Abstract
Male insects rarely collaborate with each other, but pollinator fig wasps (Hymenoptera: Agaonidae) are said to be an exception. Immature fig wasps feed on galled ovules located inside figs, the inflorescences of Ficus species (Moraceae). After mating, adult pollinator males chew communal exit-holes that allow mated females (which are often also their siblings) to escape. Figs also support non-pollinating fig wasps (NPFWs), some of which produce exit-holes independently. We determined whether collaboration between pollinator males (Kradibia tentacularis from Ficus montana) was necessary for the release of their females, and used the relationship between male numbers and likelihood of success to measure the extent of cooperation during exit-hole production. These attributes were then compared with those of an NPFW (Sycoscapter sp.) from the same host plant. Pollinators were more abundant than NPFW, but their more female-biased sex ratio meant male pollinator densities were only slightly higher. Individual males of both species could produce an exit-hole. Single males of the NPFW were just as successful as single male pollinators, but only male pollinators cooperated effectively, becoming more successful as their numbers increased. The lack of cooperation among NPFW may be linked to their earlier period of intense inter-male aggression.
Keywords: aggression, cooperation, fighting, male–male interactions, mutualism, parasitoid
1. Introduction
The evolution of cooperation is a central problem in biology [1]. Interactions among conspecific male insects are usually antagonistic, involving direct or indirect competition for opportunities to successfully mate with females and to fertilize their eggs. An element of antagonism is also often present in male–female interactions because optimal outcomes of encounters are not necessarily shared by the sexes [2]. Conversely, ecological situations where male insects interact to their mutual benefit are very rare, as are situations where males act to the benefit of females that they have not necessarily mated with, and to which they may be unrelated [3–6]. The unique environment occupied by fig wasps is believed to have generated an exception.
Pollinator fig wasp (PFW) females (Hymenoptera, Agaonidae) pollinate the 800 plus species of fig trees (Ficus, Moraceae). They lay their eggs inside galled ovules of the numerous tiny flowers that line the inside of the Ficus inflorescence—the fig [7]. PFW progeny sex ratios are female biased, but typically become less biased when increasing numbers of foundress females enter a fig [8]. Adult male PFW are short-lived and flightless. They emerge first, then mate with females that are still in their ovules. PFW males then chew one or more exit-holes through the outer wall of the figs, through which the females can make their escape. Because most figs are entered by a small number of foundresses, the females being assisted will include siblings of the males, with which they may also have mated. In some species, male PFW display additional behaviours such as helping the females to emerge from the galls [5,9], releasing pollen from male flowers [10,11] and reducing predation by ants [12]. In combination, these behaviours can be equated to parental investment by the males, as they improve the likelihood of survival of their gametes that are being carried by the females. They also have an inclusive fitness component, as the females will often be close relatives [13].
Numerous species of non-pollinating fig wasps (NPFWs; ovule gallers and parasitoids) also develop inside Ficus ovules [14]. Most NPFW females lay their eggs in several figs, from the outside, rather than first entering figs like the pollinators. Consequently, relatedness among progeny sharing a fig is less than with pollinators. Reflecting this, their sex ratios are usually less strongly female biased [15]. The total numbers of NPFW offspring per fig are also typically lower. NPFW usually depend on pollinator males to construct an exit-hole [7], but some are independent [16].
Here, we examine the extent of collaboration between male fig wasps from an Asian fig tree. We determine whether PFW males do collaborate to release their females, whether collaboration is necessary for females to escape and whether an NPFW displays the same abilities as its associated pollinator.
2. Material and methods
A glasshouse population of Ficus montana Blume was maintained at the experimental gardens of Leeds University, UK, together with its pollinator Kradibia (i.e. Liporrhopalum) tentacularis (Grandi) and associated undescribed parasitoid, Sycoscapter sp. They originated from Indonesia. Ficus montana is dioecious, with fig wasps only developing on male plants. Sycoscapter sp. is widely distributed and is usually the only NPFW found in field collections of F. montana figs (S. G. Compton 1994–2005, unpublished data). Females lay eggs from the outside of the figs two to three weeks after pollinator entry. The NPFW males are wingless and most never leave their natal figs. They are nonetheless capable of independent production of exit-holes. Like those of the PFW, the exit-holes they produce are small, permitting sequential emergence of females one after the other. Under our greenhouse conditions, wasps successfully complete exit-holes and emerge from about 95 per cent of figs, but success is reduced in figs that have been removed from the trees. Sycoscapter sp., males are aggressive to each other, with fights often resulting in damaged legs and mandibles. Kradibia tentacularis males do not fight.
