Abstract
Although we are relatively naked in comparison with other primates, the human body is covered in a layer of fine hair (vellus and terminal hair) at a relatively high follicular density. There are relatively few explanations for the evolutionary maintenance of this type of human hair. Here, we experimentally test the hypothesis that human fine body hair plays a defensive function against ectoparasites (bed bugs). Our results show that fine body hair enhances the detection of ectoparasites through the combined effects of (i) increasing the parasite's search time and (ii) enhancing its detection.
Keywords: vellus hair, terminal hair, bed bug, ectoparasite detection
1. Introduction
Humans are unique among all primate species in that we are superficially glabrous [1] (i.e. hairless). There are no shortage of theories for the loss of human body hair, e.g. thermoregulatory advantages [1,2], sexual selection [3,4], ectoparasite reduction [4,5], an aquatic phase [6], noonday foraging, hunting, neoteny and allometry [6]. However, despite our hairless appearance, the human body has the same density of hair follicles as would be expected of an ape of the same size [7].
The fine body hair of humans, composed of vellus hair and terminal hair, has little role in thermoregulation [8] and plays no significant part in sexual selection: it is frequently described as non-functional [2] and consequently provides the logic for the assumption that vellus hair is an expression of this loss of function. However, these hairs are known to have some minor functions in sweat gland maintenance [9] and are mechanoreceptive [10]. We experimentally test whether human fine body hair functions to enhance detection of ectoparasites. We use the human bed bug, Cimex lectularius, a common haemotophagous parasite of humans [11], which can detect a host from several metres away through the use of heat cues, host kairomones and carbon dioxide [11]. Exactly, what determines the parasite's host choice behaviour is unclear, but the nature and duration of search behaviour is probably under strong negative selection, as contact with the host constitutes a high mortality risk [11,12]. From a human host's perspective, the ability to detect and remove ectoparasites would be beneficial as haemotophagous insects cause damage and irritation through allergic reactions, blood loss and the risk of pathogen transmission [13].
We used a pair-wise experimental design that compared parasite search time on the shaved and unshaved arms of the same host, as well as the host's ability to detect the presence of an ectoparasite on each arm. Moreover, by measuring individual host variation in ‘hairiness’, we examined the relationship between hairiness, search time and detection ability.
2. Material and methods
(a). Hosts
Twenty-nine student volunteers in the University of Sheffield (10 females and 19 males) were recruited through opportunity sampling on the social networking site, Facebook. They were aged between 19 and 27 years (median age 21 years). All participants were made aware of the risk of being bitten (no volunteers were bitten during the course of this study) and the potential risks of adverse dermatological reactions if they were bitten [14]. Each volunteer was assigned an identifying number and all data collected were anonymized with respect to host. We followed the University of Sheffield ethics regulations [15] throughout.
(b). Parasites
The bed bugs used in this study originated from recently (2007) field-collected populations that have retained natural behaviours despite being reared in the laboratory. We used only imaginal females in this study. All were fed to satiation using standard protocols [11] exactly a week before a trial took place. This time frame matches C. lectularius natural feeding habits [11,16] and ensured all experimental bed bugs were (i) ready to feed and (ii) of similar ‘hunger’ status. Bed bugs which did not attempt to feed were excluded from the data. Because each host was tested twice, and because we wanted to use the same five bed bugs at each host's test, we gave each bed bug a unique acrylic paint spot at the back of its abdomen (applied with the tip of a wooden cocktail stick).
(c). Experimental treatment
The experimental procedure required that each host was tested on a shaved and unshaved arm (randomized with respect to arm and temporal sequence). The treatment arm was shaved on the upper surface between the wrist and the elbow with a new razor (Gillette Mach 4), while using the same brand of perfume- and colour-free soap (Simple). A rectangle measuring 5 × 10 cm was then drawn on the shaved area with a marker pen and vaseline (a barrier to bed bug locomotion) applied to the marked boundary. This ensured each host experienced the same potential surface contact with the parasites. Testing on the unshaved (control) arm was preceded by washing the unshaven arm with the same soap as the treatment, and generating a vaseline-delimited rectangle as in the treatment.
