Abstract
Optimal foraging models predict that large predators should concentrate on large prey in order to maximize their net gain of energy intake. Here, we show that the largest species of sea turtle, Dermochelys coriacea, does not strictly adhere to this general pattern. Field observations combined with a theoretical model suggest that a 300 kg leatherback turtle would meet its energetic requirements by feeding for 3–4 h a day on 4 g jellyfish, but only if prey were aggregated in high-density patches. Therefore, prey abundance rather than prey size may, in some cases, be the overriding parameter for foraging leatherbacks. This is a classic example where the presence of small prey in the diet of a large marine predator may reflect profitable foraging decisions if the relatively low energy intake per small individual prey is offset by high encounter rates and minimal capture and handling costs. This study provides, to our knowledge, the first quantitative estimates of intake rate for this species.
Keywords: plankton, trophic-niche, allometry, food web, carnivores, filter feeders
1. Introduction
Optimal foraging models predict that large predators should concentrate on large prey in order to maximize their net gain of energy intake [1]. Accordingly, a positive relationship between predator–prey body size is found for both terrestrial and marine predators [2,3]. For example, small terrestrial mammalian carnivores (less than 20 kg) feed mostly on prey that is less than or equal to 45 per cent of their own mass, whereas larger terrestrial mammalian carnivores (more than 20 kg) specialize in feeding on large prey nearly equal to their own mass. Similarly, prey size generally increases with predator size in marine ecosystems [2], even though a simple relationship between predator–prey body size cannot usually be assumed without taking prey behaviour and density into account.
In accordance with the prediction of optimal foraging models, the giant leatherback sea turtle, Dermochelys coriacea (adult body mass approx. 250–450 kg), is thought to feed on large species of jellyfish, such as Rhizostoma sp. (typical body mass: 2–20 kg), Chrysaora sp. (0.4–1 kg) and Cyanea capillata (0.5–5 kg) [4]. Virtually, all feeding observations occurred at high-latitude foraging grounds—vast distances from leatherback nesting beaches in the tropics—where these large species of jellyfish are often common [4,5]. However, previous studies have also suggested that female leatherbacks may feed during the nesting season [6,7], although no direct observations of such behaviour have been reported so far.
Here, we report on feeding dynamics of male leatherback turtles that are novel for two reasons: (i) the animals were directly observed foraging in warm tropical waters, close to known nesting beaches just prior to the peak of leatherback nesting in this area, and (ii) they were feeding on an ultra-dense aggregation of very small jellyfish. The novelty of these direct observations broadens our understanding of the feeding ecology of this large marine reptile, but seems to contradict predictions of optimal foraging models regarding predator–prey size relationships.
2. Material and methods
(a). Field observations
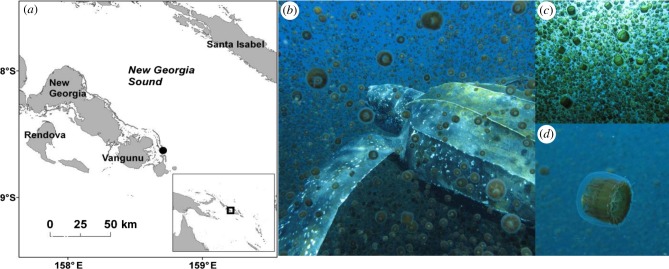
Field observations took place on 30 November 2010, approximately 0.8 km east of Karujeu Island (8°37.674′ S, 158°12.398′ E), Western Province, Solomon Islands (figure 1). A large aggregation (i.e. approx. 1 km long, 10–15 m wide and 3 m thick) of jellyfish (Linuche unguiculata, approx. 3 cm estimated diameter; approx. 4 g estimated wet mass [8]) was observed at the site, from a small vessel. At 11.05 (Greenwich mean time (GMT) + 11), a male leatherback was seen from the vessel and followed. During observations, it swam slowly through the jellyfish aggregation while feeding. Another male leatherback was spotted at 11.36 (GMT + 11) within 500 m of the first turtle while behaving in a similar manner to the first individual. Photographs and video footage of both the jellyfish aggregation and the turtles were recorded by an underwater observer (A.R.L.) equipped with standard SCUBA gear and a high-resolution (14.7 Megapixel) waterproofed digital camera (Canon PowerShot G10, Canon, Inc.) (figure 1).