Single mature, softening figs that were expected to have wasps emerge the next day were collected at two weekly intervals from 20 randomly chosen plants in the population of 160 male F. montana. The figs were placed in mesh-topped containers to let the wasps emerge (n = 600 figs). Emergence was deemed to have failed if it had not taken place within 3 days. The contents of all figs were recorded.
3. Results
One hundred and eighty six figs contained males of only one species. The presence of just one male PFW or NPFW was often enough to cut an exit-hole to let the females emerge (table 1). Overall, emergence rates were significantly higher among figs where only male PFW were present compared with figs containing only male NPFWs. In figs with a single male, exit-holes were just as likely to be generated by NPFWs as PFWs, but whereas extra PFW males improved success rates, extra NPFWs reduced them (table 1).
Table 1.
The likelihood of successful production of exit-holes in figs containing only male pollinators or only male NPFWs.
| presence of completed exit-hole |
||||||||
|---|---|---|---|---|---|---|---|---|
| only male pollinators |
only male NPFWs |
|||||||
| males present inside the figs | yes | no | % success | yes | no | % success | 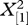 |
p |
| 1 | 12 | 7 | 63.2 | 9 | 6 | 60.0 | 0.03 | 0.85 |
| 2 | 17 | 6 | 73.9 | 5 | 7 | 41.7 | 3.51 | 0.06 |
| 3 | 12 | 5 | 70.6 | 4 | 5 | 44.4 | 1.70 | 0.19 |
| 4 | 8 | 1 | 88.9 | 3 | 3 | 50.0 | 2.78 | 0.10 |
| more than four | 51 | 8 | 86.4 | 9 | 8 | 52.9 | 8.91 | <0.001 |
| all figs combined | 100 | 27 | 78.7 | 30 | 29 | 50.8 | 14.37 | <0.001 |
No more than one exit-hole was produced, through the ostiole, irrespective of wasp numbers and species. No male fig wasps were present in 26 figs—none had exit-holes produced (table 2). Female PFW were much more common than female NPFW (means + s.d. per fig = 21.34 + 18.32 and 7.83 + 9.80, respectively). The overall parasitism rate was 29.8 per cent (proportion of total wasps as NPFW), but because the sex ratio (proportion of males) was lower in pollinators (0.19 versus 0.36), the numbers of male PFW per fig only averaged about one more than male NPFW (means + s.d. per fig = 4.94 + 8.07 and 3.92 + 3.74, respectively; ANOVA, F1,822 = 11.08, p < 0.001).
Table 2.
The numbers of adult male fig wasps in figs of F. montana.
| frequency (no. of figs) |
|||
|---|---|---|---|
| total numbers of males | pollinator males | NPFW males | both species combined |
| 0 | 59 | 37 | 26 |
| 1 | 19 | 15 | 34 |
| 2 | 23 | 12 | 20 |
| 3 | 17 | 9 | 23 |
| 4 | 9 | 6 | 36 |
| 5 | 10 | 6 | 41 |
| 6 | 8 | 4 | 37 |
| >6 | 41 | 7 | 225 |
No exit-holes were produced in 187 of the 600 figs (31%), with likelihood of failure negatively related to the total number of wasps present (logistic regression, Wald (W) = 28.52, p < 0.001) and to the total number of male wasps present (both species combined, W = 5.52, p = 0.02). Parasitism rates in successfully emerged figs were 27.7 per cent (n = 413), compared with 34.3 per cent (n = 187) in figs where no exit tunnel was completed. At low densities, at least, males of the two species did not interfere with each other because the likelihood of failed emergence from figs containing one male of each species was similar to that in figs where two male PFW were present alone (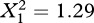 , p = 0.26).
, p = 0.26).