Each host was given a tally-counter and asked to look away as a bed bug was placed within the vaseline rectangle on their arm. Prior to release, all bed bugs experienced the same handling conditions. The volunteer was instructed to use the tally-counter every time they perceived the presence of something on their arm. The experimenter timed the duration of search behaviour of the bed bug on the host's arm and removed the insect as it extended its proboscis (the stereotyped pre-feeding behaviour). The search time was recorded as the time between placement on the host and extension of the proboscis. Host tally-counts were used as an index of parasite ‘detections’.
Bed bugs that did not extend the proboscis after 300 s were removed from the participant and these data were omitted from the analyses. In all cases, these bed bugs showed none of the stereotypical behaviours associated with searching for a feeding site.
Each volunteer was tested twice: once on one arm, and then a week later on the other. The bed bugs were fed immediately after their first trial and so were in the same state of ‘hunger’ on the second trial. Results from bed bugs that did not feed, or died, between the two trials were excluded from subsequent analyses.
(d). Hair index and calculations
We devised a method for assessing the ‘hairiness’ of the arms of our volunteers that captured the density of follicles and the length of hairs on the forearm. (N.B., we did not distinguish between vellus and terminal hairs in this study.) Our ‘hair index’ is the product of hair follicular density per square centimetre and mean hair length (figure 1). We took a digital photograph of each volunteer's arm and counted the mean number of hair follicles in three different 1 cm2 areas taken from within the marked experimental rectangle. Mean hair length was calculated from the length of five hairs selected from within the rectangle. Hair index = mean density of follicles × mean hair length. Means are expressed ±1 s.e.
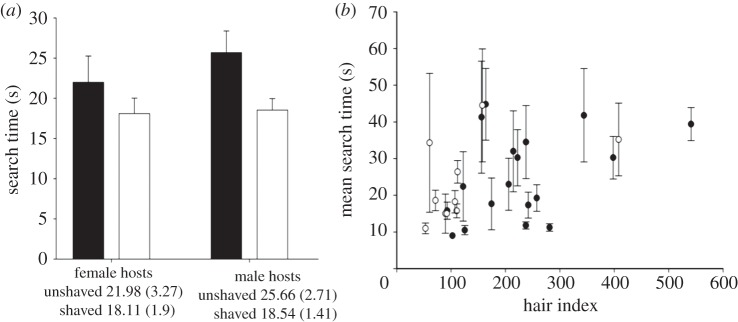
Figure 1.
(a) Comparison of search times of Cimex lectularius on male and female human hosts. Search time was significantly longer on control arms compared with treatment arms on male hosts (paired t-test: t = 3.79, d.f. = 18, p = 0.001) but not female hosts (paired t-test: t = 1.39, d.f. = 9, p = 0.199; black bars, unshaved; white bars, shaved). (b) Hair index of hosts was correlated with the search time of C. lectularius on control (unshaved) arms (r = 0.426, n = 29, p = 0.021; filled circles, male host; open circles, female host).
3. Results
The individual identification marks on the bed bugs (Wilk's lambda = 0.84, d.f. = 16, F = 1.34, p = 0.17) whether the left or right arm was shaved (Wilk's lambda = 0.93, F = 1.3, d.f. = 4, p = 0.1) and whether first exposure of the bed bug was to a shaved or control arm (Wilk's lambda =0.92, F = 1.2, d.f. = 8, p = 0.31) each had no effect on the response variables.
(a). Hair index
Male volunteers had a significantly higher hair index (238.3 ± 26.7) than female volunteers (89.6 ± 10.9; t = 5.16, d.f. = 23, p < 0.001).
(b). Search time
Bed bugs had significantly longer search times on unshaved arms compared with shaved arms on male (paired t-test: t = 3.79, d.f. = 18, p = 0.001) but not female hosts (paired t-test: t = 1.39, d.f. = 9, p = 0.199; figure 1a).
