Abstract
We report on a newly discovered cockroach (Saltoblattella montistabularis) from South Africa, which jumps and therefore differs from all other extant cockroaches that have a scuttling locomotion. In its natural shrubland habitat, jumping and hopping accounted for 71 per cent of locomotory activity. Jumps are powered by rapid and synchronous extension of the hind legs that are twice the length of the other legs and make up 10 per cent of the body weight. In high-speed images of the best jumps the body was accelerated in 10 ms to a take-off velocity of 2.1 m s−1 so that the cockroach experienced the equivalent of 23 times gravity while leaping a forward distance of 48 times its body length. Such jumps required 38 µJ of energy, a power output of 3.4 mW and exerted a ground reaction force through both hind legs of 4 mN. The large hind legs have grooved femora into which the tibiae engage fully in advance of a jump, and have resilin, an elastic protein, at the femoro-tibial joint. The extensor tibiae muscles contracted for 224 ms before the hind legs moved, indicating that energy must be stored and then released suddenly in a catapult action to propel a jump. Overall, the jumping mechanisms and anatomical features show remarkable convergence with those of grasshoppers with whom they share their habitat and which they rival in jumping performance.
Keywords: cockroach, jumping, kinematics, Saltoblattella
1. Introduction
Cockroaches are a highly adaptable group of about 4000 species belonging to a monophyletic group (Blattodea) that includes two distinct clades; one containing termites and primitive, semi-social cockroaches (Cryptocercidae), and the other the more typical cockroaches [1]. Most modern cockroaches show evolutionary conservatism in body form, and their characteristic scuttling gait is propelled by three pairs of legs of similar size and structure. No previously described extant species jump. We now show that a newly discovered wingless cockroach, Saltoblattella montistabularis (Blattodea, Blattellidae) [2] from Table Mountain, South Africa has enlarged hind legs that it uses to jump powerfully. It coexists with grasshoppers and shares their ability to jump accurately between grass and sedge culms. This isolated evolutionary innovation is reflected in a body form unique among cockroaches, which shows remarkable convergence to both anatomical and functional mechanisms used by grasshoppers in jumping.
2. Material and methods
Field observations were made at Silvermine Nature Reserve, Table Mountain, South Africa (34°04′30″ S, 18°23′55″ E, 450 m altitude), a montane fynbos habitat containing low-growing Restionaceae, and Ericaceae, and higher, bushy Proteaceae.
In the laboratory, jump heights and distances were recorded with a Canon MVX40 video camera (Canon Inc., Japan) and jump performance and kinetics were derived from sequential images captured at rates of 2000 frames s−1, using a Photron 1024 PCI camera and then saved directly to a computer for subsequent analysis. Recordings of electrical activity of flexor and extensor tibiae muscles of the hind legs were made from freely moving and restrained cockroaches. The possible presence of the rubber-like protein resilin was revealed by its two key signatures, its characteristic blue fluorescence when illuminated with specific wavelengths of ultraviolet light (UV), and the sensitivity of this fluorescence to experimentally induced changes in the pH of a bathing saline [3]. Leg lengths of Saltoblattella, similar-sized nymphs of the grasshopper Acrida acuminata from the same habitat, and German cockroaches Blattella germanica were measured and compared. Full details of the experimental techniques used are given in electronic supplementary material, methods.
3. Results
In the field, observations of the behaviour of 16 S. montistabularis (two males and 14 females; figure 1a) for a total of 64 min (median 2.5 min per individual, range 0.5–23) showed that locomotion accounted for 62.6 per cent of the observation time, and comprised 198 discrete locomotory events that were divided into three categories. First, 66 jumps (median 2.5 for each individual, range 0–17), defined as a 5–20 cm horizontal displacement; second, 72 hops (median 2, range 0–20), a 2–5 cm horizontal displacement; and third, 60 scuttles (median 2, range 0–18), during which the cockroach did not become airborne. Jumping and hopping comprised 71 per cent of all locomotory activity, and scuttling only 29 per cent.