Figure 1.
(a) The map shows the field site (black circle) where (b) two male leatherback turtles were observed feeding on (c) an ultra-dense aggregation of (d) Linuche unguiculata offshore Karujeu Island, Solomon Islands.
(b). Video analysis
Eight post-graduates from Swansea University watched the video footage (total duration 39 s, electronic supplementary material, video S1) 10 times each, using Real Player, and reported the following information for each review trial: (i) number, start and end time of the feeding session(s), (ii) number of mouth-opening events during each feeding session, and (iii) number of jellyfish ingested during a mouth-opening event. A feeding session was considered as finished when the turtle was not observed eating for greater than or equal to 5 s. These data were used to determine the turtles' feeding rate (i.e. jellyfish consumed per second) and estimates for daily energy intake.
(c). Photo analysis
Estimates for jellyfish density were determined from the underwater, high-resolution photographs by analysis with the software Tracker: Video Analysis and Modelling Tool (v. 4.05, http://www.cabrillo.edu/~dbrown/tracker/). Jellyfish in the bloom were of a very consistent size estimated at 3 cm bell width. To provide a crude measure of the jellyfish density, we first assumed that individuals appearing as the same size (±10%) in the images were the same distance from the camera, and then for such jellyfish we used their assumed actual size (3 cm) to estimate their distance apart. In this way, the separation distances of 17 pairs of jellyfish were measured, from which the overall density was estimated.
3. Results
All video analysers recorded two feeding sessions (see the electronic supplementary material, video S1). The mean duration of the first and second feeding sessions was 6.9 ± 2.0 s and 13.5 ± 2.8 s (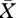 ± s.d., n = 8 video analysers), respectively, while the mean time between feeding sessions was 8.4 ± 3.4 s. The mean number of mouth-opening events was 6.6 ± 1.1 and 11.5 ± 1.3 during the first and second feeding session respectively, with one to three jellyfish ingested per mouth-opening event. We calculated a minimum effective ingestion rate of one jellyfish every 2.3 s, based on an average of 6.6 mouth-opening events between the beginning of the first feeding session and the beginning of the second one (i.e. in 15.3 s).
± s.d., n = 8 video analysers), respectively, while the mean time between feeding sessions was 8.4 ± 3.4 s. The mean number of mouth-opening events was 6.6 ± 1.1 and 11.5 ± 1.3 during the first and second feeding session respectively, with one to three jellyfish ingested per mouth-opening event. We calculated a minimum effective ingestion rate of one jellyfish every 2.3 s, based on an average of 6.6 mouth-opening events between the beginning of the first feeding session and the beginning of the second one (i.e. in 15.3 s).