Search time was positively correlated with hair index on control (unshaved) arms (r = 0.426, n = 29, p = 0.021; there was no difference between male and female correlations; figure 1b) but there was no relationship on shaved arms (r = 0.299, n = 29, p = 0.115).
(c). Host's detection abilities
Host ‘detections’ were more frequent on unshaved when compared with shaved arms on male (paired t-test: t = 4.37, d.f. = 18, p < 0.001) and female (paired t-test: t = 4.11, d.f. = 9, p = 0.003) hosts (figure 2a).
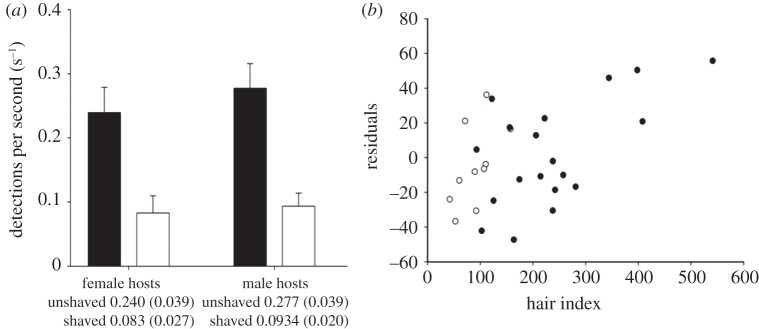
Figure 2.
(a) Detection of ectoparasites on unshaved (black bars) and shaved (white bars) arms. Hosts detected ectoparasites significantly more frequently on control arms compared with shaved arms in female (paired t-test: t = 4.11, d.f. = 9, p = 0.003) and male hosts (paired t-test: t = 4.37, d.f. = 18, p < 0.001). (b). Residual detection rate (derived from detection versus search time on unshaved arms) was positively correlated with hair index (r = 0.533, n = 29, p = 0.003). Analysis is from pooled data (male (filled circles) and female (open circles) correlations did not differ), but male and female data are shown separately.
The cumulative detection rate by the host was positively associated with cumulative search time on both arms (shaved arm: y = 16.5 + 1.47x, F = 9.21, d.f. = 1,119, p = 0.003; unshaved arm: y = 13.7 + 1.28x, F = 346.21, d.f. 1,119, p < 0.001).
Residual detection rate (of search time versus detection rate) was positively correlated with hair index on unshaved arms (r = 0.533, n = 29, p = 0.003; figure 2b) but not on shaved arms (r = 0.353, n = 29, p = 0.060).
4. Discussion
Our results show that the presence of fine body hair (i) prolongs the search behaviour of C. lectularius and (ii) enhances the detection of searching ectoparasites. Moreover, a higher hair index makes a host more likely to detect an ectoparasite. Because males have a higher hair index compared with females this result has implications for sex-differences in parasite detection. Unfortunately, we could not distinguish between vellus hair and terminal hair in our study and sexual dimorphism in terminal hairs may be partly responsible for the higher hair index in males in this study. Enhanced male detection of ectoparasites may be an evolutionary response driven by female mate choice directed at parasite-free males [17] and/or sexual dimorphism in investment in immune function [18].
Increased search times are likely to be disadvantageous to ectoparasites because of increased energy demands and, as our data have shown, increased probability of detection and consequently mortality risk [11,12]. Our results show that detection ability increased with host hair index: similarly, Freeland [19] found that parasite-induced grooming increased with hair length in primates.
Cimicids (the bed, bat and bird bugs) prefer to bite hairless sites on bats and the featherless areas of birds [11] and there is evidence that mosquito bites occur primarily on the relatively hairless underside of wrists and ankles [20]. Although these areas may be more frequently exposed, our findings suggest that selection may also favour parasites that avoid the risks associated with foraging on the hairier areas of hosts.
Changes in grooming behaviour have been linked to ectoparasite loads in a number of animals, including birds [21], impala [22], bats [23], cats [24] and non-human primates [25,26] with grooming frequency increasing in individuals with a higher ectoparasite load. An increase in grooming frequency also reduces these parasite loads [22,24].
