Abstract
Experimental autoimmune encephalomyelitis (EAE) is an inflammatory disease of the central nervous system (CNS) and has been used as an animal model for study of the human demyelinating disease, multiple sclerosis (MS). EAE is characterized by pathologic infiltration of mononuclear cells into the CNS and by clinical manifestation of paralytic disease. Similar to MS, EAE is also under genetic control in that certain mouse strains are susceptible to disease induction while others are resistant. Typically, C57BL/6 (H-2b) mice immunized with myelin basic protein (MBP) fail to develop paralytic signs. This unresponsiveness is certainly not due to defects in antigen processing or antigen presentation of MBP, as an experimental protocol described here had been used to induce severe EAE in C57BL/6 mice as well as other reputed resistant mouse strains. In addition, encephalitogenic T cell clones from C57BL/6 and Balb/c mice reactive to MBP had been successfully isolated and propagated.
The experimental protocol involves using a cellular adoptive transfer system in which MBP-primed (200 μg/mouse) C57BL/6 donor lymph node cells are isolated and cultured for five days with the antigen to expand the pool of MBP-specific T cells. At the end of the culture period, 50 million viable cells are transferred into naive syngeneic recipients through the tail vein. Recipient mice so treated normally do not develop EAE, thus reaffirming their resistant status, and they can remain normal indefinitely. Ten days post cell transfer, recipient mice are challenged with complete Freund adjuvant (CFA)-emulsified MBP in four sites in the flanks. Severe EAE starts to develop in these mice ten to fourteen days after challenge. Results showed that the induction of disease was antigenic specific as challenge with irrelevant antigens did not induce clinical signs of disease. Significantly, a titration of the antigen dose used to challenge the recipient mice showed that it could be as low as 5 μg/mouse. In addition, a kinetic study of the timing of antigenic challenge showed that challenge to induce disease was effective as early as 5 days post antigenic challenge and as long as over 445 days post antigenic challenge. These data strongly point toward the involvement of a "long-lived" T cell population in maintaining unresponsiveness. The involvement of regulatory T cells (Tregs) in this system is not defined.
Keywords: Immunology, Issue 62, Autoimmune diseases, experimental autoimmune encephalomyelitis, immunization, myelin basic protein, adoptive transfer, paralysis
Protocol
1. Preparing CFA-emulsified Antigen
Dissolve guinea pig MBP (prepared according to the method of Swanborg1 and stored in lyophilized form at 4 °C) in phosphate buffer solution (PBS) at a concentration of 2 mg/mL. If synthesized MBP peptides (MBP60-80, S H H A A R T T H Y G S L P Q K S Q R)2 are used, prepare a 2 mg/mL solution. Draw up an appropriate amount of antigen solution (0.1 mL per mouse to be immunized) into a glass syringe with luer-lok.
Draw up a volume of complete Freund's adjuvant H37Ra (0.1WT%) (CFA, Difco Laboratories) equal to the antigen solution into another glass syringe equipped with luer-lok.
Connect the two glass syringes with a three-way stopcock. Mix the MBP solution with the CFA by pushing the plungers back and forth. Keep the motion until an emulsion is formed.
To test if an emulsion is formed, push all of the mixture into one syringe. Disconnect the empty syringe, pull the plunger back a little and discharge a drop of the residual emulsion into a beaker of clean water at room temperature. If the emulsion drop remains intact on the surface of the water, an emulsion is formed. If the emulsion drop disperses, reconnect the syringes and continue the mixing motion.
Use a 1 mL plastic syringe for the immunization process to ensure delivery of an accurate dose of antigen. To transfer the antigen emulsion to the plastic syringe, pull out the plunger and insert the empty plastic syringe into the opening of three-way stopcock where the previous glass syringe was disconnected as in 1.4.
Fill the plastic syringe to the rim by pushing the plunger of the glass syringe. Insert the plunger of the plastic syringe back and push back excess emulsion till reaching the 1.2 mL mark on the plastic syringe. This maneuver assures that no air bubble is trapped inside the plastic syringe.
Remove the plastic syringe from the three-way stopcock. Attach a #26 gauge needle to the plastic syringe. Push the plunger to the 1 mL mark so that the emulsion now fills the void volume of the needle.
2. Immunization of Donor Mice
Note: Female C57BL/6 mice are typically injected with antigen emulsion in four sites in the flanks, following the methods originally designed by McFarlin's group3 at the National Institutes of Health. For an experienced investigator, one person can perform the whole task. For beginners, help may be solicited so that one person holds the mouse and the other person does the injection. Alternatively, the mouse can be anesthetized for the injection.
