Abstract
The control of primary sex-ratio by vertebrates has become a major focus in biology in recent years. Evolutionary theory predicts that a differential effect of maternal characteristics on the fitness of sons and daughters is an important route, whereby selection is expected to favour a bias towards the production of one sex. However, despite experimental evidence for adaptive brood sex-ratio manipulation, support for this prediction remains a major challenge in vertebrates where inconsistencies between correlative studies are frequently reported. Here, we used a large dataset (2215 nestlings over 3 years) from a wild population of tree swallows (Tachycineta bicolor) and show that variations in breeding conditions affect female sex allocation in this species. Our results also suggest that such variation in sex allocation, owing to breeding season heterogeneity, modifies the relationships between maternal characteristics and maternal investment. Indeed, we detect a positive effect of maternal age on brood sex-ratio when age also affects offspring condition (in a low-quality breeding season). Our results indicate that including measures of both breeding season quality and maternal investment will help to better understand sex allocation patterns.
Keywords: birds, breeding season heterogeneity, sex allocation, sex-ratio, tree swallow
1. Introduction
Variation in the production of sons and daughters is a key parameter in life history and evolutionary theory. Theory predicts that when fitness returns of producing sons or daughters vary with environmental or parental characteristics, selection should favour individuals capable of adjusting their offspring sex-ratio accordingly. Given this, Trivers & Willard [1] advocated that if one sex has a greater variance in reproductive success than the other, then mothers in better condition should invest more in one sex, generally males. On the other hand, mothers in poorer condition may improve their fitness by producing daughters rather than poor condition, less competitive sons. Yet, despite an impressive number of studies, results obtained from vertebrates are usually equivocal [2]. In birds, the applicability of sex-ratio theory to empirical contexts has been questioned [3] and initial findings are often debated (e.g. [4] versus [5]), leading to a lack of general interpretation of the phenomenon.
Most sex-ratio studies focus on the influence of parental characteristics without assessing variation in breeding conditions or the maternal investment in offspring. This simplification of the Trivers–Willard fundamentals can become problematic as soon as we consider that relationships between individual characteristics and individual investment are potentially unstable [6]. For example, environmental variation may mask age-related differences in female breeding performance, with individual heterogeneity being more obvious under challenging conditions [7]. Yet, despite its potential importance for elucidating key evolutionary processes, few studies have assessed the interplay among variation in female characteristics, sex allocation, maternal investment and breeding conditions.
Here, we investigate the relationship between maternal age and sex allocation under contrasted breeding seasons in tree swallows (Tachycineta bicolor). As proposed by Trivers & Willard [1], we hypothesize that sex biases should occur only when maternal characteristics also affect offspring condition.
2. Material and methods
(a). Species, study area and sampling
Tree swallows are socially monogamous birds with one of the highest levels of extra-pair paternity [8]. Variance in reproductive success of males is consequently higher than that of females [9]. Also, nestling condition influences their future reproductive success through post-fledging survival or territory ownership [10], and females are known to adjust their primary brood sex-ratio adaptively [11].
Our study area contains 400 nest-boxes equally distributed among 40 farms over 10 200 km2 in Québec, Canada [12,13]. Since 2004, all nest-boxes are monitored and both hatching date and nestling survival (proportion of hatched young that fledged) are routinely recorded. Breeding females are trapped in nest-boxes during incubation, ringed, sexed according to the presence of a brood patch and classified as second-year (SY) or after second-year (ASY) [14]. Over three breeding seasons (2006–2008), we recorded asymptotic nestling mass (±0.01 g) at 12 days old for 2215 nestlings (603 broods). Asymptotic body mass reflects parental feeding rate [15], which is a major energy and time investment in offspring [16]. A blood (or tissue) sample from each nestling (death or alive, or from un-hatched eggs with embryos) was collected for molecular sex determination. We found no sex-biased mortality between egg laying and nestling sex determination (binomial test, p = 0.217).