Belt [27] was first to suggest that a reduction of body hair would have partially alleviated Homo sapiens of ectoparasites. Aspects of our evolutionary history, i.e. group living and a fixed home base, mean we were potentially prone to high ectoparasite loads: consequently, a reduction in body hair may be an adaptation to remove ectoparasitic refuges on the body [4] and make parasites easier to find and remove. However, these traits would not have removed the opportunity for ectoparasitism. It is possible that human fine body hair is maintained by the balance between selection on it (i) being shorter/sparser (the cost being diminished ectoparasite detection) and (ii) being longer/denser (the cost being more options for ectoparasite concealment).
Our data suggest that reduced body hair in humans functions, at least partly, as a defence against ectoparasites. On the basis of our results, and our conclusion that fine body hair functions to enhance ectoparasite detection, we predict that transient ectoparasites should show feeding preferences for relatively hairless parts of their host's body.
Acknowledgements
We thank Richard Naylor for practical assistance and Klaus Reinhardt and Adam Dobson for discussions and two anonymous referees who made comments that improved the manuscript.
References
- 1.Rebora A. 2010. Lucy's pelt: when we became hairless and how we managed to survive. Int. Soc. Dermatol. 49, 17–20 10.1111/j.1365-4632.2009.04266.x (doi:10.1111/j.1365-4632.2009.04266.x) [DOI] [PubMed] [Google Scholar]
- 2.Wheeler P. E. 1984. The evolution of bipedality and loss of functional body hair in hominids. J. Hum. Evol. 13, 91–98 10.1016/S0047-2484(84)80079-2 (doi:10.1016/S0047-2484(84)80079-2) [DOI] [Google Scholar]
- 3.Darwin C. 1981. The descent of man and selection in relation to sex (reprint of 1871 edn.). Princeton, NJ: Princeton University Press [Google Scholar]
- 4.Pagel M., Bodmer W. 2003. A naked ape would have fewer parasites. Proc. R. Soc. B 270, 117–119 10.1098/rsbl.2003.0041 (doi:10.1098/rsbl.2003.0041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rantala M. J. 1999. Controversies in parasitology: human nakedness: adaptation against ectoparasites? Int. J. Parasitol. 29, 1987–1989 10.1016/S0020-7519(99)00133-2 (doi:10.1016/S0020-7519(99)00133-2) [DOI] [PubMed] [Google Scholar]
- 6.Morgan E. 1990. The scars of evolution. Worcester: Billing and Sons Ltd [Google Scholar]
- 7.Schwartz G. G., Rosenblum L. A. 1981. Allometry of primate hair density and the evolution of human hairlessness. Am. J. Phys. Anthropol. 55, 9–12 10.1002/ajpa.1330550103 (doi:10.1002/ajpa.1330550103) [DOI] [PubMed] [Google Scholar]
- 8.Randall V. A., Sundberg J. P., Philpott M. P. 2003. Animal and in vitro models for the study of hair follices. J. Invest. Dermatol. Symp. Proc. 8, 39–45 10.1046/j.1523-1747.2003.12170.x (doi:10.1046/j.1523-1747.2003.12170.x) [DOI] [PubMed] [Google Scholar]
- 9.Folk G. E., Semken A. 1991. The evolution of sweat glands. Int. J. Biometeorol. 35, 180–186 10.1007/BF01049065 (doi:10.1007/BF01049065) [DOI] [PubMed] [Google Scholar]
- 10.Paus R., Cotsarelis G. 1999. Mechanisms of disease: the biology of hair follicles. N. Engl. J. Med. 341, 491–497 10.1056/NEJM199908123410706 (doi:10.1056/NEJM199908123410706) [DOI] [PubMed] [Google Scholar]
