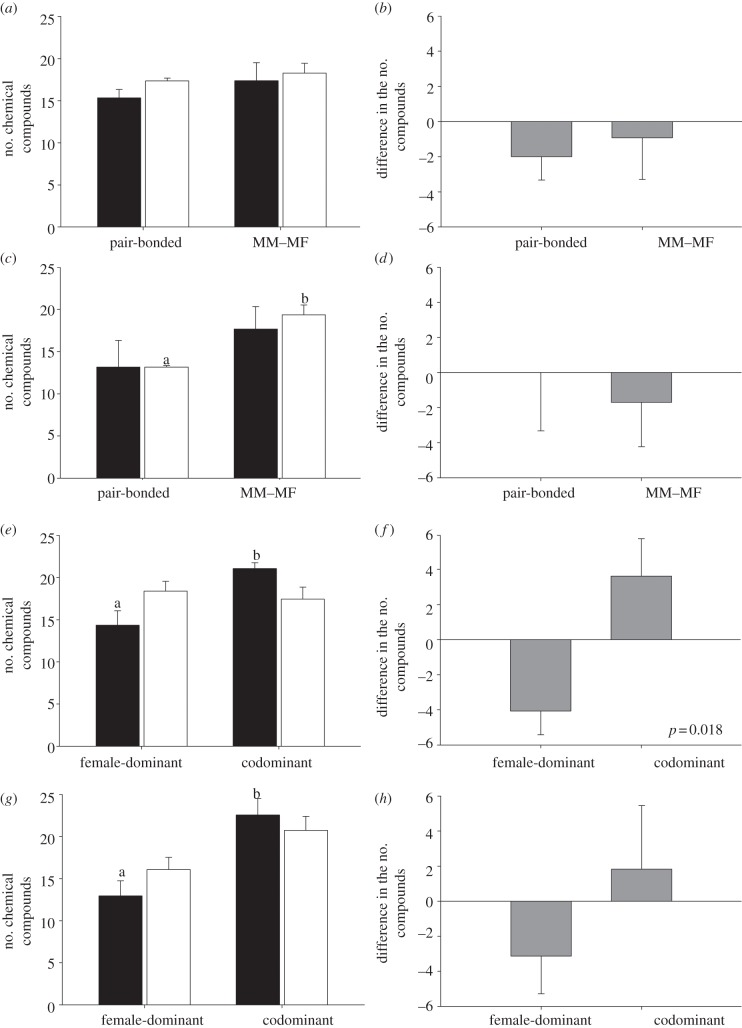
In comparing MM–MF and pair-bonded species, we found no relation between the type of social system and genital secretions in either sex (independent t-tests: p > 0.05 for separate analyses for males and females; figure 5a) or perianal secretions in males (p > 0.05; figure 5c); however, in support of the ‘social complexity hypothesis’, the chemical richness of female perianal secretions was greater in species with MM–MF social systems than in pair-bonded social systems (t6 = –5.21, p = 0.003; figure 5c). In both MM–MF species and pair-bonded species, females tended to produce more compounds than did males, but not reliably so (p > 0.05 for separate analyses considering genital and perianal secretions; figures 5b,d).
Figure 5.
Relation between (a–d) social system or (e–h) dominance structure and (a,b,e and f) the chemical richness of genital and (c,d,g and h) perianal secretions in Eulemur. Shown in the left panel is male (filled bars) and female (open bars) richness; shown in the right panel is the sex difference in richness, with positive values indicating a male bias and negative values indicating a female bias. Social systems include pair-bonded and multimale–multifemale (MM–MF); dominance structures include female-dominant and codominant. Bars and whiskers represent means and standard errors, respectively. Different letters within a panel denote a statistically significant difference.
In examining effects of social structure, we found that male chemical richness was greater in codominant species than in female-dominant species, both for genital (t6 = –2.87, p = 0.029; figure 5e) and perianal (t6 = –3.43, p = 0.01; figure 5g) secretions. Chemical richness in females, however, did not differ between female-dominant and codominant species for either secretion (p > 0.05; figure 5e,g). Interestingly, sex differences in chemical richness were reversed between the two types of social structures, with a greater male bias in codominant species, but a greater relative female bias in female-dominant species. This pattern was statistically significant for genital secretions (t6 = –3.21, p = 0.018; figure 5f), but not reliable for perianal secretions (p = 0.25; figure 5h).