(b). DNA extraction and sexing
DNA was extracted using a proteinase K digestion followed by salt extraction [17]. Nestlings were sexed by PCR-amplification of the chromo-helicase DNA-binding genes using the P2 and P8 primers [13]. PCR products were visualized under UV-light (G-Box, Syngene). We also verified the sex of 249 adults, and molecular sex always matched our phenotypic assessments.
(c). Statistical analyses
(i). Breeding season heterogeneity
Breeding seasons were compared using data on both hatching date and nestling survival. Hatching date affects several components of fitness [18] and offspring survival usually declines with date of hatching [19]. Also, the optimum reproductive investment of parents may increase with early breeding, because more time will be available for improving both adult and fledging body condition before migration. Differences in hatching date among breeding seasons were tested with a generalized linear mixed model (GLMM) with a normal distribution of errors and identity link function. Variations in nestling survival rate were tested with a GLMM with binomial errors and logit link function. Tukey contrasts were performed to account for multiple comparisons (multcomp package run in R v. 2.9.1).
(ii). Sex allocation, maternal age and investment
We modelled sex allocation (i.e. primary brood sex-ratio) from two vectors composed of the number of sons and daughters found in each brood. We fitted GLMM to those vectors using a quasi-binomial error distribution (owing to underdispersion) and a logit link function. To assess if the influence of maternal age on sex allocation varied with season, we fitted a model including an interaction between seasons and maternal age. If a significant effect of the interaction was found, similar models were run to assess differences among seasons. Both hatching date and clutch size were included as covariates. Female and farm identities were included as random terms and models were fitted in R v. 2.9.1 (lme4 package). We then assessed the effect of maternal age on nestling mass depending on sex using GLMMs with normal distribution of errors and identity link function. Again, if a significant interaction among year, female age and nestling sex was found, analyses were further conducted on each year separately. We accounted for possible effects of brood size and brood sex-ratio by including them as covariates. Nest identity was included as a random term in these models and analyses were conducted using GenStat v. 13 (VSN-intl.).
3. Results
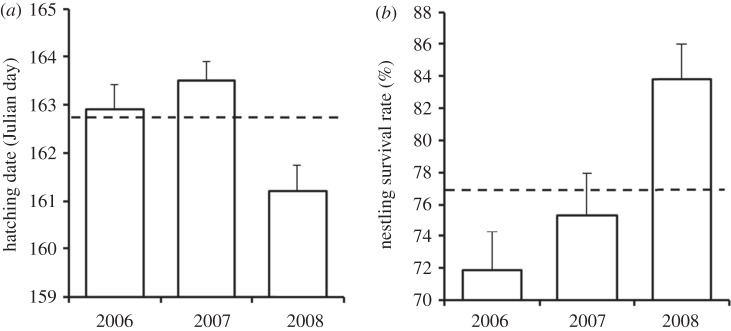
Breeding seasons differed in their quality based both on hatching date (F2,599 = 8.15, p < 0.001; figure 1a) and nestling survival (F2,694 = 5.40, p = 0.005; figure 1b). Tukey contrasts revealed that 2008 was characterized by both earlier hatching (2006–2008: z = 3.06, p = 0.006 and 2007–2008: z = 3.86, p < 0.001) and higher nestling survival (2006–2008: z = 5.01, p < 0.001 and 2007–2008: z = 3.80, p < 0.001). Both indexes were equivalent in 2006–2007 (hatching date: z = 0.94, p = 0.62 and nestling survival: z = 1.11, p = 0.51). Yet, 2006 was characterized by the lowest nestling survival rate (0.719 ± 0.024) measured in our population (mean survival rate in our system = 0.768 ± 0.009), whereas 2007 was average (0.753 ± 0.026; figure 1b).
Figure 1.
Variations in yearly (a) hatching date and (b) nestling survival (mean ± s.e.) of tree swallows breeding in nest-boxes located in southern Québec, Canada, 2006–2008. Dashed line represents the mean value in our long-term studied population (2004–2010).
