Abstract
Streptococcus pneumoniae is the leading bacterial opportunistic infection in HIV-infected individuals. Anti-retroviral treatment (ART) of HIV-infected individuals reduces their risk of invasive pneumococcal disease (IPD), however, it remains 20- to 40-fold greater compared with age-matched general population. This review summarizes the available published data on the immunogenicity, safety and efficacy of pneumococcal polysaccharide-protein conjugate vaccines (PCV) in HIV-infected children and adults.
Several studies have demonstrated that PCV are safe in the HIV-infected persons. Although PCV are immunogenic in HIV-infected infants, the antibodies produced are functionally impaired, there is possibly a lack or loss of anamnestic responses and immunity declines in later life However, quantitative and qualitative antibody responses to PCV in HIV-infected infants are enhanced when vaccination occurs whilst on ART, as well as if vaccination occurs when the CD4+ cell percentage is ≥ 25% and if the nadir CD4+ is >15%. Although the efficacy of PCV was lower, the vaccine preventable burden of hospitalization for IPD and clinical pneumonia were 18-fold and 9-fold greater, respectively, in HIV-infected children compared with –uninfected children.
In HIV-infected adults, PCV vaccination induces more durable and functional antibody responses in individuals on ART at the time of vaccination than in ART-naive adults, independently of baseline CD4+ cell count, although there does not appear to be much benefit from a second-dose of PCV. PCV has also been shown to reduce the risk of recurrent IPD by 74% in HIV-infected adults not on ART, albeit, also with subsequent decline in immunity and protection.
Keywords: Streptococcus pneumoniae, HIV, immunogenicity, pneumococcal conjugate vaccine, pneumococcal disease
Introduction
Although less than one percent of the global under-5 y of age population are HIV-infected,1 these children account for 10.8% of the approximately 870 000 annual deaths attributed to Streptococcus pneumoniae, including 19.8% of pneumococcal deaths in African children.2 In the absence of antiretroviral treatment (ART), S. pneumoniae is the leading bacterial opportunistic infection with the risk of invasive pneumococcal disease (IPD) being 40-fold greater in HIV-infected children.3-5 In settings such as Southern Africa where the prevalence of HIV in children is less than 5% more than 65% of all IPD cases occur in HIV-infected children.3,5 Although the susceptibility to IPD is reduced by 41% in HIV-infected children when treated with ART, the risk nevertheless remains 21-fold (95% CI: 16 to 28) greater compared with HIV-uninfected children.3
Similarly HIV-infected adults have 10–300 times greater susceptibility to IPD compared with HIV-uninfected individuals6-10 and are at greater risk of recurrent IPD, with up to 25% of individuals having an additional episode within the next 12 mo.11,12 In HIV-infected adults the initiation of ART has been associated with marked reductions in morbidity and mortality from opportunistic infections, including two- to 3-fold reductions in the risk of IPD.7,10 Nevertheless, in the USA the incidence of IPD in HIV-infected adults in the era of ART continued to be approximately 35-fold greater than the general population.10
The increased susceptibility of HIV-infected individuals to pneumococcal disease in part relates to impairment of both cell-mediated and humoral arms of the immune system. An immunologic response to pneumococcal polysaccharides, a T-cell independent type antigen, elicits production of serotype-specific opsonic antibodies by B lymphocytes independent of T-lymphocyte interaction.13 Both T- and B- lymphocytes are decreased and function impaired in HIV-infected individuals.14,15 This results in impaired quantitative and qualitative antibody responses to natural infections and vaccination.16,17 ART partially reconstitutes the immune system of HIV-infected individuals, by increasing B- and T- lymphocyte number and functionality. However, deficiencies in humoral response because of depleted or persistent defects in memory cell function persist after ART initiation.18
Vaccines available to protect against pneumococcal disease include a 23-valent pneumococcal polysaccharide vaccines (PPV) and polysaccharide-protein conjugate vaccines (PCV). PPV is licensed for use in adults and children older than 2 y; and particularly recommended for elderly persons and others with specified underlying medical conditions.19 In adults PPV reduces the risk of IPD and in some studies decreased the risk of pneumonia.20-22 However drawbacks of PPV vaccination include that vaccine-induced antibody concentrations declined within 1–2 y post-vaccination.23,24 In addition, PPV being processed as a T-cell independent antigen does not prime for anamnestic responses, is dominated by an IgM antibody response and may result in hypo-responsiveness following subsequent doses of vaccine.25-27 PPV vaccination in children has also not consistently being associated with a reduction in risk of nasopharyngeal colonization with vaccine-serotype pneumococci.28 In young children, PPV is associated with poor immunogenicity, especially for serotypes causing the majority of childhood pneumococcal disease, due to immaturity of the T-cell independent immune system in these children.29
PCV induces a T-cell dependent immune response, which matures while in utero, and has an improved immunogenicity profile including in groups of individuals at high risk of IPD.30-32 Currently there are three licensed PCV formulation for use in children and adolescents, including 7-valent [PCV7, Prev(e)nar™; Pfizer Inc.], 10-valent (PCV10, Synflorix™; GlaxoSmithKline) and 13-valent (Prevenar13™, Pfizer Inc.)
Previous reviews on PCV in adults has had limited emphasis to HIV-infected individuals.33,34 In addition the last review of PCV in HIV-infected children was reported in 2008,35 since when there have been a number of new studies in HIV-infected children. This review provides and updated analysis on the safety, efficacy and immunogenicity of PCV in HIV-infected individuals.
Methods
Data for this review were identified by doing a literature search on PubMed using combinations of the following search terms: “pneumococcus,” “pneumococcal,” “Streptococcus pneumoniae,” “pneumococcal conjugate vaccine,” “HIV,” “conjugate vaccine,” “immunogenicity,” “efficacy” and “safety.” Only English language studies were reviewed, no date restrictions were set and no attempt at statistical analysis was undertaken.
Results
Measures of immunogenicity of PCV in children and adults
The benchmark for measuring the immunogenicity of new formulations of PCV is based on recommendations of a WHO working group.36 This includes the proportion of subjects who attain serotype-specific antibody concentration of ≥0.35 μg/ml measured by enzyme-linked immunosorbent assay (ELISA) following a primary series of PCV for serotypes included in PCV7. This threshold of antibody is a putative measure of protection against IPD at a community-level in otherwise healthy children but does not necessarily indicate protection at an individual level nor is it serotype-specific.36,37 The same threshold of antibody, albeit not validated, has been used as a measure of immunogenicity in HIV-infected children in more recent studies.16,38,39 Immunogenicity studies in adults and older children have primarily reported on the proportion of children with a pre-defined increase in serotype-specific antibody concentration, as the majority of older individuals will have had serotype-specific antibody concentrations of ≥0.35 μg/ml through naturally acquired antibody stimulation mainly from nasopharyngeal colonization before vaccination.
The immunogenicity of PCV is also corroborated by the geometric mean antibody concentrations (GMCs) and the functionality of induced antibody measured by an opsonophagocytic activity assay (OPA). The latter includes measuring the geometric mean antibody titers (GMTs) and proportion of subjects with measurable OPA activity (i.e., OPA ≥ 8). Studies indicate that there may be a closer association using a serotype-specific threshold of OPA ≥ 8 than antibody concentration ≥0.35 μg/ml as a measure of protection against IPD at least for some serotypes such as 6B and 19F.16,40 Similarly, OPA measurements may be more important in predicting potential efficacy for additional serotypes included in newer formulation of PCV than antibody concentration thresholds, as suggested by animal model studies of PCV13 for serotype 3.41 It is also important, particularly in the context of HIV in children and other high-risk groups in whom the risk of IPD may persist beyond that in the general population, that the anamnestic responses induced by PCV be included in the evaluation of the immunogenicity of the vaccines.
Immune responses to PCV vaccination in HIV-infected compared with -uninfected children
Three different PCV formulations, all containing CRM-197 (cross-reactive material) as the carrier protein, including PCV7 and experimental 5-valent PCV (PCV5) and experimental 9-valent PCV (PCV9), have been evaluated for immunogenicity in HIV-infected children.16,38,39,42-49 In addition, studies on the safety and immunogenicity of PCV10 (which has protein-D, tetanus toxoid and diphtheria toxoid as carrier proteins) and PCV13 (also containing CRM-197) are currently underway with results expected in 2012/3. Direct comparisons between the immunogenicity studies among HIV-infected children are difficult since they varied in the dosing schedules used, vaccination age, immunological endpoints analyzed, clinical stage of HIV/AIDS disease and immunosuppression levels of the participants and the proportion of participants on ART (Table 1).