(e). Species signatures and isolating mechanisms: pairwise chemical distances in relation to pairwise phylogenetic and geographical distances
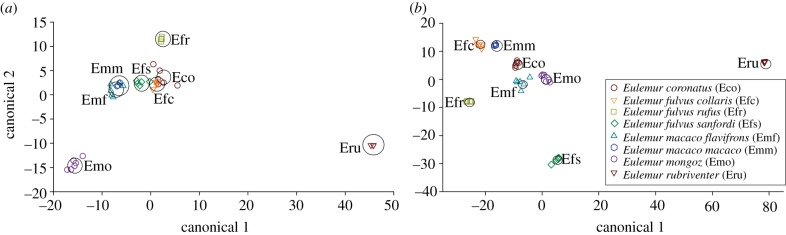
By species, the percentage of conspecifics expressing each of the 158 compounds in either their genital or perianal secretions is shown in figure 2. For each secretion type, the majority of the expressed compounds was present in only one species (figure 6). These data illustrate that each species expressed a unique set of compounds, consistent with species-specific scent signatures; however, we further tested for species signatures with PCA/LDA, which showed a significant separation of genital (Wilks' lambda = 7.06 × 10–10, p < 0.0001; figure 7a) and perianal (Wilks' lambda = 3.85 × 10−12, p < 0.0001; figure 7b) compounds based on species. Thus, Eulemur scent signals contain chemical information that could allow differentiating between species.
Figure 6.
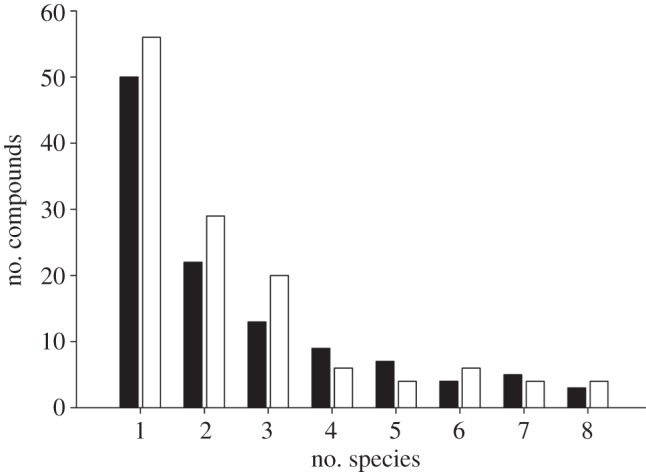
Number of species expressing the different chemical compounds detected in the genital (filled bars) and perianal (open bars) secretions of Eulemur.
Figure 7.
Species differences in the chemical composition of (a) genital and (b) perianal secretions. Large circles represent the 95% confidence limits of the means. The x- and y-axes in each panel are isometric.
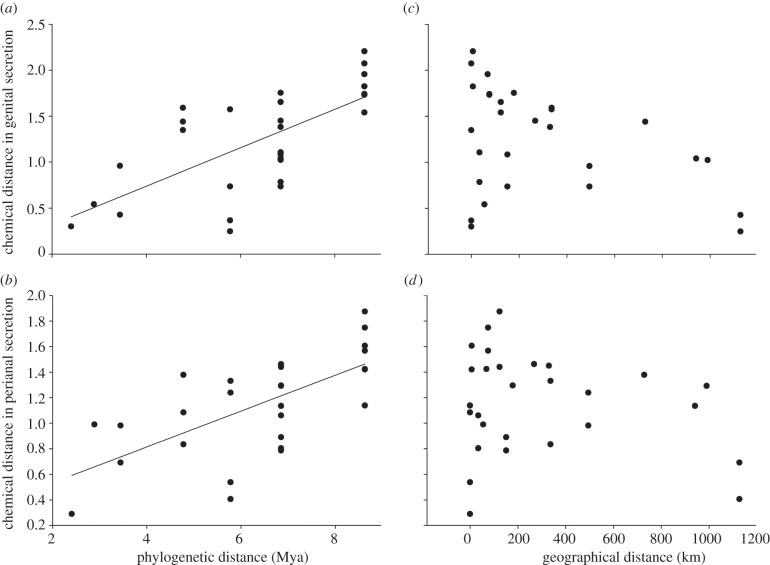
We further probed whether these species differences reflected phylogenetic placement (and hence mode of evolution) or geographical distribution. We found a positive correlation between chemical distance and phylogenetic distance between pairs of species, for both genital (r = 0.69, p = 0.009; figure 8a) and perianal (r = 0.66, p = 0.006; figure 8b) secretions. By contrast, we did not find any relationship between chemical distance and geographical distance between pairs of species, for either genital (r = −0.39, p = 0.12; figure 8c) or perianal (r = −0.21, p = 0.36; figure 8d) secretions. Therefore, chemical distances between species were better predicted by their phylogenetic distances than by their geographical distances.
Figure 8.
Relationship between the chemical distance between pairs of Eulemur species and their (a,b) phylogenetic distance versus (c,d) their geographical distance. Shown are data derived for (a,c) genital secretions and (b,d) perianal secretions. Each dot is the value of a pairwise difference and thus these figures are only graphical approximations of the Mantel tests that we performed. Lines indicate the effect of statistically significant relationships.
4. Discussion
In one of the few comparative studies of mammalian chemical signals—the first to be focused on a group of closely related species—we uncovered rich information content in Eulemur genital and perianal secretions. As in other mammals, including other strepsirrhine primates, the volatile compounds expressed in Eulemur glandular secretions contained various information about the individual signaller, including gland of origin, sex and breeding status [8], as well as information about the species' identity and phylogenetic placement [14]. Despite their close relatedness, the chemical distance between Eulemur species increased with their phylogenetic distance, supporting a gradual mode of signal evolution, even in the relatively early stages of divergence within this primate clade. More unusually, however, we found that facets of Eulemur social complexity, including type of mating system and dominance structure, were also chemically encoded within their glandular signals. These latter findings provide the first evidence that group living may have selected for increased complexity in olfactory communication.