Hold the mouse firmly by the neck skin and hold back the tail between the 4th and 5th fingers such that the thorax and abdomen are easily accessible.
Place the needle of the 1mL plastic syringe at a site in the thorax slightly above the right armpit (of the mouse) and inject 0.05 mL of the antigen emulsion subcutaneously. A little bulge of the whitish CFA can be seen through the pale skin. Repeat the same for the site in the thorax slightly above the left armpit.
Release the tail and take hold of the right foot (of the mouse), turn it and hold it between the 4thand 5th fingers. Locate the site just below the rib case in the right flank and inject 0.05 mL of the antigen emulsion.
Release the right foot, turn the mouse the other way and hold the left foot (of the mouse) between the 4th and 5th fingers. Locate the site just below the rib case in the left flank and inject 0.05 mL of the antigen emulsion.
Change to a fresh needle after injection of 10 mice, or if the needle becomes dulled.
3. Isolation of Lymph Node Cells for Tissue Culture 7 to 10 Days after Immunization
Seven to 10 days after immunization, sacrifice the mice and remove the draining lymph nodes. The preferred method of euthanasia is by CO2 inhalation. After sacrifice, lay the mouse on its back on a dissection board with the limbs stretched out and secured to the board with pins. The mice are wetted with a spray of 70% ethanol.
Cut a slit in the skin longitudinally in the middle from the lower end of the abdomen up to the chin with sterile scissors and forceps.
Cut the abdomen skin from the area of the groin down to each leg. Peel back and pin down the skin, exposing the inguinal lymph nodes.
Cut the skin along the forelimbs (left and right) so that the skin can be peeled back and pinned down to expose the axillary lymph nodes near the armpits.
With two pairs of forceps working together, pull away the connective tissues covering and around the inguinal lymph nodes. When the connective tissues are cleared, place one pair of forceps under the lymph node and pull away from the body. Place the lymph nodes in a 35 mm Petri dish filled with sterile PBS.
Pull away the connective tissues covering the two axillary lymph nodes using two pairs of forceps. Isolate the lymph nodes and place them in the Petri dish. Be very careful not to break the blood vessels crossing the area as bleeding makes identifying the lymph nodes very difficult.
After all the lymph nodes are collected, move the Petri dish to a biosafety hood. Break and tease the lymph nodes apart with the two pairs of forceps, grinding one against the other. Alternatively, the lymph nodes can be broken up by using the flat end of the plunger of a 3 mL plastic syringe pressing against the lymph nodes on the bottom of the Petri dish.
Pass the teased lymph node cell suspension through a nylon mesh to remove the membranes and debris.
Make up the lymph node cell suspension to 50 mL using sterile PBS. Centrifuge at 1000 rpm for 10 mins. Resuspend the pellet in culture medium. Do a viable cell count and adjust the concentration to 4 x 106 cells/mL. UseRPMI 1640 supplemented with 10% fetal calf serum, 2 mM L-glutamine, 1 mM MEM sodium pyruvate, 0.1 mM MEM non-essential amino acids, 100 U/mL penicillin, 100 μg/mL streptomycin, 10 mM Hepes buffer and 5 x 10-5 M 2-ME. Pre-warm the culture before resuspending the cells.
Culture the cells in 24-well culture plates with 2 mL volume per well (final cell concentration 8 x 106 cells/well). Add antigen to each well at a final concentration of 100 μg/mL. Place the culture plate in a 37 °C incubator with 5% CO2. Incubate cells for 5 days.
4. Harvesting Cultured Lymph Node Cells 5 Days after Initiation of Cultures
Mix the cells in each well by pipetting up and down with a Pasteur glass pipette or a small volume pipette. Harvest the cells to a 50 mL centrifuge tube.
Centrifuge the cells at 1000 rpm for 10 mins. Resuspend the cell pellet into a small volume of PBS.
Count cells and adjust the viable cell concentration to 2.5 x 108 cells/mL with sterile PBS. Draw the cells into a syringe fitted with a #26 gauge needle.
Place a heat lamp over the cage of recipient mice to help dilate the tail vein.
Using a mouse holder, extend the tail of the mouse through the slit in the holder. Pull to extend the tail. Locate the vein.
Inject 0.2 mL/mouse of the cell suspension, equaling 5 x 107 cells.