Table 1. PCV immunogenicity studies in HIV-infected children.
| Country and Reference | Study vaccines | Vaccine schedule# | Time of sampling | Participants | % on ART at first vaccine dose | Presented endpoints |
|---|---|---|---|---|---|---|
| US44 |
PPVa PCV5b |
Arm 1: PPV (D0) Arm 2: PCV (D0)+ PPV (D42) |
D0, D42, D84 |
2–9 y n = 30 HIV-infected n = 30 HIV-uninfected |
not mentioned in manuscript |
ELISA GMC; fold-rise in GMC; % of responders (4 fold-rise) |
| US45 |
PCV5b Placebo (16 HIV-uninfected participants only) |
Arm 1: PCV (D0)+ PCV (D60)+ PCV (D120) Arm 2: placebo at same timepoints |
D0, D60, D120, D150 |
≤ 2 y n = 18 HIV-infected n = 33 HIV-uninfected |
not mentioned in manuscript |
ELISA GMC; fold-rise in GMC; % of responders (GMC ≥ 1 μg/ml) |
| US43 Follow-up study |
PCV5b Placebo (11 HIV-uninfected participants only) |
Arm 1: PCV (D0)+ PCV (D60)+ PCV (D120) Arm 2: placebo at same timepoints |
D0, D150, D360 |
≤ 2 y n = 16 HIV-infected n = 25 HIV-uninfected |
not mentioned in manuscript |
ELISA GMC; Percent drop in GMC; % of responders (GMC ≥ 1 μg/ml) |
| US47 |
PCV7c Placebo |
Arm 1: PCV (D0)+ PCV (D56)+ PCV (D112)+ PCV (15 mo of age) Arm 2: placebo at same timepoints (arms randomized 2:1) |
D0, D240, pre-booster, post-booster, D730 |
56–180 d n = 45 HIV-infected |
71% (11% on protease inhibitor) |
ELISA GMC; fold-rise in GMC; % of responders (4-fold-rise) |
| South Africa16 |
PCV9d Placebo |
Arm 1: PCV (6 weeks of age)+ PCV (10 weeks of age)+ PCV (14 weeks of age) Arm 2: placebo at same timepoints |
1 mo after 3 dose |
n = 66 HIV-infected n = 127 HIV-uninfected |
No |
ELISA GMC; % of responders (GMC ≥ 0.35 μg/ml); OPA |
| Greece48 |
PCV7e |
HIV-infected: PCV (D0)+ PCV (D30)+ PCV (D365) HIV-uninfected: PCV (D0)+ PCV (D365) |
D0, D30, D60, D365, D395 |
128 mo (mean age) n = 14 HIV-infected n = 21 HIV-uninfected |
79% |
ELISA GMC; fold-rise in GMC; % of responders (2 fold-rise for 4 serotypes); Avidity |
| Spain49 |
PCV7e |
PCV (D0)+ PCV (D60) |
D0, D60, D150 |
11 y (mean age) n = 56 HIV-infected |
100% |
ELISA GMC for ST: 6B, 14, 23F; OPA titers; % of responders (2 fold-rise in GMC and OPA titers; from negative to positive OPA levels) |
| US42 |
PPVa PCV7e |
PCV (D0)+ PCV (56)+ PPV (D112) |
D0, D56, D112, D168, D336, D672 |
2–19 y n = 225 HIV-infected |
100% |
ELISA GMC for ST: 1, 6B, 14, 19F, 23F; % of responders (GMC ≥ 0.5 μg/mL or ≥ 1 μg/mL) |
| South Africa38 Follow-up study |
Group 1: PCV9d Group 2: Placebo |
Arm 1: PCV (6 weeks of age)+ PCV (10 weeks of age)+ PCV (14 weeks of age) Arm 2: placebo at same timepoints |
5 y after dose 3 |
n = 31 HIV-infected vaccinees n = 49 HIV-infected placebo n = 98 HIV-uninfected vaccines n = 116 HIV-uninfected placebo |
No |
% of responders for PCV7 ST (GMC ≥ 0.2 μg/ml or ≥ 0.35 μg/ml) |
| South Africa46 |
PCV7e |
PCV (5 y post-primary series) |
Pre- and post- vaccination |
n = 31 HIV-infected previous vaccinees n = 45 HIV-infected previous placebo n = 89 HIV-uninfected previous vaccines n = 110 HIV-uninfected previous placebo |
20% |
ELISA GMC; % of responders (GMC ≥ 0.35 μg/mL); OPA for ST: 6B, 9V and 19F |
| South Africa39 | PCV7e | PCV (6–12 weeks)+ PCV (9–18 weeks)+ PCV (12–24 weeks) | 1 mo after 3 dose | n = 249 HIV-infected n = 234 HIV-uninfected |
69% | ELISA GMC; % of responders (GMC ≥ 0.35 μg/mL); OPA for ST: 9V, 19F and 23F |
Notes: #, When not specified the different vaccination arms enrolled subjects 1:1; ELISA GMCs, geometric mean antibody concentrations measured by enzyme-linked immunosorbent assay; % of responders: percentage of subjects who achieved a predefine endpoint; ST, serotype; OPA, opsonophagocytic activity assay. aPnu-Immune 23 (25 μg of each capsular polysaccharide). b10 μg of capsular polysaccharide from serotypes: 6B, 14, 18C, 19F, and 23F, covalently conjugated to CRM197. c2 μg of capsular polysaccharide from serotypes 1, 4, 5, 9V, 14, 19F and 23F; 4μg of capsular polysaccharide from serotype 6B and 2 μg of oligosaccharide from serotype 18C covalently conjugated to CRM197. d2 μg of capsular polysaccharide from serotypes 4, 9V, 14, 18C, 19F and 23F and 4 μg of capsular polysaccharide from serotype 6B covalently conjugated to CRM197. ePrevnar, Wyeth-Lederle (2 μg of capsular polysaccharide from serotypes 4, 9V, 14, 18C, 19F and 23F and 4 μg of capsular polysaccharide from serotype 6B covalently conjugated to CRM197)
Comparison of immune responses to PCV vaccination between HIV-infected and -uninfected children were reported in seven studies.16,38,39,43-45,48 Quantitative comparisons were made comparing the proportion of vaccinees who achieved either a pre-defined serotype-specific antibody concentration16,38,39,43,45 or a pre-determined fold-rise in antibody concentration from baseline to post-vaccination.44,48 In addition, qualitative responses using OPA and long-term anamnestic effects have been evaluated in the South African studies (Table 1).16,39
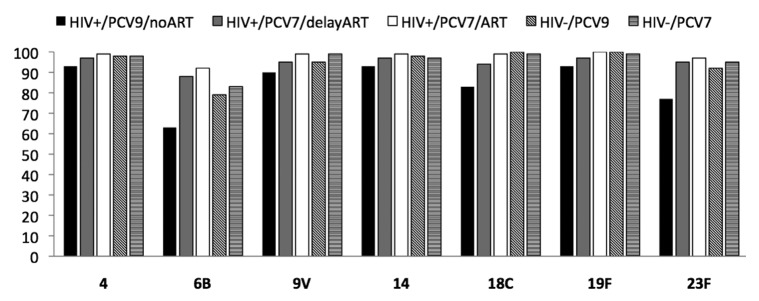
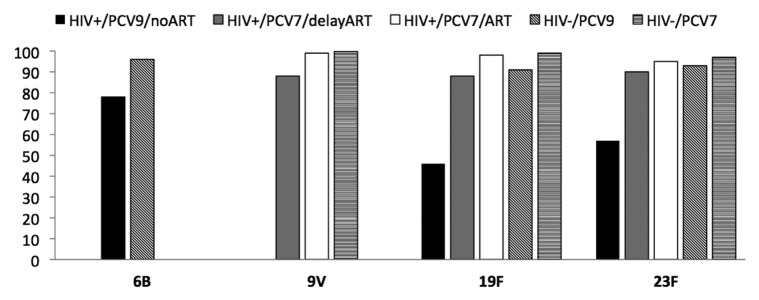
PCV vaccination, together with the other routine infant childhood vaccines, has been evaluated in two studies from South Africa and one from US.16,39,47 In the South African studies, PCV was scheduled to be given at 6, 10 and 14 weeks of age with immunogenicity measured one month after the three-dose primary series of vaccine; Table 1.16,39 The study by Nachman et al. evaluated a three-dose schedule during infancy spaced two months apart and a booster dose at 15 mo of age.47 In the absence of ART in African children, one month after a primary three-dose series of PCV9 (which included PCV7 serotypes and serotypes 1 and 5), the proportion of HIV-infected vaccinees with antibody concentrations ≥0.35 μg/ml to vaccine serotypes ranged between 63 to 93% compared with 79 to 100% in HIV-uninfected infants. This proportion was lower in HIV-infected compared with –uninfected children for serotypes 1 (p = 0.03), 5 (p = 0.03), 18C (p = 0.03) and 23F (p = 0.04) (Fig. 1). Similarly GMCs were lower in HIV-infected children for all serotypes, albeit only significantly so for serotypes 1 and 18C.16 The differences in response to PCV were, however, even more pronounced on OPA for all three analyzed serotypes (6B, 19F and 23F). The proportion of HIV-infected infants with OPA ≥8 was lower in HIV-infected compared with –uninfected infants for serotypes 6B (78% vs. 96%), 19F (46% vs. 91%) and 23F (57% vs. 93%) (Fig. 2). The proportion of HIV-infected subjects with OPA titers ≥8 was more closely associated than the proportion with antibody concentration of ≥0.35 μg/ml, in relation to 65% efficacy against vaccine-serotype IPD observed in HIV-infected children from the same population.50 In addition, HIV-infected children had lower GMTs and required higher concentration of antibody for 50% killing activity on OPA. The higher antibody concentration required for comparable OPA killing activity in HIV-infected compared with –uninfected children suggest functional impairment of antibody in HIV-infected children. Thus, the threshold of antibody concentration required for preventing IPD in HIV-infected children may be higher than the ≥0.35 μg/ml putative threshold suggested for the general population of children.
Figure 1. Proportion of responders with GMCs ≥ 0.35 μg/ml for the 7 serotypes included in PCV7. Data was derived from.16,39 HIV+/PCV9/no ART: HIV-infected children vaccinated with three PCV9 doses not on ART. HIV+/PCV7/delay ART: HIV-infected children vaccinated with three PCV7 doses initiated on ART when clinically or immunologic indicated. HIV+/PCV7/ART: HIV-infected children vaccinated with three PCV7 doses on ART at the time of vaccination. HIV-/PCV9: HIV-uninfected children vaccinated with three PCV9 doses. HIV-/PCV7: HIV-uninfected children vaccinated with three PCV7 doses.