Unlike most anthropoid primates (or indeed most other mammals), Malagasy lemurs show varying degrees of female ‘masculinization’ [62] evidenced behaviourally by female aggression or social dominance over males [63,64] and anatomically by unusual genital features [42,65]. Here, we see that female masculinization is additionally revealed in glandular morphology and semiochemistry, providing partial support for our first hypothesis. Notably, we detected greater morphological elaboration in the anogenital glands of female than male Eulemur. As anatomical complexity did not predict chemical complexity, however, it remains unclear why female glandular fields would be more elaborate than those of males. Perhaps the extensive perianal folds increase secretory surface and are implicated in enhanced production rate (which we could not measure) more so than enhanced chemical diversity. Indeed, ongoing comparative research shows pronounced scent-marking behaviour in female, relative to male, Eulemur (J. Petty and C. M. Drea 2011, unpublished observations), which might require greater odourant production rates in females.
Despite glandular elaboration, we found that relatively few compounds were expressed in the genital and perianal secretions of the various Eulemur species, regardless of sex. At most, we detected 27 compounds in the genital secretions of a male E. f. collaris. By comparison, male and female L. catta express an average of 135 and 218 compounds, respectively, in their genital secretions [20]. Although a generalized reduction in chemical richness may have occurred in Eulemur, a more plausible alternative involves an ancestral condition of chemical simplicity in the Lemur–Eulemur clade, followed by a dramatic increase in chemical richness in L. catta that possibly mirrored the latter's increased sociality.
The relative chemical simplicity of Eulemur genital and perianal secretions notwithstanding, both types of secretions were chemically distinctive, despite the close physical proximity of the two glands. Male L. catta also express distinctive secretions from their various glands [8], but compared with Eulemur, glandular ‘signatures’ are more readily anticipated in L. catta, given the more widely diverse glandular anatomy and secretory features [58]. Interestingly, male L. catta mix certain secretions prior to scent signalling, potentially to increase the longevity of the signal [8]. Likewise, scent marking in Eulemur presumably involves mixing of the distinct genital and perianal secretions [43,47], possibly to produce a more complex and informative signal. Given the positive correlations we found between the two types of secretions in both the richness and diversity of their chemical content, individuals invested similarly in their different secretions, even if the overall investment may have differed among individuals. These patterns suggest that odour cues in Eulemur may also convey unique information about the individual, such as identity [8] or genetic quality [10,11]; however, these questions were beyond the scope of the present study.
Consistent with the findings in a range of other mammals [66–69], we found full support for our second hypothesis, namely that olfactory cues in Eulemur function in sexual or reproductive advertisement. Notably, across all species studied, both types of glandular secretions differed significantly by sex and season. This chemically encoded information appears to be discernable to conspecifics, as certain Eulemur have been shown to discriminate between male and female odours [70].
More exceptionally, we also found support for our third hypothesis that social complexity drives chemical complexity. Notably, although the chemical richness of male secretions was not tied to the type of social system, females in MM–MF species had richer chemical profiles in their perianal secretions than did females in pair-bonded species. As we have previously suggested [14], increased chemical richness possibly facilitates the increased transfer of information that is required in socially complex groups.
Also as predicted for our third hypothesis, the chemical richness of genital secretions was greater in males than in females in codominant species (as would normally be the case in many mammals), but greater in females than in males in female-dominant species. This reversal of the traditional sex difference in olfactory cues in female-dominant species was mostly driven by species differences between males, given that chemical richness in males was greater in codominant species than in female-dominant species, but did not differ between females by type of dominance structure. In L. catta, a species in which females clearly dominate males [71–73], females likewise express more volatile compounds in their genital secretions than do conspecific males [8,20]. Thus, relative to other mammals, masculinization in strepsirrhine females [42,62,65] may be associated with a more pronounced role for female olfactory communication. Although this enhanced olfactory role in females may be reflected in greater glandular elaboration and/or greater chemical complexity of certain olfactory signals [8,20], it is important to recognize that we have characterized only a subset of all available odourants for any given species and that males sometimes possess glands that are absent in females [8].
We also found support for our final hypothesis that olfactory cues provide a mechanism for species isolation. As predicted, the eight Eulemur species examined differed significantly from one another in the chemical composition of both their genital and perianal secretions. These species differences in chemical composition agree with behavioural studies in Eulemur showing that individuals discriminate between conspecific and heterospecific odours [74]. Although all of the Eulemur species showed ‘signatures’, those of E. rubriventer, in particular, and also of E. mongoz, were the most different from the other species. Interestingly, whereas most Eulemur species have a MM–MF social system, both E. rubriventer [39,40] and E. mongoz [38] form pair bonds and are presumably monogamous. Perhaps monogamy played a role in the differentiation of their signals. Alternately, the unique chemical composition of E. rubriventer secretions may have been phylogenetically driven. In a recent and well-resolved Eulemur phylogeny [55], the oldest branching event in the clade separates E. rubriventer from the other Eulemur species. Indeed, phylogenetic distance between species significantly predicted the degree to which their chemical profiles differed, with more distantly related species showing the greatest chemical divergence. In contrast, we did not find that sympatric or neighbouring species had more divergent chemical profiles than did allopatric or geographically distant species. Nonetheless, given the dramatic impact that human arrival had on Malagasy ecosystems, current species distributions may differ substantially from their historical distributions [75].
