Abstract
Although only discovered in 1999, the symbiotic filamentous actinobacteria present on the integument of certain species of leaf-cutting ants have been the subject of intense research. These bacteria have been shown to specifically suppress fungal garden parasites by secretion of antibiotics. However, more recently, a wider role for these bacteria has been suggested from research revealing their generalist anti-fungal activity. Here we show, for the first time, evidence for a role of these bacteria in the defence of young worker ants against a fungal entomopathogen. Experimental removal of the bacterial bio-film using an antibiotic resulted in a significant increase in susceptibility of worker ants to infection by the entomopathogenic fungus Metarhizium anisopliae. This is the first direct evidence for the advantage of maintaining a bacterial bio-film on the cuticle as a defensive strategy of the ants themselves and not exclusively for protection of the fungus garden.
Keywords: defence mechanisms, symbiosis, pathogen, Metarhizium anisopliae, actinobacteria
1. Introduction
Leaf-cutting ants possess sophisticated defensive strategies to counter the spread of diseases between colony members but also to avoid infestations of their fungus gardens by pathogens and saprophytes. The symbiotic association with Leucocoprineae and Pterulaceae fungi, which the ants cultivate as a food source, has evolved over the last 45–50 million years [1]. The fungus garden is maintained meticulously clean by the ants, firstly by the removal of contaminants from the plant material brought into the nest by the foragers, when gardener ants lick the leaf surfaces, ingesting any contaminating material that is rendered innocuous in the infrabucal pocket [2]. The same method is used for the removal of weeds from the fungus garden [3]. Worker ants decontaminate themselves by self-grooming and other colony members by allo-grooming [4]. Apart from these behavioural tactics, ants also employ chemical defences against parasites that include the secretion of antibiotic compounds from the metapleural glands [5], and the production of antibiotics by bacteria associated with the integument [6] or present in the fungus garden [7].
Despite the highly fastidious behaviour of attine ants, their gardens are occasionally overrun by a specialized parasitic fungus of the genus Escovopsis [8]. Currie et al. [9] discovered a new level of symbiosis in the leaf-cutting ants, a bacterium present on the integument (originally identified as a Streptomyces), which produces potent antibiotic secretions with specific activity against Escovopsis. Further studies presented evidence for direct interactions between the ants, the symbiotic integumental bacteria and Escovopsis [10]. Candicidin macrolides isolated from leaf-cutting ant-associated Streptomyces inhibited Escovopsis, but demonstrated no activity against the ant's garden fungus or three species of entomopathogenic fungi [11]. However, other studies have show that the bacteria isolated from the cuticles of leaf-cutting ants were capable of inhibiting a wide range of micro-organisms in vitro including the ants own symbiotic fungus [12]. Although antagonism has been demonstrated between leafcutter cultivars and filamentous Pseudonocardia bacteria in Petri dish bioassays, this did not result in a negative impact on the fungus garden [13]. An additional role for these actinomycetes should be considered, without excluding their importance in suppressing microbial infestations of the ant's fungus gardens.
There are few reports of entomopathogenic fungi naturally attacking leaf-cutting ants [14], even though a high abundance of Metarhizium anisopliae was found in soil samples near ant colonies [15]. This could reflect their highly organized defence strategies. As entomopathogenic fungi attack their hosts by penetration of the cuticle, microbiota present on the host integument may serve as an extra line of defence. The results presented here investigate the role of these bacteria in protecting worker ants against fungal infection.
2. Material and methods
(a). Insects
Acromyrmex subterraneus subterraneus worker ants were obtained from a single colony recently collected in the field (Bom Jardim: 22°09′07″ S and 42°25′10″ W) and maintained in the laboratory as previously described [16]. Only worker ants with a head capsules of greater than 2 mm and supporting an extensive growth of bacterial bio-film on the cuticle (score 12 using the bacterial bio-film scale created by Poulsen et al. [17]) were used. Ants were separated using sterile fine forceps. One hundred and twenty ants were used for each replicate experiment (60 exposed to Metarhizium and 60 controls). The experiment was carried out three times, with a total of 360 ants used in this study.
(b). Fungal isolate and preparation of conidial suspensions
The isolate of M. anisopliae used here was obtained from the collection at ESALQ (ESALQ818; originally isolated from a soil sample) in Piracicaba (São Paulo). The fungus was cultured on sabouraud dextrose agar (SDA) at 27°C for 15 days. Fungal suspensions were prepared in 0.05 per cent Tween 80 (TW) and conidial concentration determined using a Neubauer haemocytometer. A final concentration of 1 × 108 conidia per millilitre was prepared by serial dilution. Fungal suspensions were vortexed vigorously before evenly applying 750 µl to filter paper discs (9 cm diameter) in Petri dishes using a micropipette.