Likelihood of failure was positively related to parasitism rate (logistic regression, W = 12.61, p < 0.001), driven largely by poor emergence from those figs with the highest rates of parasitism. In figs containing both PFW and NPFW males, the likelihood of exit-hole production increased with combined male numbers (W = 5.92, p = 0.01). When individual species were considered separately, only male PFW numbers had a significant effect on exit-hole production (logistic regression, W = 5.56, p = 0.02 for male pollinators and W = 0.05, p = 0.82 for male NPFWs).
4. Discussion
It has long been stated that male PFWs collaborate to produce exit-holes for females, based on observations that multiple males contribute to exit-hole production, but this is, to our knowledge, the first demonstration that their shared efforts improve the likelihood of a successful outcome. Both PFW and NPFW males were capable of releasing their females from the figs. When only a single male was present, NPFWs and PFWs were equally capable of exit-hole production, but whereas PFW males cooperated, NPFWs impeded each other. The consequences of NPFW failure to collaborate will have been magnified under our experimental conditions because exit-holes are easier to produce in figs left in situ than in figs removed from the trees.
Shared efforts to produce exit-holes are routine among males of PFW species and the successful collaboration we recorded in K. tentacularis is likely to be the norm. By contrast, Sycoscapter sp. is just one of many hundreds of NPFW that range from species where females are totally dependent on PFW males for emergence to others that, because of their larger size, must always emerge independently. Individuals sharing more genes may behave more altruistically and less aggressively towards their relatives, as may fig wasps with more individuals per fig [17,18]. NPFWs sharing a fig have a lower average relatedness and smaller brood sizes per fig than PFWs, but their reproductive success still totally depends on mated females being able to escape. Alternatively, the failure of supernumerary NPFW males to cooperate might reflect less intense selection for them to do so, because they can rely on the efforts of PFW males, yet there were no such males available in almost 10 per cent of the figs we surveyed. NPFW males vary greatly in the intensity of their competition for mates [19]. Unlike K. tentacularis, Sycoscapter sp. males are often damaged in fights, but their interactions are mild compared with the routinely fatal fighting seen in some congeners. Intense inter-male aggression when competing for mating opportunities may turn out to be incompatible with subsequent cooperative behaviour that needs to take place just a few hours later [20,21]. If so, then cooperation may also be absent in the few PFW species with males that fight intensively [11]. An inability to collaborate among species displaying intense male : male aggression may also explain the rarity of independent emergence among NPFW with fighting males: solitary males can be effective in the small, thin-walled figs of F. montana, but cooperation may be a prerequisite for success in larger figs, which have thicker walls to penetrate.
Acknowledgements
Martin Lappage provided invaluable practical assistance and two referees generated stimulating discussion. Financial support (N.S. and S.R.) was from The Ministry of Education, Pakistan.
References
- 1.Sachs J. L. 2006. Cooperation within and among species. J. Evol. Biol. 19, 1415–1418 10.1111/j.1420-9101.2006.01152.x (doi:10.1111/j.1420-9101.2006.01152.x) [DOI] [PubMed] [Google Scholar]
- 2.Chapman T. 2006. Evolutionary conflicts of interest between males and females. Curr. Biol. 16, R744–R754 10.1016/j.cub.2006.08.020 (doi:10.1016/j.cub.2006.08.020) [DOI] [PubMed] [Google Scholar]
- 3.Dugatkin L. A. 1994. Cooperation among animals: an evolutionary perspective. Science 266, 1030–1032 10.1126/science.7973654 (doi:10.1126/science.7973654) [DOI] [PubMed] [Google Scholar]