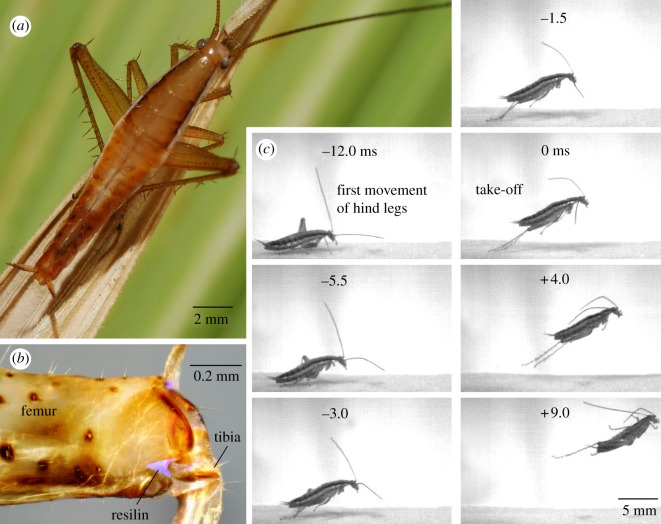
Figure 1.
(a) Male Saltoblattella montistabularis preparing to jump; the hind tibiae are being flexed before fitting into ventral grooves of the enlarged femora. (b) Femoro-tibial joint of a hind leg showing the blue fluorescence characteristic of resilin in a v-shaped notch and at the base of a dorsal spine. (c) Selected images, at the times indicated, from a jump captured at 2000 s–1 with an exposure time of 0.1 ms, arranged in two columns. Take-off occurred at time 0 ms when the hind tarsi of this female cockroach left the ground.
In the laboratory, Saltoblattella jumped a maximum forward distance of 35.1 cm (48 times body length for a female), reaching maximum heights of 18.8 cm, from an average take-off angle of 40°. Females jumped horizontally further (mean of 26.3 ± 3.1 cm, or 36 times body length, 64 jumps by six females) than males (mean of 22.3 ± 3.2 cm, or 24 times body length, 68 jumps by seven males; t-test, t11 = 2.32, p = 0.041), but males (11.6 ± 2.7 cm) jumped slightly higher than females (9.5 ± 2.4 cm; t11 = 1.48, p = 0.17). Females (16.7 ± 2.8 mg) were significantly heavier than males (14.0 ± 0.8 mg; t15 = 2.51, p = 0.024), but males were significantly longer (9.3 ± 0.5 mm, n = 7) than females (7.3 ± 0.5 mm, n = 10; t15 = 8.44, p = 4.43 × 10−7). Females (1.4 ± 0.1 mm) had relatively longer hind legs than males (1.1 ± 0.1 mm; t15 = 6.70, p = 6.99 × 10−6) when expressed as a proportion of body length.
Jumps were powered by the femoral muscles of the enlarged hind legs which were more than twice the length of the other legs (ratio of front to middle to hind of 1 : 1.2 : 2.4) and 50 per cent longer than the body (figure 1a). The large femoral muscles contributed to a 300 per cent difference between the weight of the hind and middle legs, with the hind femora alone making up 19 per cent of the body weight. German cockroaches (B. germanica) that do not jump, but belong to the same family as Saltoblattella [2], have proportionately shorter hind legs (ratio 1 : 1.3 : 1.7) that are only the same length as the body. By contrast, nymphs of the grasshopper A. acuminata from the same habitat as Saltoblattella had hind legs nearly four times the length of the other legs (1 : 1.1 : 3.7) but only 19.5 per cent longer than the body. Additional features of the hind legs that are shared with grasshoppers include: (i) a groove on the ventral surface of each hind femur that accommodates the tibia when fully flexed in preparation for jumping; (ii) strengthening of the distal end of the femur and the proximal end of the tibia at the insertions of the flexor and extensor muscles (figure 1b); and (iii) v-shaped areas in both the lateral and medial surfaces of a distal femur, which fluoresce bright blue under UV light of specific wavelengths (figure 1b). These areas were not found in B. germanica that does not jump. The fluorescence was also sensitive to the pH of a bathing saline. These are the two key signatures of the elastic protein resilin. Blue fluorescence was absent in comparable positions in the front and middle legs. Semi-lunar processes that act as energy storage devices in grasshoppers were notably absent.