(c). Antibiotic and sterile distilled water pre-treatments of ants prior to exposure to conidia
Ants were submersed for 5 s in a gentamicin (GENT) solution (8 mg ml−1; Schering-Plough, Brazil) dissolved in sterile distilled water (SDW) with the aid of sterile fine forceps. For water pre-treatment, ants were submersed in SDW for 5 s.
(d). Exposure to fungal conidia
One hour following these pre-treatments, ants were exposed to M. anisopliae conidia (MET) in Petri dishes (five ants per dish). Ants were maintained for 24 h in contact with the fungus before being transferred to sterile dishes. Controls for each treatment group were submersed in GENT or SDW and then placed in dishes with filter paper discs to which 750 µl of TW had been applied. Two cotton wool balls were added to each dish following the initial 24 h exposure to fungi, one soaked in 10 per cent sucrose and the other soaked in SDW that were changed every 48 h. Survival was evaluated on a daily basis for 10 days.
(e). Statistical analysis
A two-way ANOVA with Duncan's multiple range test was used to analyse the differences in endpoint survival rates between treatments and between Petri dishes within treatments.
3. Results
The survival curves for worker ants pre-treated with GENT or SDW followed by exposure to fungal conidia (and their respective controls) are shown in figure 1. Pre-treatment with GENT significantly increased the susceptibility of the ants to fungal infection, resulting in a mean survival rate of 15.5 per cent (s.e.m. ± 8.9) on day 10, when compared with a survival rate of 49.9 per cent (±8.8) observed for ants pre-treated with SDW and then exposed to M. anisopliae.
Figure 1.
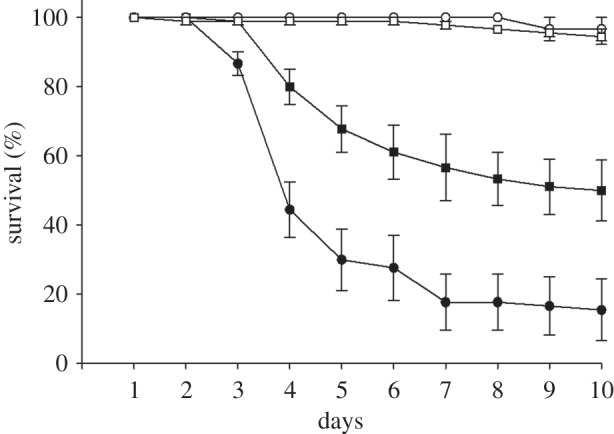
Survival curves of A. subterraneus subterraneus workers that had been pre-treated with gentamicin (GENT) or sterile distilled water (SDW) before exposure to the entomopathogenic fungus Metarhizium anisopliae (MET). Controls were pre-treated with GENT or SDW and then exposed to Tween (TW). The results are the mean survival of each group of five ants per Petri dish, with a total of 90 ants used per treatment. Error bars: s.e.m. Filled circles, GENT + MET; filled squares, SDW + MET; open circles, GENT + TWEEN; open squares, SDW + TWEEN.
A repeated two-way ANOVA showed that there was an effect of treatments (F3,8 = 17.10; p < 0.01), but no effect of experimental unit (Petri dishes; F5,30 = 0.83; p = 0.54) and no interaction between treatments × experimental unit (F15,40 = 1.04; p = 0.44). Duncan's multiple range test showed that the GENT + MET group had the lowest survival rate when compared with all other groups (p < 0.05). The results also showed that the SDW + MET group had a lower survival rate than the GENT + TWEEN and SDW + TWEEN groups (p < 0.05). There was no difference between the GENT + TWEEN and SDW + TWEEN groups (p > 0.05). Control survival rates were 94.4 per cent (±2.2) for ants pre-treated with SDW and 96.6 per cent (±3.3) for GENT pre-treated ants.
Treatment with GENT visibly reduced the bacterial population on the cuticle (figure 2a (before antibiotic treatment); figure 2b (following antibiotic treatment)), whereas the SDW pre-treatment did not have any obvious visible effect on the bacteria (figure 2c). There were no significant changes in locomotor behaviour between treatments (F3,49 = 0.036; p > 0.05; electronic supplementary material, figure S1). GENT had no effect on M. anisopliae growth when using a standard challenge bioassay (results not shown).
Figure 2.

Effects of antibiotic and water treatment on bacterial bio-films: (a) A. subterraneus subterraneus major caste workers with extensive bio-film before antibiotic treatment; (b) antibiotic treatment; (c) treatment with water. Scale bar, 2 mm.
4. Discussion
During the first 12–15 days of the adult phase, the integument of A. subterraneus subterraneus major workers supports a visible and increasingly extensive bacterial coating [17]. Removal of the bacterial bio-film from workers resulted in an increased incidence of Escovopsis infestations of the fungus garden [10]. However, these bacteria could also have a role in the protection of the colony as a whole. Currie et al. [10] briefly discussed the possibility that the bacterial bio-film could serve as a physical barrier preventing fungal spores from coming into contact with the insect's exoskeleton.