Figure 2. Proportion of responders with OPA titers ≥ 8 for serotypes 6B, 9V, 19F and 23F. Data was derived from16 for serotypes 6B, 19F and 23F and from39 for serotypes 9V, 19F and 23F. HIV+/PCV9/no ART: HIV-infected children vaccinated with three PCV9 doses not on ART. HIV+/PCV7/delay ART: HIV-infected children vaccinated with three PCV7 doses initiated on ART when clinically or immunologic indicated. HIV+/PCV7/ART: HIV-infected children vaccinated with three PCV7 doses on ART at the time of vaccination. HIV-/PCV9: HIV-uninfected children vaccinated with three PCV9 doses. HIV-/PCV7: HIV-uninfected children vaccinated with three PCV7 doses.
A follow-on study in the same setting, involved infants with access to ART.39 This included a group of HIV-infected infants who were initiated on ART immediately upon being diagnosed as having HIV infection at 4–12 weeks of age and another group with CD4+ cell percentage ≥25% at the time of receipt of the primary series of PCV, but who were only initiated on ART when clinically or immunologic indicated as per previous WHO treatment guidelines.51 The immunogenicity of PCV, measured by the proportion of vaccinees with antibody concentration of ≥0.35 μg/ml, was similar between HIV-uninfected and both groups of HIV-infected infants (Fig. 1). These data corroborated the findings from an earlier study by Nachman et al. in which there was no difference in the GMCs in HIV-infected children, 71% of who were on protein-inhibitor based ART regimen, compared with a historical control group of HIV-uninfected infants.47
The study by Nachman et al. has not reported on OPA responses in those children. However, a significantly lower proportion of HIV-infected African infants in whom ART was delayed developed OPA titers of ≥8 to the three analyzed serotypes, despite CD4+ cell percentage ≥25% at vaccination, compared with HIV-uninfected infants or HIV-infected children who were initiated on immediate ART (Fig. 2). The poorer qualitative immune response in the children in whom ART was deferred was also associated with lower OPA GMTs and higher concentration of antibody being required for 50% killing on OPA than in HIV-uninfected children.39 The response in HIV-infected infants with CD4+ cell percentage ≥25% when vaccinated but in whom ART was deferred was, however, better relative to infants without any access to ART (Fig. 1 and Fig. 2).16 This comparison may, however, be biased as only children with a CD4+ cell percentage ≥25% at time of vaccination were included in the group in whom ART was deferred whereas the CD4+ cell counts in the group without access to ART was not evaluated. In addition, 17% of the children in whom ART was deferred had been initiated on ART by the time of measuring the immune response to the primary series of PCV.39 These infant studies indicate that PCV immunization is associated with comparable immunogenicity as in HIV-uninfected infants when undertaken if CD4+ cell percentage is ≥25% and children have been initiated on early ART.
Other studies of PCV in HIV-infected children have mainly involved those older than one year of age and have evaluated different PCV schedules with or without PPV used as a booster/supplementary dose. In two small studies evaluating an investigational PCV5, which included much higher serotype polysaccharide concentration (10 μg per serotype) than in currently licensed formulations (2–4 μg per serotype), the percentage of protocol-defined responders, as per Table 1, although higher in HIV-uninfected children was not significantly different from the HIV-infected group at one month post-primary series.45,48 However, the persistence of antibody was lower in HIV-infected compared with—uninfected children one year after the initial two-doses primary series of PCV.45,48 The studies by King et al. differed to that of Spoulou et al. in that the former reported rapid and similar drop in antibody titers in HIV-infected and—uninfected children, although antibody concentrations were higher than at pre-vaccination.43 In contrast, antibody levels were sustained in HIV-uninfected children 12 mo after the primary series in the study by Spoulou et al. compared with one month post-primary series concentration, whereas HIV-infected children experienced a decay in antibody concentrations to pre-vaccination concentrations.48 Differences in vaccine formulation and study design could explain the observed differences in these studies. Differences included that Spoulou et al. recruited older children (mean age: 128 mo) most of them on ART (79%),48 whereas the study by King et al. vaccinated younger children (mean age: 12.9 mo).43 The observation that a second dose of PCV failed to induce any further increase in GMCs for all serotypes among older HIV-infected children may suggest the absence of PCV inducing anamnestic responses in these children.48 Lower antibody concentrations and greater magnitude of decline in antibody concentration was also observed in ART-naïve HIV-infected children compared with HIV-uninfected children when measured five-years after the primary PCV series during infancy.38,46
PCV booster effect in HIV-infected children
Immunologic memory in HIV-infected children previously primed with 2–3 doses of PCV was assessed by administrating a booster dose of PCV at approximately 12 mo after primary series47,48 or at 5 y post-primary series.46 When a booster dose of PCV was given at 15 mo of age after three primary doses during infancy which included 71% of participants on ART and symptomatic and asymptomatic children, anamnestic responses were detected.47 This included higher serotype-specific antibody concentrations post-booster compared with pre-booster concentration; and the mean fold-change in antibody concentration being significantly higher compared with the group who had previously received placebo. Nevertheless PCV vaccinees experienced a significant waning of serotype-specific GMCs at 24 mo of age, although they remained above pre-immunization levels.47 A booster dose of PCV in older symptomatic HIV-infected children (median age 128 mo), 79% of whom were on ART, however, induced only a modest increase in GMCs, indicating either absence of having induced or failure to sustain an anamnestic response.48
When HIV-infected children who received PCV9 (previous vaccinees) or placebo (control group) during infancy were vaccinated with one dose of PCV7 five years after the primary three dose series during infancy in South Africa, an increase in GMCs was observed for 6 of the 7 serotypes (all PCV7 serotypes except 19F) compared with pre-booster GMCs in HIV-infected children.46 However, the magnitude in fold-change in concentrations was greater for only three serotypes (serotypes 4, 6B and 14) among HIV-infected previous vaccinees compared with previous placebo recipients. In addition, the proportion of children with antibody concentrations ≥0.35 μg/ml was higher for only two serotypes (serotypes 6B and 14) in previous PCV9-vaccinees compared with controls when measured one month after receipt of PCV.46 Furthermore, irrespective of previous priming with PCV during infancy, post-booster GMCs and functional antibody activity were higher in HIV-uninfected than—infected children. These observations also demonstrated impairment in anamnestic immune responses in HIV-infected children. The study was not, however, able to determine whether the HIV-infected children had failed to develop immunologic memory after primary immunization or whether there was a subsequent loss of anamnestic responses with the HIV/AIDS disease progression.46
Association of HIV/AIDS disease stage and PCV immune response in children
Most of the studies stratified HIV-infected children accordingly to clinical stage of AIDS or level of immunosuppression based on CD4+ cell measures. HIV-infected children in the absence of ART with CDC clinical category-C AIDS had lower GMCs to five (serotypes 5, 9V, 14, 18C and 23F) of the nine PCV9 serotypes, compared with children who were CDC clinical category N/A following completion of the primary series of PCV9.16 A multicenter study in the US with HIV-infected children receiving ART, studied children aged 2–19 y who received two doses of PCV7 followed by one PPV dose eight weeks apart. This study demonstrated that higher antibody concentration at baseline, higher CD4+ cell percentage at vaccination, higher nadir CD4+ percentage, lower HIV-viral loads, longer duration of current ART regimen and younger age were predictors of better immune response in HIV-infected children.42 In particular, children vaccinated early in the course of their HIV illness, including when the CD4+ cell percentage was ≥25% at its nadir and at the time of vaccination, had the greatest antibody increase in relation to prior antibody concentration after each dose of vaccine. In addition, antibody concentrations remained high in this group of children two years later. Conversely, immunization of children when both the nadir and time of vaccination CD4+ cell percentage was <15%, showed poor antibody responses to both doses of PCV and the PPV. This study also identified a significant positive association between antibody concentration and the duration of the ART regimen.42 These results support the importance of ART in lymphocyte reconstitution and the subsequent effect thereof on immunogenicity of PCV.