- 4.Greeff J. M. 1999. Cooperative breeding, offspring packaging and biased sex ratios in allodapine bees. Behav. Ecol. 10, 141–148 10.1093/beheco/10.2.141 (doi:10.1093/beheco/10.2.141) [DOI] [Google Scholar]
- 5.Zammit J., Schwarz M. P. 2000. Intersexual sibling interactions and male benevolence in a fig wasp. Anim. Behav. 60, 695–701 10.1006/anbe.2000.1522 (doi:10.1006/anbe.2000.1522) [DOI] [PubMed] [Google Scholar]
- 6.Shelly T. E. 2001. Lek size and female visitation in two species of tephritid fruit flies. Anim. Behav. 62, 33–40 10.1006/anbe.2000.1723 (doi:10.1006/anbe.2000.1723) [DOI] [Google Scholar]
- 7.Weiblen G. D. 2002. How to be a fig wasp. Annu. Rev. Entomol. 47, 299–330 10.1146/annurev.ento.47.091201.145213 (doi:10.1146/annurev.ento.47.091201.145213) [DOI] [PubMed] [Google Scholar]
- 8.Raja S., Suleman N., Compton S. G., Moore J. C. 2008. The mechanism of sex ratio adjustment in a pollinating fig wasp. Proc. R. Soc. B 275, 1603–1610 10.1098/rspb.2008.0136 (doi:10.1098/rspb.2008.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn D. W., Yu D. W., Ridley J., Cook J. M. 2008. Longevity, size and early emergence in a pollinating fig wasp: implications for the stability of a fig-pollinator mutualism. J. Anim. Ecol. 77, 927–935 (doi:10.0.1111/j.1365-2656.2008.01416.x) [DOI] [PubMed] [Google Scholar]
- 10.Michaloud G., Carrière S., Kobbi M. 1996. Exceptions to the one : one relationship between African fig trees and their fig wasp pollinators: possible evolutionary scenarios. J. Biogeogr. 23, 513–520 10.1111/j.1365-2699.1996.tb00013.x (doi:10.1111/j.1365-2699.1996.tb00013.x) [DOI] [Google Scholar]
- 11.Greeff J. M., Van Noort S., Rasplus J.-Y., Kjellberg F. 2003. Dispersal and fighting in male pollinating fig wasps. C. R. Biol. 326, 121–130 10.1016/S1631-0691(03)00010-6 (doi:10.1016/S1631-0691(03)00010-6) [DOI] [PubMed] [Google Scholar]
- 12.Zachariades C., Schatz B., Compton S. G. 2010. Wasp emergence from the figs of Ficus sur: characteristics and predation by ants. Trop. Zool. 23, 121–138 [Google Scholar]
- 13.Sachs J. L., Mueller U. G., Wilcox T. P., Bull J. J. 2004. The evolution of cooperation. Quart. Rev. Biol. 79, 135–160 10.1086/383541 (doi:10.1086/383541) [DOI] [PubMed] [Google Scholar]
- 14.Compton S. G., Hawkins B. A. 1992. Determinants of species richness in southern African fig wasp assemblages. Oecologia 91, 68–74 [DOI] [PubMed] [Google Scholar]
- 15.Murray M. G. 1990. Comparative morphology and mate competition of flightless male fig wasps. Anim. Behav. 39, 434–443 10.1016/S0003-3472(05)80406-3 (doi:10.1016/S0003-3472(05)80406-3) [DOI] [Google Scholar]
- 16.Ramirez W. B. 1974. Coevolution of Ficus and Agaonidae. Ann. Mo. Bot. Gard. 61, 770–780 10.2307/2395028 (doi:10.2307/2395028) [DOI] [Google Scholar]
- 17.West S. A., Pen I., Griffin A. S. 2002. Cooperation and competition between relatives. Science 296, 72–75 10.1126/science.1065507 (doi:10.1126/science.1065507) [DOI] [PubMed] [Google Scholar]
- 18.West S. A., Murray M. G., Machado C. A., Griffin A. S., Herre E. A. 2001. Testing Hamilton's rule with competition between relatives. Nature 409, 510–513 10.1038/35054057 (doi:10.1038/35054057) [DOI] [PubMed] [Google Scholar]
- 19.Cook J. M., Rasplus J. Y. 2003. Mutualists with attitude: coevolving fig wasps and figs. Trends Ecol. Evol. 18, 241–248 10.1016/S0169-5347(03)00062-4 (doi:10.1016/S0169-5347(03)00062-4) [DOI] [Google Scholar]
- 20.Platt T. G., Bever T. D. 2009. Kin competition and the evolution of cooperation. Trends Ecol. Evol. 24, 370–377 10.1016/j.tree.2009.02.009 (doi:10.1016/j.tree.2009.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barta Z., McNamara J. M., Huszar D. B., Taborsky M. 2011. Cooperation among non-relatives evolves by state-dependent generalized reciprocity. Proc. R. Soc. B 278, 843–848 10.1098/rspb.2010.1634 (doi:10.1098/rspb.2010.1634) [DOI] [PMC free article] [PubMed] [Google Scholar]