The removal of the bacterial bio-film with antibiotics significantly increases the susceptibility of the ants to infection by M. anisopliae. Anti-fungal compounds secreted by actinobacteria isolated from A. subterraneus subterraneus inhibit the growth of M. anisopliae as seen in Petri dish challenge assays (electronic supplementary material, figure S2). Therefore, the removal of bacterial bio-films probably reduces the concentration of anti-fungal compounds on the cuticle surface and increases the chances of conidial germination or germ tube formation, resulting in higher infection rates.
Younger workers serve the needs of the colony for a longer time period and protecting them potentially offers the greatest return on investment in defence against disease. However, recently emerged workers may be particularly vulnerable to infection as (i) the integument has yet to fully develop its protective layers [18]; (ii) the immune system in younger ants is less active [19]; and (iii) the metapleural glands of young ants might not secrete antibiotics immediately [20]. Thus, extensive symbiotic bacterial bio-films present over the first weeks of adult life could be particularly cost-effective in protecting the most vulnerable colony members.
The role of antibiotic metapleural-gland secretions is noteworthy here, because different attine ant species appear to specialize on either using metapleural-gland secretions or integumental bacterial bio-films as their primary defence against entomopathogens. Fernández-Marín et al. [21] demonstrated that Atta and Sericomyrmex workers lacking visible bacteria bio-films responded to pathogen challenges by increasing metapleural-gland grooming rates, whereas Acromyrmex and Trachymyrmex (which maintain abundant bacteria bio-films) displayed lower metapleural-glands grooming rates in response to infection.
The results presented here support a role of actinobacteria as a first line of defence against fungal pathogen attack. The integument is known to be an effective barrier against pesticides, predators and pathogens. In the case of Acromyrmex, this defensive barrier is reinforced by bacteria-secreting anti-fungal compounds.
Acknowledgements
This research was supported by FAPERJ (E-26/110.533/2009) and CNPq (471627/2008-9). We thank Marinete Pinheiro Carrera for statistical analysis and Prof. Stuart Reynolds and Prof. Ulrich Mueller for their helpful comments. Richard Ian Samuels is a CNPq research fellow.
References
- 1.Schultz T. R., Brady S. G. 2008. Major evolutionary transitions in ant agriculture. Proc. Natl Acad. Sci. USA 105, 5435–5440 10.1073/pnas.0711024105 (doi:10.1073/pnas.0711024105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Little A. E. F., Murakami T., Mueller U. G., Currie C. R. 2006. Defending against parasites: fungus-growing ants combine specialized behaviours and microbial symbionts to protect their fungus garden. Biol. Lett. 2, 12–16 10.1098/rsbl.2005.0371 (doi:10.1098/rsbl.2005.0371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Currie C. R., Stuart A. E. 2001. Weeding and grooming of pathogens in agriculture by ants. Proc. R. Soc. Lond. B 268, 1033–1039 10.1098/rspb.2001.1605 (doi:10.1098/rspb.2001.1605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morelos-Juárez C., Walker T. N., Lopes J. F. S., Hughes W. O. H. 2010. Ant farmers practice proactive personal hygiene to protect their fungus crop. Curr. Biol. 20, R553–R554 10.1016/j.cub.2010.04.047 (doi:10.1016/j.cub.2010.04.047) [DOI] [PubMed] [Google Scholar]
- 5.Bot A. N. M., Ortius-Lechner D., Finster K., Maile R., Boomsma J. J. 2002. Variable sensitivity of fungi and bacteria to compounds produced by the metapleural glands of leaf-cutting ants. Insect. Soc. 49, 363–370 10.1007/PL00012660 (doi:10.1007/PL00012660) [DOI] [Google Scholar]
- 6.Currie C. R., Poulsen M., Mendenhall J., Boomsma J. J., Billen J. 2006. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 311, 81–83 10.1126/science.1119744 (doi:10.1126/science.1119744) [DOI] [PubMed] [Google Scholar]