5. Antigenic Challenge of Recipient Mice 20 Days Post Cell Transfer and Development of Disease Symptoms
Challenge recipient mice not showing signs of disease with the antigen. The procedures are the same as section 2 on "Immunization of Donor Mice".
- Observe development of paralytic disease 7 to 10 days after antigenic challenge.
- Disease starts with weakness in the tail. Disease is graded as 1 when the tail becomes flaccid. Disease is graded as 2 when the mouse is paralyzed in one hind limb. Disease is graded 3 when both hind limbs are paralyzed and the mouse drags both hind limbs when crawling forward. Disease is graded 4 when the mouse loses use of both forelimbs, leaving the mouse totally immobile. Disease is graded 5 when the mouse is moribund and the body curls up. Death is scored as disease grade 64.
MBP-specific EAE induced in C57BL/6 mice following this protocol is monophasic. The mice may recover from the disease but do not undergo spontaneous relapses.
6. Representative Results
Mice that received primed and in vitro-expanded donor lymph node cells did not develop EAE disease symptoms, reflecting their resistance to disease induction. However, severe disease developed after antigenic challenge (Table I), demonstrating the ability of the antigenic challenge in reversing the resistance mechanism. Two special features of the antigenic challenge noted are the doses of antigen and the kinetics of challenge to induce disease. Very low dose of antigen is needed as shown in Table III. Surprisingly, mice that received donor cells a year earlier could still develop severe disease when challenged with the antigen (Table IV). These two observations strongly suggest the involvement of "long-lived" T cells in maintaining EAE resistance. This protocol is applicable to many EAE resistant mouse strains4.
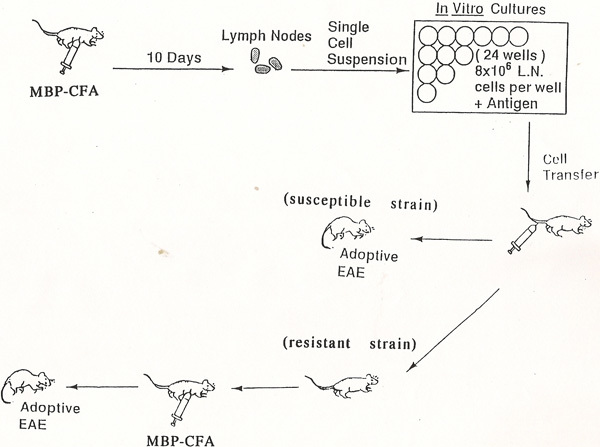 Figure 1. Flow chart of the experimental design for induction of EAE in reputed resistant mouse strains.
Figure 1. Flow chart of the experimental design for induction of EAE in reputed resistant mouse strains.
| Clinical EAE after adoptive transfer | Clinical EAE after antigenic challenge | ||||||
| Strain | Antigen | incidence | Av. disease grade (range) | Av. day of onset (range) | Incidence | Av. disease grade (range) | Av. day of onset (range) |
| SJL | MBP | 21/21 | 4.0 (3-5) | 8.0 (6-10) | N.D. | N.D. | N.D. |
| C57BL/6 | MBP | 0/10 | - - | - - | 7/7 | 3.85 (3-5) | 9.28 (9-10) |
| Balb/c | MBP | 0/7 | - - | - - | 5/5 | 3.2 (3-4) | 7.0 (7) |
| C3H/HeJ | MBP | 0/7 | - - | - - | 4/4 | 3.2 (3-4) | 8.0 (8) |
| C57BL/6 | MBP60-80 | 0/4 | - - | - - | 4/4 | 2.75 (2-4) | 10.2 (9-11) |
Table I. Induction of MBP-mediated EAE in C57BL/6 mice. Adoptive transfer of primed and in vitro-expanded donor cells did not induce EAE in C57Bl/6 mice. Antigenic challenge post cell transfer rendered the mice susceptible to disease induction. Av., average. N.D., not done.
| Clinical EAE after antigenic challenge | ||||
| Priming Antigen | Challenge antigen | Incidence | Av. disease grade (range) | Av. day of onset (range) |
| MBP-CFA | CFA alone | 0/3 | - - | - - |
| MBP-CFA | OVA-CFA | 0/3 | - - | - - |
| MBP-CFA | MBP-CFA | 3/3 | 4.0 (4.0) | 7.7 (7-9) |
Table II. Specificity of antigenic challenge. Recipient mice were challenged with the priming antigen as well as other irrelevant antigens. Only the priming antigen induced severe disease. OVA: chicken ovalbumin. Av., average.