Lastly, as we previously proposed for strepsirrhine urinary signals [14], the relationship between chemical distance and phylogenetic distance for these glandular signals is consistent with a gradual, as opposed to saltational, mode of evolution [28]. An argument against a gradual mode of evolution would be that species-specific signals should be under stabilizing selection against changes in the signal, as small deviations may render the signal ineffective [28,76]; however, in mammalian species in which chemical signals consist of a blend of many compounds, there may be gradual changes in the relative abundance or presence/absence of particular compounds without loss of information from the signal as a whole. Gradual evolution of chemical signals also has been proposed for Drosophila species, for which an accumulation of gradual changes over evolutionary time would not negatively affect the functionality of the signal [28,77].
In summary, we have shown that, despite logistical hurdles, the comparative approach can be a powerful tool in olfactory research. Here, we have used this approach to reveal consistent chemical patterns within and across species that are relevant to understanding current function, such as sex and seasonal differences underlying the signalling of reproductive state. Likewise, we have shown consistent differences between species, such as would constitute the basis of their ‘scent signatures’, that are relevant to understanding olfactory mechanisms of species isolation. Further comparison of these species' chemical profiles within a phylogenetic context have provided an insight into the processes of signal evolution, revealing that the olfactory signals of lemurs have evolved gradually, whether we look over long stretches [14] or relatively short periods of evolutionary time. Lastly, this approach has allowed us to explore, for the first time, how aspects of primate social complexity might have driven the complexity of olfactory signals. On the one hand, lemur olfactory signals may have been selected for enhanced information transfer, in a manner comparable to primate vocal, gestural and facial signals. On the other hand, lemur olfactory signals may be more uniquely linked to the unusual evolution of female-dominant social systems.
Acknowledgements
Our procedures were in accordance with regulations of the United States Department of Agriculture and the research protocols were approved by the Institutional Animal Care and Use Committee of Duke University (protocol nos. A156–04–05 and A245–03–07).
We are grateful to the DLC staff, particularly D. Brewer, J. Taylor, B. Schopler, DVM, C. Williams, DVM, and S. Zehr, PhD, for providing access to the animals and their records, as well as for assistance with animal handling. Funding was provided by National Science Foundation research grants BCS-0409367 and IOS-0719003 (to C.M.D.). This is DLC publication no. 1219.
References
- 1.Marler P. 1977. The evolution of communication. In How animals communicate (ed. Sebeok T. A.), pp. 45–70 Bloomington, IN: Indiana University Press [Google Scholar]
- 2.McComb K., Semple S. 2005. Coevolution of vocal communication and sociality in primates. Biol. Lett. 1, 381–385 10.1098/rsbl.2005.0366 (doi:10.1098/rsbl.2005.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumstein D. T., Armitage K. B. 1997. Does sociality drive the evolution of communicative complexity? A comparative test with ground-dwelling sciurid alarm calls. Am. Nat. 150, 179–200 10.1086/286062 (doi:10.1086/286062) [DOI] [PubMed] [Google Scholar]
- 4.Brown R. E., Macdonald D. W. 1985. Social odours in mammals. Oxford, UK: Oxford University Press [Google Scholar]
- 5.Johnston R. E., delBarco-Trillo J. 2009. Communication by chemical signals: behavior, social recognition, hormones and the role of the vomeronasal and olfactory systems. In Hormones, brain and behavior (eds Pfaff D. W., Arnold A. P., Etgen A. M., Rubin R. T., Fahrbach S. E.), pp. 395–440, 2nd edn. New York, NY: Elsevier [Google Scholar]
- 6.Eisenberg J. F., Kleiman D. G. 1972. Olfactory communication in mammals. Annu. Rev. Ecol. Syst. 3, 1–32 10.1146/annurev.es.03.110172.000245 (doi:10.1146/annurev.es.03.110172.000245) [DOI] [Google Scholar]