Other trials were less able to identify differences in response rates to PCV based on CD4+ cell count, immune status before ART or clinical AIDS stage.43-45,47,49 In two of these studies a substantial proportion of children were, however, also taking ART.47,49
Efficacy of PCV in HIV-infected children
The efficacy of PCV has only been evaluated in HIV-infected children in a randomized placebo-controlled trial in South Africa. This study evaluated an investigational PCV9, which included serotypes 1 and 5 and was otherwise identical to PCV7.50 The nine serotypes included in PCV9 covered 83–91% of invasive disease causing serotypes among HIV-infected children prior to the study.9,35 PCV was found to be efficacious in preventing vaccine serotype-specific IPD in HIV-infected infants, who were not on ART. The vaccine efficacy, following 2.3 y of follow-up, against IPD in HIV-infected was 65% (95% CI: 24 to 86) and lower than observed in HIV-uninfected children (83%; 95% CI: 39 to 97).50 However, because of the 40-fold greater burden of IPD pre-existent in HIV-infected children,4 despite the lower vaccine efficacy, there was an 18-fold greater reduction in the burden of vaccine-serotype IPD prevented in HIV infected compared with –uninfected children (570 vs. 32 per 100 000 children vaccinated, respectively).50
PCV vaccination was also associated with non-significant reductions in overall mortality (6%; p > 0.05) and reduction in radiologic confirmed pneumonia (13%; 95% CI: -7 to 29).50 The lack of efficacy against the endpoint of radiologic confirmed pneumonia may, however, have been confounded in that this non-specific endpoint used as a surrogate for “pneumococcal pneumonia” may have had even less specificity in HIV-infected compared with -uninfected children.52 This was corroborated in part by subsequent, post hoc analysis, in which using different criteria to define pneumonia revealed a 15% (95% CI: 5 to 24) reduction in all-cause clinical pneumonia and 59% (95% CI: 1 to 83) reduction in vaccine-serotype bacteremic pneumonia in HIV-infected children.53 Furthermore, the low sensitivity of blood cultures in the diagnosis of pneumococcal pneumonia, even in HIV-infected children, was evident in that the vaccine-attributable rate reduction was 5.3-fold greater for all-cause clinical pneumonia (2 573 cases per 100 000 child-years prevented) compared with bacteremic pneumococcal pneumonia (483 per 100 000 child-years episodes).53 Similarly to that observed for IPD, there was 9-fold greater reduction of all-cause clinical pneumonia in HIV–infected children compared with –uninfected children after 2.3 y of follow-up.50,53 The South African study, through using PCV vaccination as a probe, also identified the important role of pneumococcal co-infection as a precipitating cause of hospitalization for respiratory viral and Mycobacterium tuberculosis associated pneumonia in HIV-infected children.54,55
At 5 y of follow-up in the South African study, in the absence of a booster dose of PCV or ART access, vaccine efficacy against IPD in HIV-infected children dropped from the initial 65% estimate at 2.3 y of age to 39% (95% CI: -8 to 65). In HIV-uninfected children, efficacy of a three-dose primary series of PCV against IPD remained unchanged at 5 y post-vaccination (78%; 95% CI: 34 to 93) compared with at 2.3 y (83%).38 Despite this, the vaccine-attributable rate reduction in IPD, irrespective of serotype, in HIV-infected children (2 250 per 100 000 child-years prevented) was 59-fold greater compared with HIV-uninfected children (38 per 100 000 child-years prevented) by five years of age. The efficacy of PCV9 against any serotype IPD was, however, greater in HIV-infected children (46%) than in HIV-uninfected children (35%), mainly due to the cross-protection afforded by the PCV9 against serotype 6A.38 All these data emphasize the need for sustained protection against IPD in HIV-infected children well beyond the first two years of life, which is when most disease occurs in HIV-uninfected children. Consequently, determination of anamnestic responses and persistence thereof are important measures when evaluating the immunogenicity of PCV in HIV-infected and possibly other high risk groups of children.
Safety of PCV in HIV-infected children
Five studies from the US or South Africa explored the safety of PCV in HIV-infected children.42,44,45,47,50 In all the studies PCV was well tolerated and in the studies including a placebo arm, no significant differences in local or systemic reactions between placebo and PCV recipients were observed.45,47 Nachman et al. however reported more frequent severe signs and symptoms among PCV recipients than placebo, and these included diarrhea, rash, fever and anemia.47 In the South African study a higher rate of asthma was reported among PCV9 recipients, however no stratification by HIV-status was done for asthma rate. In addition, the 5-y follow-up period of this study revealed a lower CD4+ cell percentage, as well as a similar trend in mean CD4+ counts, in previous PCV recipients compared with past placebo recipients.38 The clinical significance of the lower CD4+ cell count, in the absence of receiving ART, among previous PCV recipients in HIV-infected children remains to be explored.
Pneumoccocal polysaccharide vaccination in HIV-infected adults
Immunization with a single dose of PPV is recommended for HIV-infected adults and adolescents as soon as possible after diagnosis of HIV infection and who have a CD4+ cell count ≥ 200 cells/μl.56-58 PPV vaccination of older HIV-infected children and adults has been associated with poor or modest immunogenicity.44,59,60 Even in HIV-infected adults on ART, PPV elicited only modest increases in functional antibody and in serotype-specific antibody concentrations, with antibody responses being lower than in healthy controls.61-63 The use of PPV in African HIV-infected adults is controversial. A randomized placebo-controlled trial in Uganda reported an increase in pneumonia in the six-month period following PPV vaccination of HIV-infected adults not on ART,60 albeit subsequently suggesting a 16% reduction in all-cause mortality.64 A recent meta-analysis by Pedersen et al., indicated marked heterogeneity in results on the efficacy of PPV against varying pneumococcal disease syndromes in HIV-infected adults and did not demonstrate any overall benefit.65 The effectiveness of PPV in HIV-infected adults, and discrepant efficacy results between studies, may be associated with HIV viral load and correspondingly ART status at the time of vaccination.60,61,64,66,67 Teshale et al. suggested that PPV related protection against pneumonia was only evident in individuals with HIV viral load of < 100 000 copies/μl irrespective of CD4+ immunologic categorization.68 Several studies have also shown that HIV-infected persons with CD4+ cell counts < 500 cells/μl have impaired antibody responses against several pneumococcal serotypes compared with less immunocompromized HIV-infected persons or healthy controls.59,69
In less immunocompromized HIV-infected individuals, the rate of antibody decline after PPV vaccination is comparable to healthy controls. However, since HIV-infected individuals have lower post-vaccination antibody concentrations, their antibody concentrations may diminish to below the estimated protective levels sooner than in HIV-uninfected adults.23,70 As there are no immunologic correlates of clinical protection against IPD for adults, immunogenicity studies in adults have used the same threshold (≥0.35 μg/ml) suggested for infants or higher thresholds such as 1 μg/ml, or the fold-increase in antibody concentration as immunologic endpoints when measuring the immunogenicity of PCV. In addition, some studies have also evaluated OPA responses.
Immunogenicity of PCV in HIV-infected adults
Table 2 summarizes the studies in which the immunogenicity and safety of PCV has been evaluated in HIV-infected adults, aimed at showing better potential than that conferred by PPV. To our knowledge only one published study reported on clinical efficacy of PCV vaccination in HIV-infected adults.80 Several other studies have, however, explored the serotype-specific immune responses induced by PCV in adults with HIV.69-79 Direct comparisons between studies were confounded in that some used PCV alone while other studies evaluated PCV in combination with PPV. Studies also varied in the definition of measuring immune responses, including the use of endpoints such as antibody concentration expressed as GMCs, functional antibody levels determined by OPA or avidity experiments and percentage of responders (fold-rise in post-vaccination antibody titers compared with pre-vaccination) (Table 2). Two of the studies69,70 presented in Table 2 measured serum antibody titers by ELISA without the currently recommended step of absorption with heterologous pneumococcal polysaccharides 22F to improve serotype-specific antibody specificity by inhibiting non-serotype specific antibodies.81
Table 2. PCV immunogenicity studies in HIV-infected adults.
| Study year, country and reference | Study vaccines | Vaccine schedule# | Time of sampling | Participants | % on ART at first vaccine dose | CD4+ cell count at baseline (cells/μL) | Previous pneumococcal vaccination | Presented endpoints |
|---|---|---|---|---|---|---|---|---|
| US (July–Dec 1993)$ 69 |
PPV23a PCV5b |
Arm 1: PPV (D0) Arm 2: PCV (D0) |
D0, D30 |
18–65 y n = 183 HIV-infected n = 99 HIV-uninfected |
47% |
< 200: 90 (group I) ≥ 200: 93 (group II) |
No PPV in past 5 y |
ELISA GMC; fold-rise in GMC; % of responders (2-fold rise) |
| The Netherlands$ 70 |
PPV23c PCV4d |
PCV (D0)+ PCV (D30)+ PPV (D300) Historical controls: PPV (D0) |
D0, D30, D60, D300, D330 Historical controls: D0, D30 |
16–62 y n = 30 HIV-infected n = 9 HIV-uninfected Historical controls: n = 50 HIV-infected n = 10 HIV-uninfected |
100% of group I |
< 200: 16 (group I); 26 historical controls ≥ 200: 14 (group II); 24 historical controls |
Not mentioned in manuscript |
ELISA GMC; fold-rise in GMC; % of responders (GMC ≥ 1 μg/ml) |
| US (Jan 1998–June 1999)71 |
PPV23e PCV7f |
Arm 1: PCV (D0)+ PCV (D56) Arm 2: PCV (D0)+ PPV (D56) Arm 3: Placebo (D0)+ PCV (D56) Arm4: Placebo (D0)+ PPV (D56) |
D0, D56, D112 |
> 17 y n = 67 HIV-infected |
42% |
≥ 200 |
No PPV in past 5 y |
ELISA GMC for ST: 4, 6B, 6V, 14 and 23F; OPA titers for ST: 4, 6B, 6V, 14 and 23F; % of responders (2-fold in GMC or 4 fold-rise in OPA) |
| Uganda (Oct 2001–June 2002)72 |
PCV7f |
PCV (D0)+ PCV (D28) (median time from previously PPV/placebo administration (1:1) 62 mo) |