(a). Sex allocation, maternal age and investment
Maternal age effect on brood sex-ratio varied significantly among seasons (table 1). While the effect of maternal age on sex-ratio was significant in 2006 (older females had more sons, effect: 0.69 ± 0.10, t = 6.88, p < 0.001; figure 2a), it was not in 2007 (effect: 0.01 ± 0.06, t = 0.19, p = 0.85; figure 2a) and 2008 (effect: 0.02 ± 0.06, t = 0.38, p = 0.71; figure 2a). Also, the effect of maternal age on nestling mass was significantly different depending on seasons and nestling sexes (F2,1862 = 4.50, p = 0.011). More specifically, there was a significant interaction between female age and nestling sex (F1,473 = 5.72, p = 0.017) in 2006, owing to younger females having smaller daughters and bigger sons than older females (figure 2b), but not in 2007 and 2008 (both p > 0.20). Nestlings of young and old females had similar mass in 2007 and 2008 (figure 2c) but younger females nevertheless had a tendency to produce smaller nestlings than older females in 2007 (F1,177 = 2.78, p = 0.097; figure 2c). Brood size had a negative effect on nestling mass in both 2006 (effect: −0.32 ± 0.14, F1,168 = 5.55, p = 0.020) and 2007 (effect: −0.31 ± 0.12, F1,194 = 7.18, p = 0.008), but not in 2008 (effect: −0.10 ± 0.11, F1,197 = 0.83, p = 0.36). Sons were heavier than daughters in each season (effect: 1.17 ± 0.06, F1,1682 = 335.82, p < 0.001). Brood sex-ratio had no effect on nestling mass (effect: −0.51 ± 0.31, F1,587 = 2.71, p = 0.10).
Table 1.
Breeding season and maternal characteristics effects on tree swallow primary brood sex-ratio (proportion of males). Estimates were obtained from a mixed-effects logistic regression model with female and farm identities as random terms.
| fixed effects | estimate | s.e. | t-value | p-value |
|---|---|---|---|---|
| hatching date | −0.002 | 0.002 | 1.26 | 0.21 |
| clutch size | 0.081 | 0.012 | 6.95 | <0.001 |
| maternal age (ASY 2006) | 0.775 | 0.092 | 8.39 | <0.001 |
| season 2007 | 0.750 | 0.106 | 7.11 | <0.001 |
| season 2008 | 0.791 | 0.105 | 7.54 | <0.001 |
| maternal age × season 2007 | −0.705 | 0.109 | 6.46 | <0.001 |
| maternal age × season 2008 | −0.938 | 0.109 | 8.62 | <0.001 |
Figure 2.
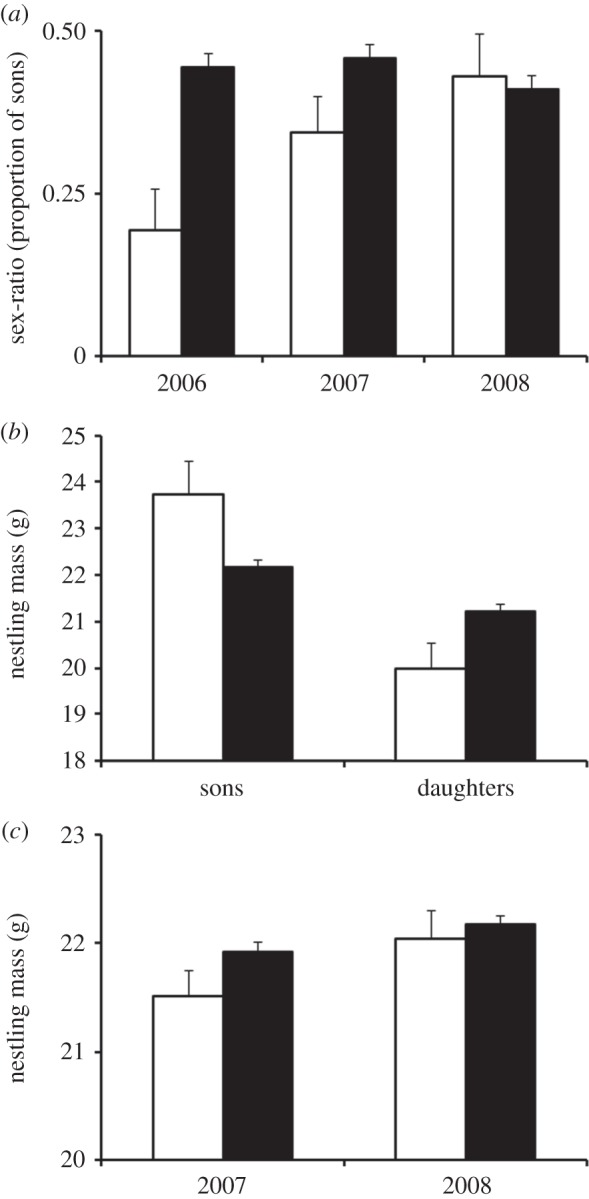
Variations in (a) brood sex-ratio and nestling mass at day 12 (mean ± s.e.) according to (b) nestling sex and maternal age in 2006 and (c) maternal age in 2007 and 2008. Second-year females are represented by white bars and after second-year by black bars.