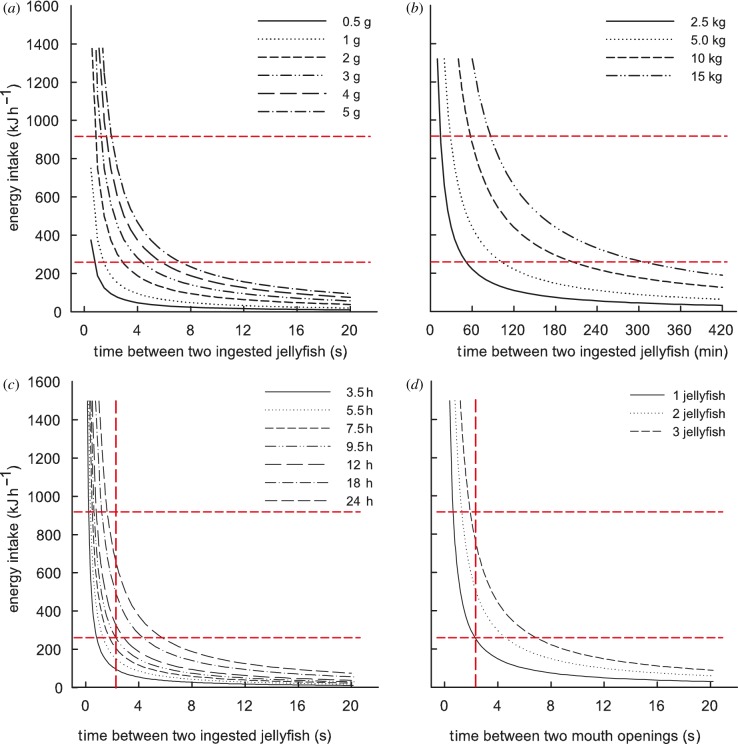
We built a theoretical model to determine if an adult leatherback turtle (body mass = 300 kg) could meet its minimum daily energy requirement (ER) by only feeding on small jellyfish, such as L. unguiculata, based on our video analysis and parameters available in the literature (see figure 2 for details about model parameters). For comparison, Rhizostoma octopus was included in the model as a large prey item.
Figure 2.
A leatherback turtle's estimated hourly energy intake obtained by eating: (a) a 0.5–5 g jellyfish such a Linuche unguiculata [8] (gross energy (GE) density = 0.13 ± 0.04 kJ g wet mass−1 [9]) every 0.5–20 s for 24 h d−1; (b) a 2.5–15 kg jellyfish such as Rhizostoma octopus (GE density = 0.11 ± 0.04 kJ g wet mass−1 [9]) every 10–420 min for 24 h d−1; (c) a 4 g jellyfish (GE density = 0.13 ± 0.04 kJ g wet mass−1) every 0.5–20 s, over a period of 3.5–24 h each day; and (d) one, two or three 4 g jellyfish (GE density = 0.13 ± 0.04 kJ g wet mass−1) per mouth opening every 0.5–20 s, over a period of 9.5 h each day. The horizontal red lines show the minimum and maximum hourly energy requirement for a 300 kg leatherback turtle (i.e. 259 kJ h−1 and 918 kJ h−1, respectively, considering an assimilation efficiency of 80 per cent [10,11]). The vertical red line on panels (c) and (d) show the effective ingestion rate estimated from the video (i.e. one mouth-opening event every 2.3 s). The minimum processing time was estimated to be 0.5 s for a small jellyfish (see §3), and 10 min for a large jellyfish [4].
Based on these data, we estimated a leatherback would need to eat a 4 g L. unguiculata every 6 s or a 5 kg R. octopus every 6300 s (105 min) to meet its minimum ER (figure 2a,b). Furthermore, a leatherback would need to eat a 4 g L. unguiculata approximately every 1.6 s or a 5 kg R. octopus every 1680 s (28 min) to meet its maximum ER. If a turtle eats a 4 g L. unguiculata every 2.3 s, as suggested by our empirical results, it would need to feed for 9.5 h d−1 to meet its minimum ER (figure 2c). Assuming that a turtle swims along a straight path at a cruising speed of 0.3 m s−1 [10], we can crudely estimate a required patch density of at least one L. unguiculata every 0.7 m to sustain an ingestion rate of one jellyfish every 2.3 s. In addition, our video analysis shows that a turtle can eat up to two to three jellyfish per mouth-opening event, while foraging on an aggregation with an estimated density of approximately 5832 jellyfish m−3. In this case, and an effective ingestion rate of one mouth-opening every 2.3 s, a turtle would need to ingest 52 or 76 jellyfish per minute for 4.75 or 3.25 h d−1, respectively (i.e. approx. 14 820 4 g jellyfish a day) to meet its minimum ER (figure 2d).