D0, D28, D56 |
24–79 y n = 109 HIV-infected |
No |
< 200: 43 (group I) 200–499: 42 (group II) ≥ 500: 23 (group III) |
Previous PPV or placebo recipients |
ELISA GMC; fold-rise in GMC; % of responders (2 fold-rise or GMC > 0.35 μg/ml or GMC > 1 μg/ml) |
| France (Dec 2002–Dec 2003)73 |
PPV23g PCV7f |
Arm 1: PCV (D0)+ PPV (D28) Arm 2: PPV (D28) |
D0, D28, D56, D168 |
44 y (mean age) n = 208 HIV-infected |
87% |
200–500 |
No PPV in past 5 y |
ELISA GMC; % of responders (2 fold-rise and GMC ≥ 1 μg/mL) |
| Malawi74 |
PCV7 |
Arm 1: PCV7 (D0) Arm 2: Placebo (D0) |
D0, D30, D180 |
32 y (mean age) n = 19 HIV-infected n = 22 HIV-uninfected |
No |
> 200 |
Not mentioned in manuscript |
ELISA GMC in serum and Bronchoalveolar lavage for ST: 6B, 14, 19F and 23F |
| Uganda (Jan–June 2005)75 |
PPV23h PCV7f |
PCV (D0)+ PPV (D60) |
D0, D60, D90, D240 |
37 y (mean age) n = 58 HIV-infected n = 29 HIV-uninfected |
No |
200–499: 30 (group I) ≥ 500: 28 (group II) |
Not mentioned in manuscript |
ELISA GMC and OPA titers for ST: 4 and 14 |
| Spain (Dec 2007–Apr 2008)76 |
PPV23 PCV7 |
Arm 1: PCV (D0)+ PPV (D28) Arm 2: PPV (D28) |
D0, D28, D56 |
44 y (mean age) n = 202 HIV-infected |
98% PCV group 91% PPV group |
200–500 |
No previous PPV |
ELISA GMC; % of responders (2 fold-rise and GMC ≥ 1 μg/ml); Avidity |
| US (Feb 2006–Sep 2008)77 |
PPV23h PCV7f |
Arm 1: PCV (D0) Arm 2: PPV (D0) (arms randomized 2:1) |
D0, D14, D60, D180 |
18–60 y old n = 131 HIV-infected PCV n = 73 HIV-infected PPV n = 25 HIV-uninfected |
82% |
< 200: 7 (group I) 200–499: 84 (group II) ≥ 500: 113 (group III) |
PPV vaccinated 3–8 y earlier |
ELISA GMC for ST: 4, 9V, 14 and 19F; fold-rise in GMC; % of responders (2 fold-rise for at least 2 serotypes and GMC ≥ 1 μg/ml) |
| Denmark (Jan–Mar 2008)78,79 | PPVi PCV7f |
PCV (D0)+ PCV (D90)+ PPV (D270) (± CPG 7909* to each vaccine dose) |
D0, D90, D120, D270, D300 | ≥ 18 y old n = 97 HIV-infected |
79% | ≥ 200 | No PPV in past 5 y | ELISA GMC; % of responders (2 fold-rise for at least 5 serotypes and GMC ≥ 1μg/ml); OPA titers (ST 6B, 14, 19F and 23F) |
Notes: #, When not specified the different vaccination arms enrolled subjects 1:1; *, Toll-like receptor agonist and vaccine adjuvant; ELISA GMCs, geometric mean antibody concentrations measured by enzyme-linked immunosorbent assay; % of responders, percentage of subjects who achieved a predefine endpoint; ST, serotype; OPA, opsonophagocytic activity assay. $ELISA performed not including absorption with heterologous 22F serotype. aPnu-Immune 23, Lederle, Wayne, NJ (25 μg of each capsular polysaccharide). bLederle-Praxis Biologics, Rochester, NY (10 μg of capsular polysaccharide from serotypes: 6B, 14, 18C, 19F, and 23F, covalently conjugated to CRM197). cPneumovax 23, Pasteur Merieux MSD, Amstelveen, The Netherlands (25 μg of each capsular polysaccharide). dMerck and Co. Inc. West Point, PA (2 μg of capsular polysaccharide from serotypes 6B and 14 in one syringe and 2 μg of serotypes 19F and 23F in another, covalently conjugated to an outer membrane complex from Neisseria meningitis). eWyeth-Lederle (25 μg of each capsular polysaccharide). fPrevenar Wyeth-Lederle (2 μg of capsular polysaccharide from serotypes 4, 9V, 14, 18C, 19F and 23F and 4 μg of capsular polysaccharide from serotypes 6B covalently conjugated to CRM197). gPneumo 23, Aventis Pasteur MSD. hPneumovax, Merck-Banyu (25 μg of each capsular polysaccharide). iPneumo Novum; sanofi-pasteur MSD.
Studies that included a PCV-vaccinated HIV-uninfected group as a control consistently detected a better antibody response in HIV-uninfected than in HIV-infected individuals.69,70,74,75,77 An exception was the study by Chen, et al. where no difference in GMCs for serotype 4 between the HIV-infected and –uninfected groups was detected at any timepoint, although only two serotypes (4 and 14) were evaluated.75
PCV immunogenicity vs. PPV in HIV-infected adults
A 4-arm randomized trial with two doses of vaccines and/or placebo administered to HIV-infected subjects 8 weeks apart (PCV7-PCV7, PCV7-PPV23, placebo-PPV23 and placebo-placebo groups) reported that antibody concentration and OPA 16 weeks after the initial vaccine dose were significantly higher in the groups that received PCV compared with the group that just received PPV for four of the five serotypes tested.71 In addition, there was a trend for higher proportion of responders in the PCV-vaccinated group compared with the placebo-PPV group with regard to obtaining more than 2 fold-rise in GMCs or 4 fold-rise in OPA titers, albeit only significant for serotype 4. A second PCV dose eight weeks after the first PCV7 dose did not produced any further increase in antibody response.71
Another study with a shorter dosing schedule (4 weeks between vaccinations) but a longer follow-up period also found that the response profile was better in the arm that received a PCV dose followed by PPV 4 weeks later compared with the PPV-only group.73 These differences were, however, only evident after the PCV group had received their PPV dose. Superiority of the PCV-PPV arm considering both antibody levels and percentage of responders was sustained at least until 24 weeks post-first dose.73
PCV vaccination of HIV-infected adults previously vaccinated with PPV 3–8 y earlier transiently induced a better immune response than PPV revaccination at 60 d post-vaccination.77 However, at 180 d post-vaccination there was no difference in antibody concentration between the two groups, indicating that PCV vaccination provided little additional immunologic benefit compared with PPV re-vaccination.77 While PCV elicited good antibody responses and in several studies higher than that induced by PPV at particular timepoints these differences were not markedly different. A study in Spain where PPV was given alone or 4 weeks after PCV did not find any difference between the two vaccination arms in serotype-specific antibody avidity and proportion of responders (defined as a 2 fold-rise in antibody titers and antibody concentration of at least 1 μg/ml), except for serotype 23F, in the PCV-group at 8 weeks post-PCV.76
PCV priming of the immune system in HIV-infected adults
Vaccination with PCV followed by PPV allowed several studies to explore the possibility of priming of the immune system by PCV. Even though the interval between vaccines appears to play a role, evidences of inducing anamnestic responses are sparse.70,71,73,75,76,78 In a study in Denmark where HIV-infected adults were immunized twice 3 mo apart with double the standard dose of PCV7 and received PPV nine months after the first-PCV7 dose, GMCs did not increase at one month post-PPV compared with one month post-second PCV dose levels. However, OPA titers were higher at one month post-PPV for three (i.e., 14, 19F and 23F) of the four serotypes assessed.78 Since this study did not include a PPV-only arm, it is not clear if the increase in OPA titers post-PPV were due to anamnestic responses induced through priming with PCV.78 Lesprit et al., however, reported that PPV given four weeks after PCV resulted in better immune response measured four weeks later, compared with HIV-infected adults only vaccinated with PPV. This included higher percentage of responders (defined as a 2-fold rise in GMCs from baseline and GMC ≥ 1ug/mL) and higher GMCs for six (i.e., 4, 9V, 14, 18C, 19F and 23F) of the serotypes common to PCV7 and PPV at four weeks post-PPV. The higher antibody concentration in PCV7 recipients was maintained at least until 20 weeks post-PPV for four serotypes, although the percentages of responders dropped in each group.73 As evident by the results of these studies the dosing schedule and timing of vaccination of HIV-infected adults with pneumococcal vaccines still needs further investigation however a schedule with a dose of PCV followed by PPV seems the most adequate in possibly enabling induction of anamnestic immune responses by PCV as well as by expanding serotype coverage of those serotypes included in the 23-valent PPV but absent in PCV.
Factors associated with PCV response in HIV-infected adults
Pneumococcal vaccines induced antibody responses are probably dependent on CD4+ cell function. Most of the studies that included HIV-infected persons with CD4+ cell counts less than 200 cells/μl, observed a lower antibody response to PCV compared with persons with counts higher than 200 cells/μl analyzing either absolute antibody concentrations70,72 or fold-rise in antibody levels.70 However it has been suggested that the association between CD4+ cell count and antibody response is mainly due to a direct association between CD4+ cell count and baseline antibody concentration.72 When searching for factors associated with PCV-specific immunologic response CD4+ cell count, virologic status or receipt of ART at initiation of vaccine series were not significantly associated with differences in antibody concentration and OPA titers during follow-up until 60–112 d.71,77 However, in one study after administration of two doses of double-strength PCV7 three months apart, HIV-infected adults on ART achieved a more durable antibody response, of higher magnitude than ART-naive individuals, independent of pre-vaccination CD4+ cell count.78 Both ART-experienced and ART-naïve subjects achieved comparable initial responses to PCV, but after a period of 9 mo post-first PCV dose significant differences were apparent in the proportion of vaccine responders (defined as a 2-fold rise in GMCs from baseline to ≥ 1ug/mL for at least 5 serotypes) in the two ART groups.78
In search for a more immunogenic pneumococcal vaccine for use in HIV-infected adults the addition of an adjuvant was explored by Sogaard et al.79 This study evaluated the inclusion of a toll-like receptor agonist and vaccine adjuvant (CPG7909) to PCV7 and PPV. In this study the proportion of vaccine high responders (defined as a 2-fold increase in antibody levels to > 1 μg/mL for at least 5 serotypes) was higher in the CPG7909 group than among controls at 4, 9 and 10 mo. OPA titers were also elevated in the CPG7909 group. The enhanced production of antibodies by CPG7909 was, however, negatively correlated with HIV viral load indicating that this strategy was unlikely to benefit HIV-infected individuals with detectable HIV-viremia. Even though vaccination with the addition of CPG7909 was generally well tolerated, mild systemic and injection site reactions to first and/or second PCV dose were more common in the CGP7909 group than in the control group. After PPV immunization influenza-like symptoms were more common in the CPG7909 group.