4. Discussion
We showed that maternal age can affect brood sex-ratio in tree swallows. Importantly, such an effect was detectable in a low-quality breeding season, when maternal age also affected offspring condition. Tree swallows show a standard pattern of senescence [20] and as a result, ASY females are expected to allocate resources towards maintaining nestling mass, enduring the costs of reproduction. In contrast, SY females have higher residual reproductive value and are not yet experienced, and thus should be less likely to sustain significant costs themselves [21]. Variations in SY female strategies may be explained by the strong heterogeneity observed during these breeding seasons. For example, in contrast with 2006, the 2008 season was characterized by high-quality breeding conditions. Such conditions have probably masked any age-experience-related differences in female investment (and associated sex-ratio adjustment), as well as brood size effect on nestling mass. Interestingly, our analyses support a rarely verified assumption: that when a bias in sex-ratio occurs according to parental characteristic, parental investments between nestlings should also be sex-biased [1]. Hence, in 2006, young females (compared with ASY) reduced the direct costs of reproduction, by producing more daughters (i.e. the lighter sex), but improved their potential fitness return by maintaining the condition of their sons. However, in other seasons, both old and young females produced broods with similar sex-ratios and nestlings with comparable fledging weights.
Our findings emphasize that including measures of both breeding season heterogeneity and maternal investment in studies of sex-ratio adjustment is a valuable and relatively simple way to account for potential environmental variation. We thus strongly encourage authors to assess such links when analysing sex-ratio variation in the wild.
Acknowledgements
We thank the research assistants and the 40 farm owners. We also thank three anonymous reviewers for comments on previous versions of this manuscript. This work was supported by Natural Sciences and Engineering Research Council (NSERC) of Canada discovery and strategic grants (D.G., M.B., Jade Savage and Jacques Brodeur), by the Canada Research Chair in Spatial and Landscape Ecology (M.B.) and by the Canadian Foundation for Innovation (D.G. and M.B.).
References
- 1.Trivers R. L., Willard D. E. 1973. Natural selection of parental ability to vary the sex-ratio of offspring. Science 179, 90–92 10.1126/science.179.4068.90 (doi:10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- 2.Cockburn A., Legge S., Double M. C. 2002. Sex-ratios in birds and mammals: can the hypotheses be disentangled? In Sex-ratios: concepts and research methods (ed. Hardy I.), pp. 266–286 Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Komdeur J., Pen I. 2002. Adaptive sex allocation in birds: the complexities of linking theory to practice. Phil. Trans. R. Soc. Lond. B 357, 373–380 10.1098/rstb.2001.0927 (doi:10.1098/rstb.2001.0927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howe H. F. 1977. Sex-ratio adjustment in the common grackle. Science 198, 744–746 10.1126/science.198.4318.744 (doi:10.1126/science.198.4318.744) [DOI] [Google Scholar]
- 5.Maddox J. D., Weatherhead P. J. 2009. Seasonal sex allocation by Common Grackles? Revisiting a foundational study. Ecology 90, 3190–3196 10.1890/08-2180.1 (doi:10.1890/08-2180.1) [DOI] [PubMed] [Google Scholar]