- 7.Belcher A. M., Smith A. B. I., Jurs P. C., Lavine B., Epple G. 1986. Analysis of chemical signals in a primate species (Saguinus fuscicollis): use of behavioral, chemical and pattern recognition methods. J. Chem. Ecol. 12, 513–531 10.1007/BF01020570 (doi:10.1007/BF01020570) [DOI] [PubMed] [Google Scholar]
- 8.Scordato E. S., Dubay G., Drea C. M. 2007. Chemical composition of scent marks in the ringtailed lemur (Lemur catta): glandular differences, seasonal variation, and individual signatures. Chem. Senses 32, 493–504 10.1093/chemse/bjm018 (doi:10.1093/chemse/bjm018) [DOI] [PubMed] [Google Scholar]
- 9.Smith T. E., Tomlinson A. J., Mlotkiewicz J. A., Abbott D. H. 2001. Female marmoset monkeys (Callithrix jacchus) can be identified from the chemical composition of their scent marks. Chem. Senses 26, 449–458 10.1093/chemse/26.5.449 (doi:10.1093/chemse/26.5.449) [DOI] [PubMed] [Google Scholar]
- 10.Charpentier M. J. E., Boulet M., Drea C. M. 2008. Smelling right: the scent of male lemurs advertises genetic quality and relatedness. Mol. Ecol. 17, 3225–3233 10.1111/j.1365-294X.2008.03831.x (doi:10.1111/j.1365-294X.2008.03831.x) [DOI] [PubMed] [Google Scholar]
- 11.Boulet M., Crawford J., Charpentier M. J. E., Drea C. M. 2010. Honest olfactory ornamentation in a female-dominant primate. J. Evol. Biol. 23, 1558–1563 10.1111/j.1420-9101.2010.02007.x (doi:10.1111/j.1420-9101.2010.02007.x) [DOI] [PubMed] [Google Scholar]
- 12.Penn D., Potts W. K. 1998. Chemical signals and parasite-mediated sexual selection. Trends Ecol. Evol. 13, 391–396 10.1016/S0169-5347(98)01473-6 (doi:10.1016/S0169-5347(98)01473-6) [DOI] [PubMed] [Google Scholar]
- 13.delBarco-Trillo J., Gulewicz K., Johnston R. E. 2009. Medial amygdala involvement in discrimination of same-species and closely-related-species male stimuli in estrous female Mesocricetus hamsters. Behav. Neurosci. 123, 758–763 10.1037/a0016439 (doi:10.1037/a0016439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.delBarco-Trillo J., Burkert B. A., Goodwin T. E., Drea C. M. 2011. Night and day: the comparative study of strepsirrhine primates reveals socioecological and phylogenetic patterns in olfactory signals. J. Evol. Biol. 24, 82–98 10.1111/j.1420-9101.2010.02145.x (doi:10.1111/j.1420-9101.2010.02145.x) [DOI] [PubMed] [Google Scholar]
- 15.Mykytowycz R. 1968. Territorial marking by rabbits. Sci. Am. 218, 116–126 [DOI] [PubMed] [Google Scholar]
- 16.Hurst J. L., Beynon R. J. 2004. Scent wars: the chemobiology of competitive signalling in mice. Bioessays 26, 1288–1298 10.1002/bies.20147 (doi:10.1002/bies.20147) [DOI] [PubMed] [Google Scholar]
- 17.Safi K., Kerth G. 2003. Secretions of the interaural gland contain information about individuality and colony membership in the Bechstein's bat. Anim. Behav. 65, 363–369 10.1006/anbe.2003.2067 (doi:10.1006/anbe.2003.2067) [DOI] [Google Scholar]
- 18.Harvey S., Jemiolo B., Novotny M. 1989. Pattern of volatile compounds in dominant and subordinate male-mouse urine. J. Chem. Ecol. 15, 2061–2072 10.1007/BF01207438 (doi:10.1007/BF01207438) [DOI] [PubMed] [Google Scholar]
- 19.Mateo J. M., Johnston R. E. 2000. Kin recognition and the ‘armpit effect’: evidence of self-referent phenotype matching. Proc. R. Soc. Lond. B 267, 695–700 10.1098/rspb.2000.1058 (doi:10.1098/rspb.2000.1058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulet M., Charpentier M. J. E., Drea C. M. 2009. Decoding an olfactory mechanism of kin recognition and inbreeding avoidance in a primate. BMC Evol. Biol. 9, 281. 10.1186/1471-2148-9-281 (doi:10.1186/1471-2148-9-281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller-Schwarze D. 2006. Chemical ecology of vertebrates. New York, NY: Cambridge University Press [Google Scholar]
- 22.Wyatt T. D. 2003. Pheromones and animal behaviour. Communication by smell and taste . Cambridge, UK: Cambridge University Press [Google Scholar]