Studies that report on HIV viral loads after vaccination, have not observed significant increases above pre-vaccination levels after one or two PCV doses.71,77
Clinical efficacy of PCV in HIV-infected adults
Although immunogenicity studies provide evidence of relative response between PCV and PPV, the absence of serologic correlates of protection against disease in HIV-infected individuals and in adults, requires demonstration of the efficacy of PCV against pneumococcal disease in HIV-infected adults. To date, there are no head-to-head randomized trials which compared the efficacy of PCV to that of PPV in HIV-infected adults. There have, however, been two separate double-blind, randomized, placebo-controlled clinical trials, one comparing PPV efficacy with placebo against pneumonia as discussed earlier and the other comparing PCV7 efficacy with placebo for protecting against recurrent vaccine-serotype IPD.60,80
The efficacy of PCV7 was assessed in Malawi from 2003 to 2007 in HIV-infected adolescents and adults (15 y of age or older) who had recovered from an IPD episode.80 The primary end point was a further episode of IPD caused by PCV7 serotypes or serotype 6A. Two doses of PCV7 or placebo were given 4 weeks apart and subjects were followed up for a mean of 1.2 y. Overall 496 subjects were enrolled, 88% being HIV-infected and of those 13% were on ART at baseline. Twenty-four (36%) of all new IPD episodes (n = 67) were PCV7 or 6A serotype and the unadjusted vaccine efficacy was 74% (95% CI: 30 to 90) with a hazard ratio of 0.26 (95% CI: 0.10 to 0.70). Vaccine efficacy, however, decreased from 85% in the first year post-vaccination to 25% thereafter. Protection against IPD was also evident in the subgroup of participants with CD4+ cell counts < 200 cells/μl, with a vaccine efficacy of 86% (95% CI: 41 to 97).80 The overall protection against IPD, irrespective of serotype, had a hazard ratio of 0.72 (95% CI: 0.42 to 1.25). In addition, no overall effect was observed in mortality between PCV7 recipients (29%) and placebo recipients [25%; adjusted hazard ratio: 1.24 (95% CI: 0.9 to 1.8)]. CD4+ cell count at enrolment was the strongest risk factor for IPD in this study in a multivariable analysis. Compared with subjects with CD4+ cell counts higher than 500 cells/μl, patients with CD4+ cell counts < 200 cells/μl had 7-times higher risk of a recurrent IPD episode. A lower CD4+ cell count was also associated with death and pneumonia from any cause.80 The study was not powered to investigate the interaction between PCV and the use of ART. The greater diversity of serotypes causing IPD in adults may, however, require formulations of PCV with broadened serotype coverage than that included in the current formulation of PCV targeted primarily at serotypes highly prevalent in children.
Safety of PCV in HIV-infected adults
The safety and tolerability of PCV administration in HIV-infected adults was comparable to PPV vaccination, both vaccines being generally well tolerated. Individuals who received PCV tended to reported more local pain at injection site than PPV recipients in two studies.69,71 The frequency of other reactions including fever, redness, swelling and tenderness were similar in the two vaccine arms. The majority of reaction reported were self-limited. In the PCV efficacy trial in Malawi, serious adverse events were significantly more common in the placebo arm.80 In the revaccination study by Crum-Cianflone et al., one HIV-infected PCV-vaccinated subject developed encephalitis 41 d after revaccination which was attributed as possibly being related to the vaccine.77
PCV and indirect protection
In addition to the direct effect of PCV, vaccination of young children has also been associated with reduction of vaccine-serotype IPD in the general population of unvaccinated individuals.82,83 This reduction is attributed to the effectiveness of PCV in reducing the risk of nasopharyngeal acquisition of the targeted vaccine serotypes in vaccinated children, who are traditionally considered the most important source of transmission of pneumococci within communities.84 In addition to indirect effect observed in the general US population,85 widespread childhood PCV7 immunization has also been temporally associated with reduction in vaccine-serotype IPD in HIV-infected adults in US (91%).86 The decline in overall IPD has, however, been offset by an increase in non-vaccine serotype IPD (28%) in this population with a net overall reduction of IPD being 41%.86,87 In the analysis by Cohen et al. from 2004–2007, the incidence of IPD (cases per 100 000) in HIV-infected adults remained 40-times higher than among HIV-uninfected adults.86 Although these data indicate the potential of childhood PCV immunization benefiting HIV-infected individuals from developing IPD, these findings need further exploration in African settings where there may be a greater diversity of serotypes associated with IPD compared with in the US. In addition, lower levels of childhood immunization coverage, differing dosing schedules and more limited catch-up campaigns of immunizing older children and possible differences in the dynamics of pneumococcal transmission in developing countries may affect the indirect potential of vaccines in some settings with a high prevalence of HIV-infection.
Conclusions
Studies on PCV demonstrate promise for directly and indirectly protecting HIV-infected individuals against developing IPD and pneumonia. PCV vaccination of HIV-infected children on ART, particularly when immunologic competent when immunized, indicate similar quantitative and qualitative antibody responses as in HIV-uninfected children. However, the immunogenicity, efficacy and durability of protection of HIV-infected children not receiving ART indicates the need for possible booster doses of PCV later in life, albeit preferably after they been initiated on antiretroviral treatment. The frequency and timing of these additional booster doses in HIV-infected children independently of ART usage still needs to be determined to improve vaccine effectiveness. Furthermore long-term anamnestic responses to PCV are yet to be established in HIV-infected children vaccinated while receiving ART.
The introduction of PCV into national children immunization programs in settings with high HIV burden should be careful designed and should include catch-up campaigns targeting HIV-infected children not vaccinated during infancy to optimize the prevention of pneumococcal disease.
Although some studies in adults are inconsistent on the immunologic advantage of PCV over PPV, PCV has nevertheless been associated with protection against vaccine serotype IPD in high risk HIV-infected adults even largely in the absence of ART. However the limited serotype coverage of PCV (seven to 13 serotypes) requires HIV-infected individuals to also receive PPV in addition to PCV, to expand the coverage of potential disease causing serotypes.
The greatest benefit of PCV immunization programs against IPD may, however, be realized through the indirect effect of childhood vaccination against adult disease. This needs to be further explored, including for some of the newer serotypes included in PCV13 (e.g., 1, 3, 5 and 7F) and not PCV7 which may differ in the dynamics of transmission within communities. The indirect effect of PCV in protecting adults in settings with a high prevalence of HIV, particularly in Africa, needs further study since significant differences exist between these settings and developed countries regarding immunization practices and vaccine coverage. The magnitude of the herd effect was not confirmed on a small study in South Africa, a country with a high prevalence of adult HIV.88 The divergences with the South African study can be due to the absence of a booster dose of PCV and to an overall PCV coverage of < 20%. It is known that herd immunity efficacy is affected by serotype distribution, actual proportion of children vaccinated and also by social factors.
In addition, the effect of possible replacement disease due to non-vaccine serotypes negating any decrease in vaccine-serotype disease also requires evaluation in settings with high HIV burden after PCV introduction.
Acknowledgments
MCN had financial support from the University of the Witwatersrand. SAM receives financial support from the National Research Foundation through the South African Research Chair Initiative.
Conflict of interest
SAM receives research grant support from GSK and Pfizer. Served on speakers bureau and received honoraria for GSK and Pfizer. Received consultancy fees from GSK, Pfizer, Novartis and MERCK.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/18432
References
- 1.UNICEF. Percentage of under fi ves with suspected pneumonia taken to appropriate health provider. http://www.childinfo.org/pneumonia_countrydatacare.php accessed July, 2011.