- 7.Santos A. V., Dillon R. J., Dillon V. M., Reynolds S. E., Samuels R. I. 2004. Occurrence of the antibiotic producing bacterium Burkholderia sp. in colonies of the leaf-cutting ant Atta sexdens rubropilosa. FEMS Microbiol. Lett. 239, 319–323 10.1016/j.femsle.2004.09.005 (doi:10.1016/j.femsle.2004.09.005) [DOI] [PubMed] [Google Scholar]
- 8.Currie C. R. 2001. Prevalence and impact of a virulent parasite on a tripartite mutualism. Oecologia 128, 99–106 10.1007/S004420100630 (doi:10.1007/S004420100630) [DOI] [PubMed] [Google Scholar]
- 9.Currie C. R., Scott J. A., Summerbell R. C., Malloch D. 1999. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 398, 701–704 10.1038/19519 (doi:10.1038/19519) [DOI] [Google Scholar]
- 10.Currie C. R., Bot A. N. M., Boomsma J. J. 2003. Experimental evidence of a tripartite mutualism: bacteria protect ant fungus gardens from specialized parasites. Oikos 101, 91–102 10.1034/j.1600-0706.2003.12036.x (doi:10.1034/j.1600-0706.2003.12036.x) [DOI] [Google Scholar]
- 11.Haeder S., Wirth R., Herz H., Spiteller D. 2009. Candicidin-producing Streptomyces support leaf-cutting ants to protect their fungus garden against the pathogenic fungus Escovopsis. Proc. Natl Acad. Sci. USA 106, 4742–4746 10.1073/pnas.0812082106 (doi:10.1073/pnas.0812082106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen R., Ishaka H. D., Estrada D., Dowd S. E., Hong E., Mueller U. G. 2009. Generalized antifungal activity and 454-screening of Pseudonocardia and Amycolatopsis bacteria in nests of fungus-growing ants. Proc. Natl Acad. Sci. USA 106, 17 805–17 810 10.1073/pnas.0904827106 (doi:10.1073/pnas.0904827106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulsen M., Currie C. R. 2010. Symbiont interactions in a tripartite mutualism: exploring the presence and impact of antagonism between two fungus-growing ant mutualists. PLoS ONE 5, e8748. 10.1371/journal.pone.0008748 (doi:10.1371/journal.pone.0008748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes D. P., Evans H. C., Hywel-Jones N., Boomsma J. J., Armitage S. A. O. 2009. Novel fungal disease in complex leaf-cutting ant societies. Ecol. Entomol. 34, 214–220 10.1111/j.1365-2311.2008.01066.x (doi:10.1111/j.1365-2311.2008.01066.x) [DOI] [Google Scholar]
- 15.Hughes W. O. H., Thomsen L., Eilenberg J., Boomsma J. J. 2004. Diversity of entomopathogenic fungi near leaf-cutting ant nests in a neotropical forest, with particular reference to Metarhizium anisopliae var. anisopliae. J. Invert. Pathol. 85, 46–53 10.1016/j.jip.2003.12.005 (doi:10.1016/j.jip.2003.12.005) [DOI] [PubMed] [Google Scholar]
- 16.Moreira D. D. O., Viana-Bailez A. M., Erthal M., Jr, Bailez O., Carrera M. P., Samuels R. I. 2010. Resource allocation among worker castes of the leaf-cutting ants Acromyrmex subterraneus subterraneus through trophallaxis. J. Insect Physiol. 56, 1665–1670 10.1016/j.jinsphys.2010.06.018 (doi:10.1016/j.jinsphys.2010.06.018) [DOI] [PubMed] [Google Scholar]
- 17.Poulsen M., Bot A. N. M., Currie C. R., Nielsen M. G., Boomsma J. J. 2003. Within-colony transmission and the cost of a mutualistic bacterium in the leaf-cutting ant Acromyrmex octospinosus. Funct. Ecol. 17, 260–269 10.1046/j.1365-2435.2003.00726.x (doi:10.1046/j.1365-2435.2003.00726.x) [DOI] [Google Scholar]
- 18.Reynolds S. E., Samuels R. I. 1996. Physiology and biochemistry of insect moulting fluid. Adv. Insect Physiol. 26, 157–232 10.1016/S0065-2806(08)60031-4 (doi:10.1016/S0065-2806(08)60031-4) [DOI] [Google Scholar]
- 19.Armitage S. A. O., Boomsma J. J. 2010. The effects of age and social interactions on innate immunity in a leaf-cutting ant. J. Insect Physiol. 56, 780–787 10.1016/j.jinsphys.2010.01.009 (doi:10.1016/j.jinsphys.2010.01.009) [DOI] [PubMed] [Google Scholar]
- 20.Poulsen M., Bot A. N. M., Boomsma J. J. 2003. The effect of metapleural gland secretion on the growth of a mutualistic bacterium on the cuticle of leaf-cutting ants. Naturwissenschaften 90, 406–409 10.1007/s00114-003-0450-3 (doi:10.1007/s00114-003-0450-3) [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Marín H., Zimmerman J. K., Nash D. R., Boomsma J. J., Wcislo W. T. 2009. Reduced biological control and enhanced chemical pest management in the evolution of fungus farming in ants. Proc. R. Soc. B 276, 2263–2269 10.1098/rspb.2006.3492 (doi:10.1098/rspb.2006.3492) [DOI] [PMC free article] [PubMed] [Google Scholar]


