Abstract
Protein is a limiting resource that is essential to the growth, maintenance and reproduction of tropical frugivores, yet few studies have examined how wild animals maintain protein balance. During chronic periods of fruit scarcity, Bornean orangutans (Pongo pygmaeus) often catabolize their own fat reserves despite unusually low metabolic requirements. Such energy deficits suggest a marginal existence, and raise the possibility that orangutans also endure periods of negative protein balance. To test this hypothesis, we conducted the first study of protein cycling in a wild primate. Our five year analysis of urinary metabolites revealed evidence of protein recycling when fruit was scarce. During these periods, orangutans consumed more leaves and bark, proteinaceous but tough foods that yielded a mean daily intake of 1.4 g protein kg−1 metabolic mass. Such an amount is inadequate for humans and one-tenth the intake of mountain gorillas, but sufficient to avert, perhaps narrowly, a severe protein deficit. Our findings highlight the functional and adaptive value of traits that maximize protein assimilation during periods of ecological exigency.
Keywords: orangutans, Borneo, protein, δ15N, urea
1. Introduction
Weathered soils and a prevalence of trees in the Dipterocarpaceae distinguish Borneo from other large Sunda-Shelf islands [1]. These factors shape the structure of the floral community and limit the production of fruit [2]; as a result, the rainforests of Borneo are widely viewed as impoverished habitats for vertebrate frugivores [3]. For one of the largest frugivores, the orangutan (Pongo pygmaeus), the evolution of extreme hypometabolism [4] is a testament to its marginal existence. Yet Bornean orangutans can still lapse into energy deficits [5–7], raising the possibility that they also endure states of somatic catabolism (tissue wasting) during prolonged periods of food scarcity.
Orangutans must obtain sufficient dietary protein for growth and maintenance of body tissue. This truism extends to all organisms; thus protein, as measured by nitrogen (N) concentration, is a basic limiting resource for frugivores across the tropics [8,9], including primates [10]. Yet, the dynamics and behavioural consequences of protein cycling in wild animals are scarcely known. With new analytical techniques [11], the excreta of wild animals may inform our understanding of how animals cope with protein limitation.
The N balance of vertebrates is positive when dietary N exceeds non-microbial output in urine and faeces [12] (electronic supplementary material, figure S1). Such a surplus results in higher concentrations of urinary urea [13,14]. If N intake falls below endogenous rates of N loss in urine and faeces, an animal can use lipid stores to maintain a steady-state negative N balance [12]. This state results in reduced levels of urinary urea and %N due to N-recycling, but stable values of δ15N because protein stores are intact. After a prolonged period of inadequate N intake and the depletion of fat reserves, an animal is expected to enter an unsteady state of negative N balance, during which body protein is catabolized for cell maintenance [12]. During this state of tissue wasting, both urea concentration and %N will be at their nadir, whereas δ15N will increase sharply [15].
This model of protein flux agrees well with studies of ungulates: urinary δ15N is highest during periods of food deprivation [14–16]. Such findings indicate the recycling of tissue-derived amino-N and highlight the potential value of urine for assessing protein balance in wild animals. Here, we use the urine of wild Bornean orangutans to test whether individuals were protein limited during a five year period.
2. Material and methods
Data were collected in Gunung Palung National Park, Borneo [17] (1°13′ S, 110°7′ E) on 27 orangutans (Pongo pygmaeus wurmbii; electronic supplementary material, table S1) observed between 1994 and 1999. The percentage of 1368 tagged trees in fruit, termed the fruit abundance index (FAI), was calculated each month. Our method for quantifying dietary protein is detailed elsewhere [5,11]. To estimate the total mass of protein consumed per feeding bout, we calculated dry mass protein per food-item × number of items ingested per minute × total bout minutes.
Total grams of protein consumed per day per individual were calculated by summing each individual's daily feeding bouts from 604 h of focal feeding data collected from 1994–1996. We tested 90 urine samples collected opportunistically, typically from first-morning voids. Our methods for preserving and measuring urinary compounds are detailed elsewhere [11]. All fruit and urine samples, feeding data and phenological data were collected concurrently.