High-speed images of jumping (electronic supplementary material, video), from which the kinematics of the movements were measured, showed that the hind femora were first rotated anteriorly, and the hind tibiae flexed fully about the femur (figure 1c). These preparatory movements were followed by the rapid and simultaneous extension of both hind tibiae so that take-off was achieved after an acceleration period of 10.6 ± 1.0 ms (mean of means of 63 jumps by five male cockroaches). Best jumps achieved a take-off velocity of 2.1 m s−1 (mean of means 1.5 ± 0.3 m s−1 for five males), and an average acceleration of 220 m s−2 (over the acceleration period) equivalent to 23g. These jumps required 38 µJ of energy, a power output of 3.4 mW and exerted a ground reaction force of 4 mN through both hind legs. To power a jump by direct contractions, the extensor tibiae muscles would need to generate 1100 W kg−1 (assuming the extensor tibiae muscles are the largest contributor to the weight of a hind femur). This value is beyond the maximum active contractile limits of striated muscles, which have averaged values of 250–500 W kg−1 [4–7]. Energy must therefore be stored in advance of the rapid movements of the hind legs.
Recordings of the electrical activity of a hind extensor tibiae muscle showed that its motor neurons were active for an average of 224 ± 79.7 ms (33 jumps by seven cockroaches) before the hind legs moved, during which phasically acting motor neurons spiked 14 ± 4.6 times. These contractions would enable the power requirements to be met if energy were stored in advance of a jump.
4. Discussion
The different mechanisms used by insects for jumping are associated with deep phylogenetic nodes, possibly suggestive of evolutionary conservatism. Fleas [8], plant-sucking bugs (Auchenorrhyncha) [9,10] and grasshoppers [11], store energy by prolonged muscle contractions and release it suddenly in a catapult action, but each uses a unique set of adaptations. In contrast, bush crickets use direct muscle contractions to move their exceptionally long hind legs [12]. While jumping in Saltoblattella represents a functional evolutionary novelty [13] for Blattodea, the various jumping adaptations are largely modifications of existing structures. This nevertheless provides it with a body plan unique among cockroaches [2], and convergent in many aspects with that of the distantly related Orthoptera. The vestigial wings indicate a reliance on jumping and walking locomotion, and the unique hemispherically protruding eyes probably confer a broad field of vision, useful for accurate positional landings on grass culms after jumps. The novel, second articulation point on the first antennal segment [2] may stabilize the long antennae while they are swept backwards during take-off (figure 1c). The presence of the elastic protein resilin in the hind legs of Saltoblattella may act to restore the shape of the hind femora after the powerful contractions of the extensor tibiae muscles. In fleas [8] and plant-sucking bugs [14], resilin is closely associated with energy storage devices, possibly preventing fracture of hard cuticle (in which most of the energy is stored), and restoring body shape [14]. Saltoblattella is an accomplished jumper, achieving forward displacements of 48 times its body length in its best jumps, thus outperforming locusts (20 body lengths) [11] but not froghoppers (114 body lengths) [9].
Why should jumping have evolved in just this one species of cockroach?
An Upper Jurassic fossil cockroach ancestor (Skok svaba) [15] had enlarged hind legs, but less well developed than those in Saltoblattella, suggesting that jumping may have arisen before in the cockroach lineage, but did not result in an adaptive radiation. While jumping appears to be synapomorphic for some insect orders, such as the Siphonaptera (fleas), Orthoptera (grasshoppers) and Archaeognatha (bristletails)—in other orders it is less common. In Hemiptera, it occurs infrequently in lineages, such as the Heteroptera, but is common in the Auchenorrhyncha [9,10]. In Coleoptera, jumping is best developed in the speciose clade of flea beetles (Alticinae) but is rare in the many other clades. A recent study [16] provides evidence for the multiple origin of the metafemoral spring used in jumping in flea beetles. The same study [16] also suggested that jumping in flea beetles is a defensive, anti-predator response, and that groups having such well-developed defensive adaptations would be ‘more prone to speciation’. Jumping in Saltoblattella would serve both for rapid locomotion within a vertically stratified microhabitat of grass and sedge culms (it was the dominant form of locomotory activity in its natural habitat), but it may also be used in predator avoidance. However, the absence of any radiation of the Saltoblattella clade suggests that locomotion rather than defense may be the primary function of jumping in this species.
Acknowledgements
SANParks and CapeNature (permit AAA004-00026-0035) granted permission to work in the Table Mountain National Park. This work was funded by a University of Cape Town URC grant (M.P.). We thank Cambridge colleagues for help during the experimental work and for comments on the manuscript.