- 11.Reinhardt K., Siva-Jothy M. T. 2007. Biology of the bedbugs (Cimicidae). Annu. Rev. Entomol. 52, 351–374 10.1146/annurev.ento.52.040306.133913 (doi:10.1146/annurev.ento.52.040306.133913) [DOI] [PubMed] [Google Scholar]
- 12.Hassell P. M., Southwood R. E. 1978. Foraging strategies of insects. Annu. Rev. Ecol. Syst. 9, 75–98 10.1146/annurev.es.09.110178.000451 (doi:10.1146/annurev.es.09.110178.000451) [DOI] [Google Scholar]
- 13.Reinhardt K., Isaac D., Naylor R. 2010. Estimating the feeding rate of the bedbug Cimex lectularius in an infested room: an inexpensive method and a case study. Med. Vet. Entomol. 24, 46–54 10.1111/j.1365-2915.2009.00847.x (doi:10.1111/j.1365-2915.2009.00847.x) [DOI] [PubMed] [Google Scholar]
- 14.Reinhardt K., Kempke D., Naylor R., Siva-Jothy M. T. 2009. Sensitivity to bites by the bedbug, Cimex lectularius. Med. Vet. Entomol. 23, 163–166 10.1111/j.1365-2915.2008.00793.x (doi:10.1111/j.1365-2915.2008.00793.x) [DOI] [PubMed] [Google Scholar]
- 15.University of Sheffield 2009. Ethics policy governing research involving human participants, personal data and human tissue. See http://www.shef.ac.uk/content/1/c6/11/12/77/Full%20Ethics%20Policy.pdf
- 16.Usinger R. L. 1966. Monograph of Cimicidae (Hemiptera-Heteroptera). College Park, MD: The Thomas Say Foundation Entomological Society of America [Google Scholar]
- 17.Følstad I., Karter A. J. 1992. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 139, 603–622 10.1086/285346 (doi:10.1086/285346) [DOI] [Google Scholar]
- 18.Rolff J. 2002. Bateman's principle and immunity. Proc. R. Soc. Lond. B 269, 867–872 10.1098/rspb.2002.1959 (doi:10.1098/rspb.2002.1959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeland W. J. 1981. Functional aspects of primate grooming. Ohio J. Sci. 81, 173–177 [Google Scholar]
- 20.Karunamoorthi K., Sabesan S. 2009. Relative efficacy of repellent-treated wristbands against three major mosquito (Diptera: Culicidae) vectors of disease, under laboratory conditions. Int. Health 1, 173–177 10.1016/j.inhe.2009.08.005 (doi:10.1016/j.inhe.2009.08.005) [DOI] [PubMed] [Google Scholar]
- 21.Cotgreave P., Clayton D. H. 1994. Comparative analysis of time spent grooming on birds in relation to parasite load. Behaviour 141, 172–187 [Google Scholar]
- 22.Mooring M. S., McKenzie A. A., Hart B. L. 1996. Grooming in impala: role of oral grooming in removal of ticks and effects of ticks in increasing grooming rate. Physiol. Behav. 59, 965–971 10.1016/0031-9384(95)02186-8 (doi:10.1016/0031-9384(95)02186-8) [DOI] [PubMed] [Google Scholar]
- 23.Giorgi M. S., Arlettaz R., Christe P., Vogel P. 2001. The energetic grooming costs imposed by a parasitic mite (Spinturnix myoti) upon its bat host (Myotis myotis). Proc. R. Soc. Lond. B 268, 2071–2075 10.1098/rspb.2001.1686 (doi:10.1098/rspb.2001.1686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckstein R. A., Hart B. L. 2000. Grooming and control of fleas in cats. Appl. Anim. Behav. Sci. 68, 141–150 10.1016/S0168-1591(00)00095-2 (doi:10.1016/S0168-1591(00)00095-2) [DOI] [PubMed] [Google Scholar]
- 25.Dunbar R. I. M. 1991. Functional significance of social grooming in primates. Folia Primatol. 57, 121–131 10.1159/000156574 (doi:10.1159/000156574) [DOI] [Google Scholar]
- 26.Hutchins M., Barash D. P. 1976. Grooming in primates: implications for its utilitarian function. Biomed. Life Sci. 17, 145–150 [Google Scholar]
- 27.Belt T. 1911. The Naturalist in Nicaragua. London: J.M. Dent & Sons [Google Scholar]