- 23.Soini H. A., Schrock S. E., Bruce K. E., Wiesler D., Ketterson E. D., Novotny M. V. 2007. Seasonal variation in volatile compound profiles of preen gland secretions of the dark-eyed junco (Junco hyemalis). J. Chem. Ecol. 33, 183–198 10.1007/s10886-006-9210-0 (doi:10.1007/s10886-006-9210-0) [DOI] [PubMed] [Google Scholar]
- 24.Zhang J. X., Soini H. A., Bruce K. E., Wiesler D., Woodley S. K., Baum M. J., Novotny M. V. 2005. Putative chemosignals of the ferret (Mustela furo) associated with individual and gender recognition. Chem. Senses 30, 727–737 10.1093/chemse/bji065 (doi:10.1093/chemse/bji065) [DOI] [PubMed] [Google Scholar]
- 25.Albone E. S. 1984. Mammalian semiochemistry. The investigation of chemical signals between mammals . Chichester, UK: Wiley [Google Scholar]
- 26.Novotny M. V., Soini H. A. 2008. Volatile mammalian chemosignals: structural and quantitative aspects. In Chemical signals in vertebrates 11 (eds Hurst J. L., Beynon R. J., Roberts S. C., Wyatt T. D.), pp. 13–23 New York, NY: Springer [Google Scholar]
- 27.Burger B. V. 2005. Mammalian semiochemicals. Top. Curr. Chem. 240, 231–278 10.1007/b98318 (doi:10.1007/b98318) [DOI] [Google Scholar]
- 28.Symonds M. R. E., Elgar M. A. 2008. The evolution of pheromone diversity. Trends Ecol. Evol. 23, 220–228 10.1016/j.tree.2007.11.009 (doi:10.1016/j.tree.2007.11.009) [DOI] [PubMed] [Google Scholar]
- 29.Roelofs W. L., Brown R. L. 1982. Pheromones and evolutionary relationships of Tortricidae. Annu. Rev. Ecol. Syst. 13, 395–422 10.1146/annurev.es.13.110182.002143 (doi:10.1146/annurev.es.13.110182.002143) [DOI] [Google Scholar]
- 30.Jürgens A., Feldhaar H., Feldmeyer B., Fiala B. 2006. Chemical composition of leaf volatiles in Macaranga species (Euphorbiaceae) and their potential role as olfactory cues in host-localization of foundress queens of specific ant partners. Biochem. Syst. Ecol. 34, 97–113 10.1016/j.bse.2005.08.005 (doi:10.1016/j.bse.2005.08.005) [DOI] [Google Scholar]
- 31.Magro A., Ducamp C., Ramon-Portugal F., Lecompte E., Crouau-Roy B., Dixon A., Hemptinne J.-L. 2010. Oviposition deterring infochemicals in ladybirds: the role of phylogeny. Evol. Ecol. 24, 251–271 10.1007/s10682-009-9304-6 (doi:10.1007/s10682-009-9304-6) [DOI] [Google Scholar]
- 32.Symonds M. R. E., Moussalli A., Elgar M. A. 2009. The evolution of sex pheromones in an ecologically diverse genus of flies. Biol. J. Linnean Soc. 97, 594–603 10.1111/j.1095-8312.2009.01245.x (doi:10.1111/j.1095-8312.2009.01245.x) [DOI] [Google Scholar]
- 33.Löfstedt C., Herrebout W. M., Menken S. B. J. 1991. Sex pheromones and their potential role in the evolution of reproductive isolation in small ermine moths (Yponomeutidae). Chemoecology 2, 20–28 10.1007/bf01240662 (doi:10.1007/bf01240662) [DOI] [Google Scholar]
- 34.Martin S. J., Carruthers J. M., Williams P. H., Drijfhout F. P. 2010. Host specific social parasites (Psithyrus) indicate chemical recognition system in bumblebees. J. Chem. Ecol. 36, 855–863 10.1007/s10886-010-9805-3 (doi:10.1007/s10886-010-9805-3) [DOI] [PubMed] [Google Scholar]
- 35.Martin S. J., Helanterä H., Drijfhout F. P. 2008. Evolution of species-specific cuticular hydrocarbon patterns in Formica ants. Biol. J. Linnean Soc. 95, 131–140 10.1111/j.1095-8312.2008.01038.x (doi:10.1111/j.1095-8312.2008.01038.x) [DOI] [Google Scholar]
- 36.Bininda-Emonds O. R. P., Decker-Flum D. M., Gittleman J. L. 2001. The utility of chemical signals as phylogenetic characters: an example from the Felidae. Biol. J. Linnean Soc. 72, 1–15 10.1111/j.1095-8312.2001.tb01297.x (doi:10.1111/j.1095-8312.2001.tb01297.x) [DOI] [Google Scholar]
- 37.Zabaras R., Richardson B. J., Wyllie S. G. 2005. Evolution in the suite of semiochemicals secreted by the sternal gland of Australian marsupials. Aust. J. Zool. 53, 257–263 10.1071/ZO04070 (doi:10.1071/ZO04070) [DOI] [Google Scholar]
- 38.Curtis D. J., Zaramody A. 1999. Social structure and seasonal variation in the behaviour of Eulemur mongoz. Folia Primatol. 70, 79–96 10.1159/000021679 (doi:10.1159/000021679) [DOI] [PubMed] [Google Scholar]
- 39.Overdorff D. J., Tecot S. R. 2006. Social pair-bonding and resource defense in wild red-bellied lemurs (Eulemur rubriventer). In Lemurs: ecology and adaptation (eds Gould L., Sauther M. L.), pp. 235–254 New York, NY: Springer [Google Scholar]