- 2.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 3.Nunes MC, von Gottberg A, de Gouveia L, et al. The impact of antiretroviral treatment on the burden of invasive pneumococcal disease in South African children: a time series analysis. AIDS. 2011;25:453–62. doi: 10.1097/QAD.0b013e328341b7f1. [DOI] [PubMed] [Google Scholar]
- 4.Madhi SA, Petersen K, Madhi A, Wasas A, Klugman KP. Impact of human immunodeficiency virus type 1 on the disease spectrum of Streptococcus pneumoniae in South African children. Pediatr Infect Dis J. 2000;19:1141–7. doi: 10.1097/00006454-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Madhi SA, Madhi A, Petersen K, Khoosal M, Klugman KP. Impact of human immunodeficiency virus type 1 infection on the epidemiology and outcome of bacterial meningitis in South African children. Int J Infect Dis. 2001;5:119–25. doi: 10.1016/S1201-9712(01)90085-2. [DOI] [PubMed] [Google Scholar]
- 6.Schuchat A, Broome CV, Hightower A, Costa SJ, Parkin W. Use of surveillance for invasive pneumococcal disease to estimate the size of the immunosuppressed HIV-infected population. JAMA. 1991;265:3275–9. doi: 10.1001/jama.1991.03460240071030. [DOI] [PubMed] [Google Scholar]
- 7.Nuorti JP, Butler JC, Gelling L, Kool JL, Reingold AL, Vugia DJ. Epidemiologic relation between HIV and invasive pneumococcal disease in San Francisco County, California. Ann Intern Med. 2000;132:182–90. doi: 10.7326/0003-4819-132-3-200002010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Jordano Q, Falco V, Almirante B, et al. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis. 2004;38:1623–8. doi: 10.1086/420933. [DOI] [PubMed] [Google Scholar]
- 9.Jones N, Huebner R, Khoosal M, Crewe-Brown H, Klugman K. The impact of HIV on Streptococcus pneumoniae bacteraemia in a South African population. AIDS. 1998;12:2177–84. doi: 10.1097/00002030-199816000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Heffernan RT, Barrett NL, Gallagher KM, et al. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995-2000. J Infect Dis. 2005;191:2038–45. doi: 10.1086/430356. [DOI] [PubMed] [Google Scholar]
- 11.McEllistrem MC, Mendelsohn AB, Pass MA, et al. Recurrent invasive pneumococcal disease in individuals with human immunodeficiency virus infection. J Infect Dis. 2002;185:1364–8. doi: 10.1086/339882. [DOI] [PubMed] [Google Scholar]
- 12.Gilks CF, Ojoo SA, Ojoo JC, et al. Invasive pneumococcal disease in a cohort of predominantly HIV-1 infected female sex-workers in Nairobi, Kenya. Lancet. 1996;347:718–23. doi: 10.1016/S0140-6736(96)90076-8. [DOI] [PubMed] [Google Scholar]
- 13.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–92. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 14.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–8. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 15.Scriba TJ, Zhang HT, Brown HL, et al. HIV-1-specific CD4+ T lymphocyte turnover and activation increase upon viral rebound. J Clin Invest. 2005;115:443–50. doi: 10.1172/JCI23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madhi SA, Kuwanda L, Cutland C, Holm A, Kayhty H, Klugman KP. Quantitative and qualitative antibody response to pneumococcal conjugate vaccine among African human immunodeficiency virus-infected and uninfected children. Pediatr Infect Dis J. 2005;24:410–6. doi: 10.1097/01.inf.0000160942.84169.14. [DOI] [PubMed] [Google Scholar]
- 17.De Milito A, Morch C, Sonnerborg A, Chiodi F. Loss of memory (CD27) B lymphocytes in HIV-1 infection. AIDS. 2001;15:957–64. doi: 10.1097/00002030-200105250-00003. [DOI] [PubMed] [Google Scholar]
- 18.Eley B. Immunization in patients with HIV infection: are practical recommendations possible? Drugs. 2008;68:1473–81. doi: 10.2165/00003495-200868110-00001. [DOI] [PubMed] [Google Scholar]
- 19.Recommended adult immunization schedule–United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1–4. [PubMed] [Google Scholar]
- 20.Musher DM, Rueda-Jaimes AM, Graviss EA, Rodriguez-Barradas MC. Effect of pneumococcal vaccination: a comparison of vaccination rates in patients with bacteremic and nonbacteremic pneumococcal pneumonia. Clin Infect Dis. 2006;43:1004–8. doi: 10.1086/507699. [DOI] [PubMed] [Google Scholar]
- 21.Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938–43. doi: 10.1001/archinte.167.18.1938. [DOI] [PubMed] [Google Scholar]
- 22.Vila-Corcoles A, Salsench E, Rodriguez-Blanco T, et al. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in middle-aged and older adults: a matched case-control study. Vaccine. 2009;27:1504–10. doi: 10.1016/j.vaccine.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Musher DM, Groover JE, Rowland JM, et al. Antibody to capsular polysaccharides of Streptococcus pneumoniae: prevalence, persistence, and response to revaccination. Clin Infect Dis. 1993;17:66–73. doi: 10.1093/clinids/17.1.66. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen H, Kvinesdal B, Benfield TL, Lundgren JD, Konradsen HB. Rapid loss of specific antibodies after pneumococcal vaccination in patients with human immunodeficiency virus-1 infection. Scand J Infect Dis. 1998;30:597–601. doi: 10.1080/00365549850161160. [DOI] [PubMed] [Google Scholar]
- 25.Musher DM, Manof SB, Liss C, et al. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. 2010;201:516–24. doi: 10.1086/649839. [DOI] [PubMed] [Google Scholar]
- 26.Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49:1–35. [PubMed] [Google Scholar]
- 27.O'Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis. 2007;7:597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 28.Sloyer JL, Jr., Ploussard JH, Karr LJ, Schiffman GD. Immunologic response to pneumococcal polysaccharide vaccine in infants. Ann Otol Rhinol Laryngol Suppl. 1980;89:351–6. doi: 10.1177/00034894800890s382. [DOI] [PubMed] [Google Scholar]
- 29.Douglas RM, Miles HB. Vaccination against Streptococcus pneumoniae in childhood: lack of demonstrable benefit in young Australian children. J Infect Dis. 1984;149:861–9. doi: 10.1093/infdis/149.6.861. [DOI] [PubMed] [Google Scholar]
- 30.Vernacchio L, Neufeld EJ, MacDonald K, et al. Combined schedule of 7-valent pneumococcal conjugate vaccine followed by 23-valent pneumococcal vaccine in children and young adults with sickle cell disease. J Pediatr. 1998;133:275–8. doi: 10.1016/S0022-3476(98)70235-5. [DOI] [PubMed] [Google Scholar]
- 31.Chan CY, Molrine DC, George S, et al. Pneumococcal conjugate vaccine primes for antibody responses to polysaccharide pneumococcal vaccine after treatment of Hodgkin's disease. J Infect Dis. 1996;173:256–8. doi: 10.1093/infdis/173.1.256. [DOI] [PubMed] [Google Scholar]
- 32.Storek J, Mendelman PM, Witherspoon RP, McGregor BA, Storb R. IgG response to pneumococcal polysaccharide-protein conjugate appears similar to IgG response to polysaccharide in bone marrow transplant recipients and healthy adults. Clin Infect Dis. 1997;25:1253–5. doi: 10.1086/516965. [DOI] [PubMed] [Google Scholar]
- 33.Abraham-Van Parijs B. Review of pneumococcal conjugate vaccine in adults: implications on clinical development. Vaccine. 2004;22:1362–71. doi: 10.1016/j.vaccine.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis. 2011;52:633–40. doi: 10.1093/cid/ciq207. [DOI] [PubMed] [Google Scholar]
- 35.Bliss SJ, O'Brien KL, Janoff EN, et al. The evidence for using conjugate vaccines to protect HIV-infected children against pneumococcal disease. Lancet Infect Dis. 2008;8:67–80. doi: 10.1016/S1473-3099(07)70242-6. [DOI] [PubMed] [Google Scholar]
- 36.Feavers I, Knezevic I, Powell M, Griffiths E. Challenges in the evaluation and licensing of new pneumococcal vaccines, 7-8 July 2008, Ottawa, Canada. Vaccine. 2009;27:3681–8. doi: 10.1016/j.vaccine.2009.03.087. [DOI] [PubMed] [Google Scholar]
- 37.Siber GR, Chang I, Baker S, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine. 2007;25:3816–26. doi: 10.1016/j.vaccine.2007.01.119. [DOI] [PubMed] [Google Scholar]
- 38.Madhi SA, Adrian P, Kuwanda L, et al. Long-term immunogenicity and efficacy of a 9-valent conjugate pneumococcal vaccine in human immunodeficient virus infected and non-infected children in the absence of a booster dose of vaccine. Vaccine. 2007;25:2451–7. doi: 10.1016/j.vaccine.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Madhi SA, Adrian P, Cotton MF, et al. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. J Infect Dis. 2010;202:355–61. doi: 10.1086/653704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jódar L, Butler J, Carlone G, et al. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine. 2003;21:3265–72. doi: 10.1016/S0264-410X(03)00230-5. [DOI] [PubMed] [Google Scholar]
- 41.Fernsten P, Mason KW, Yu X, et al. 13-valent pneumococcal conjugate vaccine immune sera protects against pneumococcal serotype 1, 3 and 5 bacteremia in a neonatal rat challenge model. Hum Vaccin. 2011;7:75–84. doi: 10.4161/hv.7.0.14566. [DOI] [PubMed] [Google Scholar]
- 42.Abzug MJ, Pelton SI, Song LY, et al. Immunogenicity, safety, and predictors of response after a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2006;25:920–9. doi: 10.1097/01.inf.0000237830.33228.c3. [DOI] [PubMed] [Google Scholar]
- 43.King JC, Jr., Vink PE, Chang I, et al. Antibody titers eight months after three doses of a five-valent pneumococcal conjugate vaccine in HIV and non-HIV-infected children less than two years of age. Vaccine. 1998;16:361–5. doi: 10.1016/S0264-410X(97)80914-0. [DOI] [PubMed] [Google Scholar]
- 44.King JC, Jr., Vink PE, Farley JJ, et al. Comparison of the safety and immunogenicity of a pneumococcal conjugate with a licensed polysaccharide vaccine in human immunodeficiency virus and non-human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1996;15:192–6. doi: 10.1097/00006454-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 45.King JC, Jr., Vink PE, Farley JJ, Smilie M, Parks M, Lichenstein R. Safety and immunogenicity of three doses of a five-valent pneumococcal conjugate vaccine in children younger than two years with and without human immunodeficiency virus infection. Pediatrics. 1997;99:575–80. doi: 10.1542/peds.99.4.575. [DOI] [PubMed] [Google Scholar]
- 46.Madhi SA, Klugman KP, Kuwanda L, Cutland C, Kayhty H, Adrian P. Quantitative and qualitative anamnestic immune responses to pneumococcal conjugate vaccine in HIV-infected and HIV-uninfected children 5 years after vaccination. J Infect Dis. 2009;199:1168–76. doi: 10.1086/597388. [DOI] [PubMed] [Google Scholar]
- 47.Nachman S, Kim S, King J, et al. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants with human immunodeficiency virus type 1 infection. Pediatrics. 2003;112:66–73. doi: 10.1542/peds.112.1.66. [DOI] [PubMed] [Google Scholar]
- 48.Spoulou VI, Tsoumas DL, Papaevangelou VG, Mostrou GI, Theodoridou MC. Immunogenicity and immunological memory induced by a 7-valent pneumococcal CRM197 conjugate vaccine in symptomatic HIV-1 infected children. Vaccine. 2005;23:5289–93. doi: 10.1016/j.vaccine.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Tarragó D, Casal J, Ruiz-Contreras J, et al. Assessment of antibody response elicited by a 7-valent pneumococcal conjugate vaccine in pediatric human immunodeficiency virus infection. Clin Diagn Lab Immunol. 2005;12:165–70. doi: 10.1128/CDLI.12.1.165-170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–8. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 51.doh.gov.za. http://wwwdohgovza/docs/factsheets/guidelines/artguidelines04/intropdf.