References
- 1.Ware J. L., Litman J., Klass K.-D., Spearman L. A. 2008. Relationship among the major lineages of Dictyoptera: the effect of outgroup selection on dictyopteran tree topology. Syst. Entomol. 33, 429–450 10.1111/j.1365-3113.2008.00424.x (doi:10.1111/j.1365-3113.2008.00424.x) [DOI] [Google Scholar]
- 2.Bohn H., Picker M. D., Klass K.-D., Colville J. F. 2010. A jumping cockroach from South Africa, Saltoblattella montistabularis, gen. nov., spec. nov. (Blattodea: Blattellidae). Arthropod Syst. Phylogeny 68, 53–69 [Google Scholar]
- 3.Neff D., Frazier S. F., Quimby L., Wang R.-T., Zill S. 2001. Identification of resilin in the leg of cockroach, Periplaneta americana: confirmation by a simple method using pH dependence of UV fluorescence. Arthropod Struct. Dev. 29, 75–83 10.1016/S1467-8039(00)00014-1 (doi:10.1016/S1467-8039(00)00014-1) [DOI] [PubMed] [Google Scholar]
- 4.Askew G. N., Marsh R. L. 2002. Muscle designed for maximum short-term power output: quail flight muscle. J. Exp. Biol. 205, 2153–2160 [DOI] [PubMed] [Google Scholar]
- 5.Ellington C. P. 1985. Power and efficiency of insect flight muscle. J. Exp. Biol. 115, 293–304 [DOI] [PubMed] [Google Scholar]
- 6.Josephson R. K. 1993. Contraction dynamics and power output of skeletal muscle. Annu. Rev. Physiol. 55, 527–546 10.1146/annurev.ph.55.030193.002523 (doi:10.1146/annurev.ph.55.030193.002523) [DOI] [PubMed] [Google Scholar]
- 7.Weis-Fogh T., Alexander R. M. 1977. The sustained power output from striated muscle. In Scale effects in animal locomotion (ed. Pedley T. J.), pp. 511–525 London, UK: Academic Press [Google Scholar]
- 8.Bennet-Clark H. C., Lucey E. C. A. 1967. The jump of the flea: a study of the energetics and a model of the mechanism. J. Exp. Biol 47, 59–76 [DOI] [PubMed] [Google Scholar]
- 9.Burrows M. 2003. Froghopper insects leap to new heights. Nature 424, 509. 10.1038/424509a (doi:10.1038/424509a) [DOI] [PubMed] [Google Scholar]
- 10.Burrows M. 2009. Jumping performance of planthoppers (Hemiptera, Issidae). J. Exp. Biol. 212, 2844–2855 10.1242/jeb.032326 (doi:10.1242/jeb.032326) [DOI] [PubMed] [Google Scholar]
- 11.Bennet-Clark H. C. 1975. The energetics of the jump of the locust Schistocerca gregaria. J. Exp. Biol. 63, 53–83 [DOI] [PubMed] [Google Scholar]
- 12.Burrows M., Morris O. J. 2003. Jumping and kicking in bush crickets. J. Exp. Biol. 206, 1035–1049 10.1242/jeb.00214 (doi:10.1242/jeb.00214) [DOI] [PubMed] [Google Scholar]
- 13.Wagner G. P., Lynch V. J. 2010. Evolutionary novelties. Curr. Biol. 20, 48–52 10.1016/j.cub.2009.11.010 (doi:10.1016/j.cub.2009.11.010) [DOI] [PubMed] [Google Scholar]
- 14.Burrows M., Shaw S. R., Sutton G. P. 2008. Resilin and cuticle form a composite structure for energy storage in jumping by froghopper insects. BMC Biol. 6, 41. 10.1186/1741-7007-6-41 (doi:10.1186/1741-7007-6-41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vrsansky P. 2007. Jumping cockroaches (Blattaria, Skokidae fam. n.) from the late Jurassic of Karatau in Kazakhstan. Biologia 62, 588–592 10.2478/s11756-007-0116-2 (doi:10.2478/s11756-007-0116-2) [DOI] [Google Scholar]
- 16.Ge D., Chesters D., Gómez-Zurita J., Zhang L., Yang X., Vogler A. P. 2011. Anti-predator defence drives parallel morphological evolution in flea beetles. Proc. R. Soc. B 278, 2133–2141 10.1098/rspb.2010.1500 (doi:10.1098/rspb.2010.1500) [DOI] [PMC free article] [PubMed] [Google Scholar]