- 6.Sheldon B. C., West S. A. 2004. Maternal dominance, maternal condition, and offspring sex-ratio in ungulate mammals. Am. Nat. 163, 40–54 10.1086/381003 (doi:10.1086/381003) [DOI] [PubMed] [Google Scholar]
- 7.Laaksonen T., Korpimäki E., Hakkarainen H. 2002. Interactive effects of parental age and environmental variation on the breeding performance of Tengmalm's owls. J. Anim. Ecol. 71, 23–31 10.1046/j.0021-8790.2001.00570.x (doi:10.1046/j.0021-8790.2001.00570.x) [DOI] [Google Scholar]
- 8.Griffiths S. C., Owens I. P. F., Thuman K. A. 2002. Extra-pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212 10.1046/j.1365-294X.2002.01613.x (doi:10.1046/j.1365-294X.2002.01613.x) [DOI] [PubMed] [Google Scholar]
- 9.Dunn P. O., Whittingham L. A., Lifjeld J. T., Robertson R. J., Boag P. T. 1994. Effects of breeding density, synchrony and experience on extra-pair paternity in tree swallows. Behav. Ecol. 5, 123–129 10.1093/beheco/5.2.123 (doi:10.1093/beheco/5.2.123) [DOI] [Google Scholar]
- 10.McCarty J. P. 2001. Variation in growth of nestling tree swallows across multiple temporal and spatial scales. Auk 118, 176–190 10.1642/0004-8038(2001)118[0176:VIGONT]2.0.CO;2 (doi:10.1642/0004-8038(2001)118[0176:VIGONT]2.0.CO;2) [DOI] [Google Scholar]
- 11.Whittingham L. A., Dunn P. O. 2000. Offspring sex-ratios in tree swallows: females in better condition produce more sons. Mol. Ecol. 9, 1123–1129 10.1046/j.1365-294x.2000.00980.x (doi:10.1046/j.1365-294x.2000.00980.x) [DOI] [PubMed] [Google Scholar]
- 12.Ghilain A., Belisle M. 2008. Breeding success of tree swallows along a gradient of agricultural intensification. Ecol. Appl. 18, 1140–1154 10.1890/07-1107.1 (doi:10.1890/07-1107.1) [DOI] [PubMed] [Google Scholar]
- 13.Porlier M., Bélisle M., Garant D. 2009. Non-random distribution of individual genetic diversity along an environmental gradient. Phil. Trans. R. Soc. B 364, 1543–1554 10.1098/rstb.2009.0010 (doi:10.1098/rstb.2009.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussel D. 1983. Age and plumage color in female tree swallows. J. Field Ornithol. 54, 312–318 [Google Scholar]
- 15.Ricklefs R. E. 1983. Avian postnatal development. In Avian biology, vol. 7 (eds Farner D. S., King J. R.), pp. 1–83 New York, NY: Academic Press [Google Scholar]
- 16.Ricklefs R. E. 1974. Energetics of reproduction in birds. In Avian energetics (ed. Paynter R. A.). Cambridge, MA: Publ. Nuttall Ornith; Club No. 15 [Google Scholar]
- 17.Aljanabi S. M., Martinez I. 1997. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 25, 4692–4693 10.1093/nar/25.22.4692 (doi:10.1093/nar/25.22.4692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nilsson J. A. 1994. Energetic bottle-necks during breeding and the reproductive cost of being too early. J. Anim. Ecol. 63, 200–208 10.2307/5595 (doi:10.2307/5595) [DOI] [Google Scholar]
- 19.Hochachka W. 1990. Seasonal decline in reproductive performance of Song Sparrows. Ecology 71, 1279–1288 10.2307/1938265 (doi:10.2307/1938265) [DOI] [Google Scholar]
- 20.Robertson R. J., Rendell W. B. 2001. A long-term study of reproductive performance in tree swallows: the influence of age and senescence on output. J. Anim. Ecol. 70, 1014–1031 10.1046/j.0021-8790.2001.00555.x (doi:10.1046/j.0021-8790.2001.00555.x) [DOI] [Google Scholar]
- 21.Stearns S. C. 1992. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]