- 52.Madhi SA, Cumin E, Klugman KP. Defining the potential impact of conjugate bacterial polysaccharide-protein vaccines in reducing the burden of pneumonia in human immunodeficiency virus type 1-infected and -uninfected children. Pediatr Infect Dis J. 2002;21:393–9. doi: 10.1097/00006454-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Madhi SA, Kuwanda L, Cutland C, Klugman KP. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin Infect Dis. 2005;40:1511–8. doi: 10.1086/429828. [DOI] [PubMed] [Google Scholar]
- 54.Moore DP, Klugman KP, Madhi SA. Role of Streptococcus pneumoniae in hospitalization for acute community-acquired pneumonia associated with culture-confirmed Mycobacterium tuberculosis in children: a pneumococcal conjugate vaccine probe study. Pediatr Infect Dis J. 2010;29:1099–04. doi: 10.1097/INF.0b013e3181eaefff. [DOI] [PubMed] [Google Scholar]
- 55.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–3. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kovacs JA, Masur H. Prophylaxis against opportunistic infections in patients with human immunodeficiency virus infection. N Engl J Med. 2000;342:1416–29. doi: 10.1056/NEJM200005113421907. [DOI] [PubMed] [Google Scholar]
- 57.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. [PubMed] [Google Scholar]
- 58.1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. U.S. Public Health Service (USPHS) and Infectious Diseases Society of America (IDSA) MMWR Recomm Rep. 1999;48:1–59, 61-6. [PubMed] [Google Scholar]
- 59.Kroon FP, van Dissel JT, Ravensbergen E, Nibbering PH, van Furth R. Antibodies against pneumococcal polysaccharides after vaccination in HIV-infected individuals: 5-year follow-up of antibody concentrations. Vaccine. 1999;18:524–30. doi: 10.1016/S0264-410X(99)00240-6. [DOI] [PubMed] [Google Scholar]
- 60.French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355:2106–11. doi: 10.1016/S0140-6736(00)02377-1. [DOI] [PubMed] [Google Scholar]
- 61.López-Palomo C, Martin-Zamorano M, Benitez E, et al. Pneumonia in HIV-infected patients in the HAART era: incidence, risk, and impact of the pneumococcal vaccination. J Med Virol. 2004;72:517–24. doi: 10.1002/jmv.20045. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez-Barradas MC, Alexandraki I, Nazir T, et al. Response of human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy to vaccination with 23-valent pneumococcal polysaccharide vaccine. Clin Infect Dis. 2003;37:438–47. doi: 10.1086/375841. [DOI] [PubMed] [Google Scholar]
- 63.Hung CC, Chen MY, Hsieh SM, Hsiao CF, Sheng WH, Chang SC. Clinical experience of the 23-valent capsular polysaccharide pneumococcal vaccination in HIV-1-infected patients receiving highly active antiretroviral therapy: a prospective observational study. Vaccine. 2004;22:2006–12. doi: 10.1016/j.vaccine.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 64.Watera C, Nakiyingi J, Miiro G, et al. 23-Valent pneumococcal polysaccharide vaccine in HIV-infected Ugandan adults: 6-year follow-up of a clinical trial cohort. AIDS. 2004;18:1210–3. doi: 10.1097/00002030-200405210-00018. [DOI] [PubMed] [Google Scholar]
- 65.Pedersen RH, Lohse N, Ostergaard L, Sogaard O. The effectiveness of pneumococcal polysaccharide vaccination in HIV-infected adults: a systematic review. HIV Med. 2011;12:323–33. doi: 10.1111/j.1468-1293.2010.00892.x. [DOI] [PubMed] [Google Scholar]
- 66.Grau I, Pallares R, Tubau F, et al. Epidemiologic changes in bacteremic pneumococcal disease in patients with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Intern Med. 2005;165:1533–40. doi: 10.1001/archinte.165.13.1533. [DOI] [PubMed] [Google Scholar]
- 67.Dworkin MS, Ward JW, Hanson DL, Jones JL, Kaplan JE. Pneumococcal disease among human immunodeficiency virus-infected persons: incidence, risk factors, and impact of vaccination. Clin Infect Dis. 2001;32:794–800. doi: 10.1086/319218. [DOI] [PubMed] [Google Scholar]
- 68.Teshale EH, Hanson D, Flannery B, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine on pneumonia in HIV-infected adults in the United States, 1998–2003. Vaccine. 2008;26:5830–4. doi: 10.1016/j.vaccine.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 69.Ahmed F, Steinhoff MC, Rodriguez-Barradas MC, Hamilton RG, Musher DM, Nelson KE. Effect of human immunodeficiency virus type 1 infection on the antibody response to a glycoprotein conjugate pneumococcal vaccine: results from a randomized trial. J Infect Dis. 1996;173:83–90. doi: 10.1093/infdis/173.1.83. [DOI] [PubMed] [Google Scholar]
- 70.Kroon FP, van Dissel JT, Ravensbergen E, Nibbering PH, van Furth R. Enhanced antibody response to pneumococcal polysaccharide vaccine after prior immunization with conjugate pneumococcal vaccine in HIV-infected adults. Vaccine. 2000;19:886–94. doi: 10.1016/S0264-410X(00)00232-2. [DOI] [PubMed] [Google Scholar]
- 71.Feikin DR, Elie CM, Goetz MB, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine. 2001;20:545–53. doi: 10.1016/S0264-410X(01)00347-4. [DOI] [PubMed] [Google Scholar]
- 72.Miiro G, Kayhty H, Watera C, et al. Conjugate pneumococcal vaccine in HIV-infected Ugandans and the effect of past receipt of polysaccharide vaccine. J Infect Dis. 2005;192:1801–5. doi: 10.1086/497144. [DOI] [PubMed] [Google Scholar]
- 73.Lesprit P, Pedrono G, Molina JM, et al. Immunological efficacy of a prime-boost pneumococcal vaccination in HIV-infected adults. AIDS. 2007;21:2425–34. doi: 10.1097/QAD.0b013e3282887e91. [DOI] [PubMed] [Google Scholar]
- 74.Gordon SB, Kayhty H, Molyneux ME, et al. Pneumococcal conjugate vaccine is immunogenic in lung fluid of HIV-infected and immunocompetent adults. J Allergy Clin Immunol. 2007;120:208–10. doi: 10.1016/j.jaci.2007.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen M, Ssali F, Mulungi M, et al. Induction of opsonophagocytic killing activity with pneumococcal conjugate vaccine in human immunodeficiency virus-infected Ugandan adults. Vaccine. 2008;26:4962–8. doi: 10.1016/j.vaccine.2008.06.093. [DOI] [PubMed] [Google Scholar]
- 76.Peñaranda M, Payeras A, Cambra A, Mila J, Riera M. Conjugate and polysaccharide pneumococcal vaccines do not improve initial response of the polysaccharide vaccine in HIV-infected adults. AIDS. 2010;24:1226–8. doi: 10.1097/QAD.0b013e3283389de5. [DOI] [PubMed] [Google Scholar]
- 77.Crum-Cianflone NF, Huppler Hullsiek K, Roediger M, et al. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. J Infect Dis. 2010;202:1114–25. doi: 10.1086/656147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Søgaard OS, Schonheyder HC, Bukh AR, et al. Pneumococcal conjugate vaccination in persons with HIV: the effect of highly active antiretroviral therapy. AIDS. 2010;24:1315–22. doi: 10.1097/QAD.0b013e328339fe0b. [DOI] [PubMed] [Google Scholar]
- 79.Søgaard OS, Lohse N, Harboe ZB, et al. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clin Infect Dis. 2010;51:42–50. doi: 10.1086/653112. [DOI] [PubMed] [Google Scholar]
- 80.French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med. 2010;362:812–22. doi: 10.1056/NEJMoa0903029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Concepcion NF, Frasch CE. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2001;8:266–72. doi: 10.1128/CDLI.8.2.266-272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 83.Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 84.Klugman KP. Efficacy of pneumococcal conjugate vaccines and their effect on carriage and antimicrobial resistance. Lancet Infect Dis. 2001;1:85–91. doi: 10.1016/S1473-3099(01)00063-9. [DOI] [PubMed] [Google Scholar]
- 85.Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–51. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 86.Cohen AL, Harrison LH, Farley MM, et al. Prevention of invasive pneumococcal disease among HIV-infected adults in the era of childhood pneumococcal immunization. AIDS. 2010;24:2253–62. doi: 10.1097/QAD.0b013e32833d46fd. [DOI] [PubMed] [Google Scholar]
- 87.Albrich WC, Baughman W, Schmotzer B, Farley MM. Changing characteristics of invasive pneumococcal disease in Metropolitan Atlanta, Georgia, after introduction of a 7-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44:1569–76. doi: 10.1086/518149. [DOI] [PubMed] [Google Scholar]
- 88.Albrich WC, Madhi SA, Lafond KE, Klugman KP. Herd immunity after pneumococcal conjugate vaccination. Lancet. 2007;370:218–9, author reply 9-20. doi: 10.1016/S0140-6736(07)61119-2. [DOI] [PubMed] [Google Scholar]