- 40.Marolf B., McElligott A. G., Müller A. E. 2007. Female social dominance in two Eulemur species with different social organizations. Zoo Biol. 26, 201–214 10.1002/zoo.20135 (doi:10.1002/zoo.20135) [DOI] [PubMed] [Google Scholar]
- 41.Roeder J.-J., Fornasieri I. 1995. Does agonistic dominance imply feeding priority in lemurs? A study in Eulemur fulvus mayottensis. Int. J. Primatol. 15, 629–642 10.1007/bf02735285 (doi:10.1007/bf02735285) [DOI] [Google Scholar]
- 42.Hill W. C. O. 1953. Primates: comparative anatomy and taxonomy. I. Strepsirhini. London, UK: Edinburgh University Press [Google Scholar]
- 43.Chandler C. F. 1975. Development and function of marking and sexual behaviour in the Malagasy prosimian primate, Lemur fulvus. Primates 16, 35–47 10.1007/BF02381798 (doi:10.1007/BF02381798) [DOI] [Google Scholar]
- 44.Kappeler P. M. 1988. A preliminary study of olfactory behavior of captive Lemur coronatus during the breeding season. Int. J. Primatol. 9, 135–146 10.1007/BF02735733 (doi:10.1007/BF02735733) [DOI] [Google Scholar]
- 45.Tattersall I., Sussman R. W. 1975. Observations on the ecology and behaviour of the mongoose lemur Lemur mongoz mongoz Linneaus (Primates, Lemuriformes) at Ampijoroa, Madagascar. Anthropol. Pap. Am. Mus. Nat. Hist. 52, 195–216 [Google Scholar]
- 46.Harrington J. E. 1978. Diurnal behavior of Lemur mongoz at Ampijoroa, Madagascar. Folia Primatol. 29, 291–302 10.1159/000155848 (doi:10.1159/000155848) [DOI] [PubMed] [Google Scholar]
- 47.Fornasieri I., Roeder J.-J. 1992. Marking behaviour in two lemur species (L. fulvus and L. macaco): relation to social status, reproduction, aggression and environmental change. Folia Primatol. 59, 137–148 10.1159/000156651 (doi:10.1159/000156651) [DOI] [PubMed] [Google Scholar]
- 48.Gröning J., Hochkirch A. 2008. Reproductive interference between animal species. Q. Rev. Biol. 83, 257–282 10.1086/590510 (doi:10.1086/590510) [DOI] [PubMed] [Google Scholar]
- 49.Smadja C., Butlin R. K. 2009. On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102, 77–97 10.1038/hdy.2008.55 (doi:10.1038/hdy.2008.55) [DOI] [PubMed] [Google Scholar]
- 50.Digby L., Kahlenberg S. 2002. Female dominance in blue-eyed black lemurs (Eulemur macaco flavifrons). Primates 43, 191–199 10.1007/bf02629647 (doi:10.1007/bf02629647) [DOI] [PubMed] [Google Scholar]
- 51.Colquhoun I. C. 1993. The socioecology of Eulemur macaco: a preliminary report. In Lemur social systems and their ecological basis (eds Kappeler P. M., Ganzhorn J. U.), pp. 11–23 New York, NY: Plenum Press [Google Scholar]
- 52.Pereira M. E., McGlynn C. A. 1997. Special relationships instead of female dominance for redfronted lemurs, Eulemur fulvus rufus. Am. J. Primatol. 43, 239–258 (doi:10.1002/(SICI)1098-2345(1997)43:3<239::AID-AJP4>3.0.CO;2-Y) [DOI] [PubMed] [Google Scholar]
- 53.Pereira M. E., Kaufman R., Kappeler P. M., Overdorff D. J. 1990. Female dominance does not characterize all of the Lemuridae. Folia Primatol. 55, 96–103 10.1159/000156505 (doi:10.1159/000156505) [DOI] [PubMed] [Google Scholar]
- 54.Kaufman R. 1996. The nature and frequency of agonism in free-ranging and semi-free-ranging brown lemurs, Eulemur fulvus. Primates 37, 335–350 10.1007/bf02381371 (doi:10.1007/bf02381371) [DOI] [Google Scholar]
- 55.Horvath J. E., Weisrock D. W., Embry S. L., Fiorentino I., Balhoff J. P., Kappeler P., Wray G. A., Willard H. F., Yoder A. D. 2008. Development and application of a phylogenomic toolkit: resolving the evolutionary history of Madagascar's lemurs. Genome Res. 18, 489–499 10.1101/gr.7265208 (doi:10.1101/gr.7265208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mittermeier R. A., et al. 2008. Lemur diversity in Madagascar. Int. J. Primatol. 29, 1607–1656 10.1007/s10764-008-9317-y (doi:10.1007/s10764-008-9317-y) [DOI] [Google Scholar]
- 57.IUCN 2011. IUCN Red List of threatened species. Version 2011.1. See http://www.iucnredlist.org
- 58.Scordato E. S., Drea C. M. 2007. Scents and sensibility: information content of olfactory signals in the ringtailed lemur, Lemur catta. Anim. Behav. 73, 301–314 10.1016/j.anbehav.2006.08.006 (doi:10.1016/j.anbehav.2006.08.006) [DOI] [Google Scholar]