| Clinical EAE after antigenic challenge | |||
| Challenge antigen doses (μg/mouse) | Incidence | Av. disease grade (range) | Av. day of onset (range) |
| 200 | 4/4 | 3.75 (3-4) | 8.25 (7-9) |
| 100 | 4/4 | 3.67 (3-4) | 7.5 (7-8) |
| 50 | 4/4 | 3.25 (3-4) | 8.25 (7-10) |
| 25 | 4/4 | 3.0 (3.0) | 8.25 (8-9) |
| 10 | 4/4 | 3.0 (3.0) | 8.76 (8-9) |
| 5 | 4/4 | 2.5 (2-3) | 9.75 (9-10) |
| 1 | 2/4 | 1.0 (1.0) | 16 (14-18) |
| 0 | 0/4 | - - | - - |
Table III. Challenge antigen doses required for disease induction. Various concentrations of MBP used to challenge recipient mice were tested. Av., average.
| Clinical EAE after antigenic challenge | ||||
| Challenge antigen | Day of Challenge | Incidence | Av. Disease grade (range) | Av. Day of onset (range) |
| MBP-CFA | 5 | 3/4 | 1.33 (1-2) | 9.67 (9-10) |
| MBP-CFA | 10 | 4/4 | 3.0 (3.0) | 8.25 (7-9) |
| MBP-CFA | 15 | 4/4 | 3.75 (3-4) | 7.25 (7-8) |
| MBP-CFA | 25 | 4/4 | 4.0 (4.0) | 8.0 (7-9) |
| MBP-CFA | 35 | 4/4 | 3.5 (3-4) | 7.5 (7-8) |
| MBP-CFA | 60 | 4/4 | 3.75 (3-4) | 9.0 (8-10) |
| - - - - - - - - - - - - - - - - - - - - - - - - - - | ||||
| MBP-CFA | 343 | 3/3 | 3.0 (3.0) | 10.33 (10-11) |
| MBP-CFA | 445 | 3/3 | 3.0 (3.0) | 7.0 (7.0) |
Table IV. Kinetics of antigenic challenge. Recipient mice were challenged at various time point post adoptive cell transfer. Av., average.
Discussion
Studies of EAE in mice often utilize the neuroantigens, myelin basic protein (MBP), proteolipid protein (PLP) or myelin oligodendrocyte protein (MOG). Earlier studies mostly used MBP. PLP stimulates strong and consistent responses from SJL mice. More recently, MOG is the common neuroantigen used because B6 mice are susceptible to disease induction with this antigen. Many of the gene targeted mouse strains are on the B6 genetic background. Interestingly, B6 mice are susceptible to EAE induction with MOG but are resistant to disease induction with MBP.
Much of the previous effort in elucidating the mechanisms of EAE centered on the induction mechanisms of disease, the effects of cytokine production and the recovery mediated by regulatory T cells5-7. How reputed EAE resistant mice can subvert the induction of disease, on the other hand, had not been widely investigated. This may be due to the difficulty of quantifying unresponsiveness. Arnon8 in 1981 first showed that treating B6 mice with cyclophosphamide rendered these mice susceptible to EAE induced with MBP. Others showed that treatment with anti-gamma interferon also successfully induced disease in B6 and Balb/c mice9,10. It should be noted that these studies all targeted non-antigen specific markers. On the other hand, the protocol described here focuses on a different aspect of EAE resistance. Here only specific antigen used in the challenge can overcome EAE unresponsiveness and allows induction of disease. This protocol encompasses both the EAE resistance phase with adoptive transfer of primed donor T cells and the susceptibility phase with antigenic challenge (Table I). Experiments can be designed to study the intrinsic factors that maintain EAE resistance as well as factors that reverse this unresponsiveness. Initially, it was shown that EAE resistant mice were able to mount autoimmune responses when immunized with MBP4. Later, it was shown that encephalitogenic T cells existed in these mice2. Some recent studies11,12 showed that treatment of B10.S mice with anti-CD25 antibodies prior to immunization of the mice to proteolipid protein (PLP) converted the mice from resistant to susceptible, implying the role of regulatory T cells (Tregs) in maintaining EAE resistance. However, this is not the case when C57BL/6 mice are similarly treated and immunized with MBP13. Most of the mice remain unresponsive. Thus, EAE resistance may be multi-factorial. It is postulated that, in the case of C57BL/6 mice responding to MBP, thymic selection has deleted most MBP-reactive T cells such that the frequencies of MBP-reactive T cells in the periphery are very low. This below-threshold low frequency would render the mice not able to respond to immunization with MBP. The challenge dose, through unknown mechanisms, is able to overcome the frequency issue and mount an autoimmune response. Further investigations of the possible mechanisms would include the role of IL-612 during antigenic challenge in affecting the susceptibility of encephalitogenic to inhibition by Tregs.