4. Discussion
Prey size is a critical parameter of the foraging strategy in both terrestrial and marine predators [2,3], with large predators usually concentrating on large prey to maximize their net energy gain [1]. However, our results demonstrate that the largest marine turtle can meet its daily ERs by foraging for only 3.25 h d−1 on small prey, when those prey are encountered at high-density. This suggests that small prey may be as profitable as larger prey for a large marine predator, as previously suggested in marine fishes [2], if the relatively low energy intake per small individual prey is offset by high encounter rates and minimal capture and handling costs.
Other large marine animals, such as baleen whales, whale sharks or manta rays also feed on high-density aggregations of very small prey [12,13]. However, these taxa are considered planktivores rather than predators, as they have adaptations for filter feeding and are not capable of selecting individual prey items. By contrast, leatherback turtles seem to be able to select individual prey items and opportunistically switch between large and small prey based on their respective availability. This strategy is more analogous to the foraging strategies by such marine species as sharks, and scrombrid fishes feeding on small schooling prey [12], or bats feeding on small insects [14], and could almost be considered as grazing in a jellyfish field.
Despite the low calorific value of jellyfish [9], their high digestibility [15] may explain the adoption of this specialized diet by the leatherback turtle. In addition, this particular feeding strategy may be facilitated by the relatively low metabolic rate of this marine reptile [10,11] and the slow-moving nature of its prey (i.e. negligible chasing costs).
The absolute calorific value of a 4 g L. unguiculata is about three orders of magnitude less than a 5 kg R. octopus and a leatherback turtle feeding on a R. octopus bloom would certainly gain more energy than by feeding on L. unguiculata. However, small jellyfish aggregated in a patch can be procured and processed more rapidly than larger jellyfish [16]. Leatherback turtles are indeed able to ingest a new prey while still processing and swallowing previous prey in the posterior region of the buccal cavity [16]. This suggests that for a leatherback turtle, prey abundance rather than prey size may be critical for maximizing its net energy gain [1]. Ultra-high jellyfish densities recorded in this study are no rarity, but have been observed in other tropical sites [17]. Albeit leatherback turtles appear to heavily rely on energy stores accrued at high-latitude foraging grounds for successful reproduction, our data show that leatherbacks also actively feed in warm, tropical waters and that they could surpass their daily ERs by feeding more than 3.25 h d−1 on aggregations of small jellyfish. This foraging strategy may afford leatherback turtles the chance to minimize energy loss during the nesting season for successful reproduction and for sustaining long-distance post-nesting migrations. However, as our results are based on a small sample size there is therefore a possibility that leatherback turtles from other populations behave differently. Accordingly, data from different populations are requisite; yet, observations of underwater predation by free-living giant vertebrates are challenging and still very rare.
Acknowledgements
All work conformed to the legal requirements of the country in which it was carried out, and to all institutions (Swansea University) guidelines.
We thank North Star Cruises, Captain Jarrad Butt and the post-graduates students from Swansea University. S.F. and G.C.H. were supported by a research grant from the Esmée Fairbairn Foundation.