- 59.Crawford J. C., Boulet M., Drea C. M. 2011. Smelling wrong: hormonal contraception in lemurs alters critical female odour cues. Proc. R. Soc. B 278, 122–130 10.1098/rspb.2010.1203 (doi:10.1098/rspb.2010.1203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCune B., Grace J. B. 2002. Analysis of ecological communities. Gleneden Beach, OR: MJM Software Design [Google Scholar]
- 61.Mittermeier R. A., et al. 2006. Lemurs of Madagascar, 2nd edn. Washington, DC: Conservation International [Google Scholar]
- 62.Drea C. M. 2009. Endocrine mediators of masculinization in female mammals. Curr. Dir. Psychol. Sci. 18, 221–226 10.1111/j.1467-8721.2009.01640.x (doi:10.1111/j.1467-8721.2009.01640.x) [DOI] [Google Scholar]
- 63.Dunham A. E. 2008. Battle of the sexes: cost asymmetry explains female dominance in lemurs. Anim. Behav. 76, 1435–1439 10.1016/j.anbehav.2008.06.018 (doi:10.1016/j.anbehav.2008.06.018) [DOI] [Google Scholar]
- 64.Kappeler P. M. 1993. Female dominance in primates and other mammals. In Perspectives in ethology (eds Bateson P. P. G., Thompson N., Klopfer P.), pp. 143–158 New York, NY: Plenum Press [Google Scholar]
- 65.Drea C. M., Weil A. 2008. External genital morphology of the ring-tailed lemur (Lemur catta): females are naturally ‘masculinized’. J. Morphol. 269, 451–463 10.1002/jmor.10594 (doi:10.1002/jmor.10594) [DOI] [PubMed] [Google Scholar]
- 66.Hagey L., Macdonald E. 2003. Chemical cues identify gender and individuality in giant pandas (Ailuropoda melanoleuca). J. Chem. Ecol. 29, 1479–1488 10.1023/A:1024225806263 (doi:10.1023/A:1024225806263) [DOI] [PubMed] [Google Scholar]
- 67.Zhang J.-X., Sun L., Zhang J.-H., Feng Z.-Y. 2008. Sex- and gonad-affecting scent compounds and 3 male pheromones in the rat. Chem. Senses 33, 611–621 10.1093/chemse/bjn028 (doi:10.1093/chemse/bjn028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vogler B. R., Goeritz F., Hildebrandt T. B., Dehnhard M. 2008. Gender specific expression of volatiles in captive fossas (Cryptoprocta ferox) during the mating season. In Chemical signals in vertebrates 11 (eds Hurst J. L., Beynon R. J., Roberts S. C., Wyatt T. D.), pp. 161–168 New York, NY: Springer [Google Scholar]
- 69.Penn D. J., et al. 2007. Individual and gender fingerprints in human body odour. J. R. Soc. Interface 4, 331–340 10.1098/rsif.2006.0182 (doi:10.1098/rsif.2006.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrington J. E. 1977. Discrimination between males and females by scent in Lemur fulvus. Anim. Behav. 25, 147–151 10.1016/0003-3472(77)90077-X (doi:10.1016/0003-3472(77)90077-X) [DOI] [PubMed] [Google Scholar]
- 71.Kappeler P. M. 1990. Female dominance in Lemur catta: more than just female feeding priority? Folia Primatol. 55, 92–95 10.1159/000156504 (doi:10.1159/000156504) [DOI] [PubMed] [Google Scholar]
- 72.Jolly A. 1966. Lemur behaviour: a Madagascar field study. Chicago, IL: University of Chicago Press [Google Scholar]
- 73.Drea C. M. 2007. Sex and seasonal differences in aggression and steroid secretion in Lemur catta: are socially dominant females hormonally ‘masculinized’? Horm. Behav. 51, 555–567 10.1016/j.yhbeh.2007.02.006 (doi:10.1016/j.yhbeh.2007.02.006) [DOI] [PubMed] [Google Scholar]
- 74.Fornasieri I., Roeder J.-J. 1992. Behavioral responses to own and other species’ scent marks in Lemur fulvus and Lemur macaco. J. Chem. Ecol. 18, 2069–2082 10.1007/BF00981928 (doi:10.1007/BF00981928) [DOI] [PubMed] [Google Scholar]
- 75.Richard A. F., Deward R. E. 1991. Lemur ecology. Annu. Rev. Ecol. Syst. 22, 145–175 10.1146/annurev.es.22.110191.001045 (doi:10.1146/annurev.es.22.110191.001045) [DOI] [Google Scholar]
- 76.Symonds M. R. E., Elgar M. A. 2004. The mode of pheromone evolution: evidence from bark beetles. Proc. R. Soc. Lond. B 271, 839–846 10.1098/rspb.2003.2647 (doi:10.1098/rspb.2003.2647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Symonds M. R. E., Wertheim B. 2005. The mode of evolution of aggregation pheromones in Drosophila species. J. Evol. Biol. 18, 1253–1263 10.1111/j.1420-9101.2005.00971.x (doi:10.1111/j.1420-9101.2005.00971.x) [DOI] [PubMed] [Google Scholar]