Disclosures
No financial disclosures.
Acknowledgments
Supported in part by grants from the National Institutes of Health (R01 NS37253 and N01 NS055167) and the National Multiple Sclerosis Society (RG 3288-A6).
References
- Swanborg RH. Experimental allergic encephalomyelitis. In: Sabato GD, editor. Methods in Enzymology. Vol. 162. New York, NY: Academic Press; 1988. pp. 413–421. [DOI] [PubMed] [Google Scholar]
- Shaw MK, Kim C, Hao HW, Chen F, Tse HY. Induction of myelin basic protein-specific experimental autoimmune encephalomyelitis in C57BL/6 mice: Mapping of T cell epitopes and T cell Vb gene segment usage. J. Neuroscience Research. 1996;45:690–699. doi: 10.1002/(SICI)1097-4547(19960915)45:6<690::AID-JNR5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- McCarron R, McFarlin DE. Adoptively transferred experimental autoimmune encephalomyelitis in SJL/J, PL/J, and (SJL/J x PL/J)F1 mice. Influence of I-A haplotypes on encephalitogenic epitope of myelin basic protein. J. Immunol. 1988;141:1143–1149. [PubMed] [Google Scholar]
- Shaw MK, Kim C, Ho K-L, Lisak RP, Tse HY. A combination of adoptive transfer and antigenic challenge induces consistent murine experimental autoimmune encephalomyelitis in C57BL/6 mice and other reputed resistant strains. J. Neuroimmunol. 1993;39:139–150. doi: 10.1016/0165-5728(92)90183-l. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy G, Saxena A, Mars LT, Domingues HS, Mentele R, Ben-Nun A, Lassmann H, Dornmair K, Kurschus FC, Liblau RS, Wekerle H. Myelin-specific T cells also recognize neuronal autoantigen in a transgenic mouse model of multiple sclerosis. Nat. Med. 2009;15:626–632. doi: 10.1038/nm.1975. [DOI] [PubMed] [Google Scholar]
- Anderson AC, Reddy J, Nazareno R, Sobel RA, Nicholson LB, Kuchroo VK. IL-10 plays an important role in the homeostatic regulation of the autoreactive reperteroire in naïve mice. J. Immunol. 2004;173:828–834. doi: 10.4049/jimmunol.173.2.828. [DOI] [PubMed] [Google Scholar]
- Anderton SM. Treg and T-effector cells in autoimmune CNS inflammation: a delicate balance easily disturbed. Eur. J. Immunol. 2010;40:332–334. doi: 10.1002/eji.201041100. [DOI] [PubMed] [Google Scholar]
- Arnon R. Experimental allergic encephalomyelitis - susceptibility and suppression. Immunol. Rev. 1981;55:5. doi: 10.1111/j.1600-065x.1981.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E, Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-g. J. Immunol. 1988;140:1506–1510. [PubMed] [Google Scholar]
- Arbomson-Leeman S, Alexander J, Bronson R, Corroll J, Southwood S, Dorf M. Experimental autoimmune encephalomyelitis-resistant mice have highly encephalitogenic myelin basic protein (MBP)-specific T cell clones that recognize a MBP peptide with high affinity for MHC class II. J. Immunol. 1995;154:388–398. [PubMed] [Google Scholar]
- Reddy J, Illes Z, Zhang X, Encinas J, Nicholson L, Sobel RA, Wucherpfennig KW, Kuchroo VK. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT. Myelin- specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nature Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw MK, Li J, Ho PP, Hao H, Lisak RP, Tse HY. Induction of myelin basic protein-specific adoptive experimental autoimmune encephalomyelitis in C57BL/6 mice. Longevity of donor T cells and lack of disease relapses. In: Broglie PV, editor. Neuroimmunology Research Focus. New York: Nova Science Publisher; 2007. pp. 175–191. [Google Scholar]