References
- 1.Stephens D. W., Krebs J. R. 1986. Foraging theory. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Costa G. C. 2009. Predator size, prey size, and dietary niche breadth relationships in marine predators. Ecology 90, 2014–2019 10.1890/08-1150.1 (doi:10.1890/08-1150.1) [DOI] [PubMed] [Google Scholar]
- 3.Carbone C., Mace G. M., Roberts S. C., Macdonald D. W. 1999. Energetic constraints on the diet of terrestrial carnivores. Nature 402, 286–288 10.1038/46266 (doi:10.1038/46266) [DOI] [PubMed] [Google Scholar]
- 4.James M. C., Herman T. B. 2001. Feeding of Dermochelys coriacea on medusae in the northwest Atlantic. Chelonian Conserv. Biol. 4, 202–205 [Google Scholar]
- 5.Witt M. J., Broderick A. C., Johns D. J., Martin C., Penrose R., Hoogmoed M. S., Godley B. J. 2007. Prey landscapes help identify potential foraging habitats for leatherback turtles in the NE Atlantic. Mar. Ecol. Prog. Ser. 337, 231–244 10.3354/meps337231 (doi:10.3354/meps337231) [DOI] [Google Scholar]
- 6.Casey J., Garner J., Garner S., Williard A. 2010. Diel foraging behavior of gravid leatherback sea turtles in deep waters of the Caribbean Sea. J. Exp. Biol. 213, 3961. 10.1242/jeb.048611 (doi:10.1242/jeb.048611) [DOI] [PubMed] [Google Scholar]
- 7.Myers A. E., Hays G. C. 2006. Do leatherback turtles Dermochelys coriacea forage during the breeding season? A combination of data-logging devices provide new insights. Mar. Ecol. Prog. Ser. 322, 259–267 10.3354/meps322259 (doi:10.3354/meps322259) [DOI] [Google Scholar]
- 8.Kremer P. 2005. Ingestion and elemental budgets for Linuche unguiculata, a scyphomedusa with zooxanthellae. J. Mar. Biol. Assoc. UK 85, 613–625 10.1017/S0025315405011549 (doi:10.1017/S0025315405011549) [DOI] [Google Scholar]
- 9.Doyle T. K., Houghton J. D. R., McDevitt R., Davenport J., Hays G. C. 2007. The energy density of jellyfish: estimates from bomb-calorimetry and proximate-composition. J. Exp. Mar. Biol. Ecol. 343, 239–252 10.1016/j.jembe.2006.12.010 (doi:10.1016/j.jembe.2006.12.010) [DOI] [Google Scholar]
- 10.Bostrom B. L., Jones D. R. 2007. Exercise warms adult leatherback turtles. Comp. Biochem. Phys. A 147, 323–331 10.1016/j.cbpa.2006.10.032 (doi:10.1016/j.cbpa.2006.10.032) [DOI] [PubMed] [Google Scholar]
- 11.Bradshaw C. J. A., McMahon C. R., Hays G. C. 2007. Behavioral inference of diving metabolic rate in free-ranging leatherback turtles. Physiol. Biochem. Zool. 80, 209–219 10.1086/511142 (doi:10.1086/511142) [DOI] [PubMed] [Google Scholar]
- 12.Michael S. W. 2005. Reef sharks and rays of the world. Annapolis, MD: ProStar Publications [Google Scholar]
- 13.Goldbogen J., Calambokidis J., Oleson E., Potvin J., Pyenson N., Schorr G., Shadwick R. E. 2011. Mechanics, hydrodynamics and energetics of blue whale lunge feeding: efficiency dependence on krill density. J. Exp. Biol. 214, 131–146 10.1242/jeb.048157 (doi:10.1242/jeb.048157) [DOI] [PubMed] [Google Scholar]
- 14.Kurta A., Kunz T. H., Nagy K. A. 1990. Energetics and water flux of free-ranging big brown bats (Eptesicus fuscus) during pregnancy and lactation. J. Mammal. 71, 59–65 10.2307/1381316 (doi:10.2307/1381316) [DOI] [Google Scholar]
- 15.Arai M. N. 2005. Predation on pelagic coelenterates: a review. J. Mar. Biol. Assoc. UK 85, 523–536 10.1017/S0025315405011458 (doi:10.1017/S0025315405011458) [DOI] [Google Scholar]
- 16.Bels V., Davenport J., Renous S. 1998. Food ingestion in the estuarine turtle Malaclemys terrapin: comparison with the marine leatherback turtle Dermochelys coriacea. J. Mar. Biol. Assoc. UK 78, 953–972 10.1017/S0025315400044908 (doi:10.1017/S0025315400044908) [DOI] [Google Scholar]
- 17.Larson K. 1992. Riding Langmuir circulations and swimming in circles: a novel form of clustering behavior by the scyphomedusa Linuche unguiculata. Mar. Biol. 112, 229–235 10.1007/BF00702466 (doi:10.1007/BF00702466) [DOI] [Google Scholar]