Linear mixed models (LMM) were fit to the data using maximum-likelihood estimation to predict four outcome measures (urea concentration (µM per µM creatinine), δ15N (‰), weight %N and total protein consumed per day (gram; electronic supplementary material, table S2). All available data were included in statistical analyses. Models were fitted with the lme (linear mixed-effect) function from the nlme package in R [18]. Variables were selected for inclusion in the model using Akaike's information criterion. For post hoc multiple comparisons of per cent protein in food items, we calculated non-parametric multiple comparisons for unequal sample sizes [19]. All probability levels are two-tailed, and the significance for all tests was α < 0.05.
3. Results
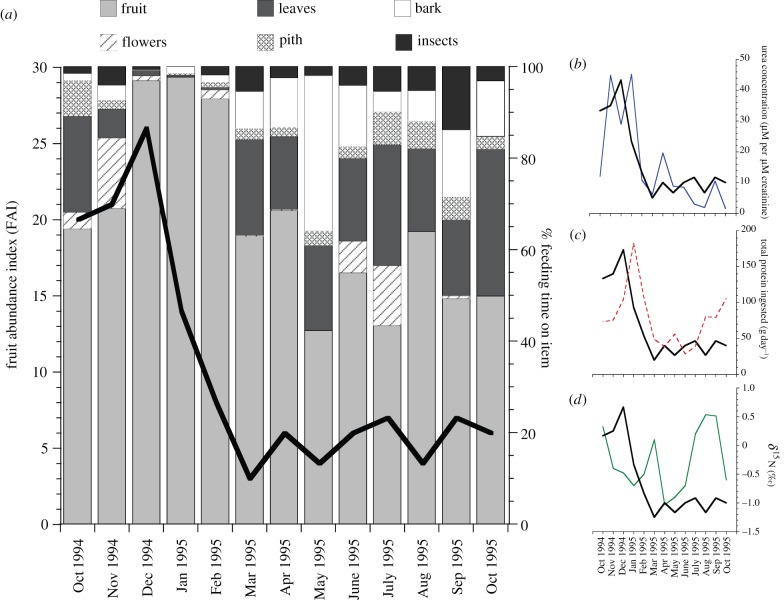
Figure 1 illustrates a continuous subset of our data and the effects of a steep decline in fruit abundance (FAI) on dietary behaviours and urinary metabolites. To detect changes in protein cycling, we measured the percentage of N (range: 0–9.2%) and the concentration of urea (range: 0.4–147.1 µM per µM creatinine) in each urine sample. We found that urea varied as a positive function of FAI (LMM: t = 3.06, d.f. = 50, p = 0.004; figure 1b) and urinary N (LMM: t = 1.93, d.f. = 40, p = 0.06; electronic supplementary material, tables S2 and S3).
Figure 1.
Monthly variation in (a) fruit abundance index (FAI) and the percentage of time consuming different food items; (b) urea concentration (µM µM−1 creatinine) as a function of FAI; (c) total daily protein ingested (gram) as a function of FAI; and (d) urinary δ15N as a function of FAI, from October 1994 to October 1995. Solid black line = FAI in all graphs.
We also calculated the average mass of protein consumed per day (gram per 100 g organic matter). After controlling for reproductive condition, which accounted for 3 per cent of the total variance, we found evidence of diminishing protein intake as FAI declined (LMM: t = 2.01, d.f. = 22, p = 0.05; figure 1c), a pattern that coincided with reduced overall food intakes and lipid catabolism [5].
To test whether this level of protein intake resulted in protein catabolism, we measured the δ15N of each urine sample. Variation in δ15N (range: −1.0 to 1.5‰) was unrelated to FAI (LMM: t = −0.86, d.f. = 44, p = 0.40; figure 1d); however, there was a weak inverse relationship with protein intake (LMM: t = −1.88, d.f. = 19, p = 0.07; electronic supplementary material, figure S2) indicating that some orangutans entered the early stages of a protein deficit.
This result highlights the role of ‘fallback foods’ [20] following periods of protein profligacy. As fruit became scarce, orangutans descended into a negative energy balance [5,6] and consumed a greater fraction of leaves (r2 = 0.30, p = 0.04) and inner-bark tissues (r2 = 0.31, p = 0.03; figure 1a). The mean protein content of leaves was twofold greater than fruit (p < 0.05), whereas the mean protein content of inner-bark tissues was comparable to fruit (9.98 ± 6.15% versus 9.48 ± 5.70% protein, respectively; figure 2).
Figure 2.
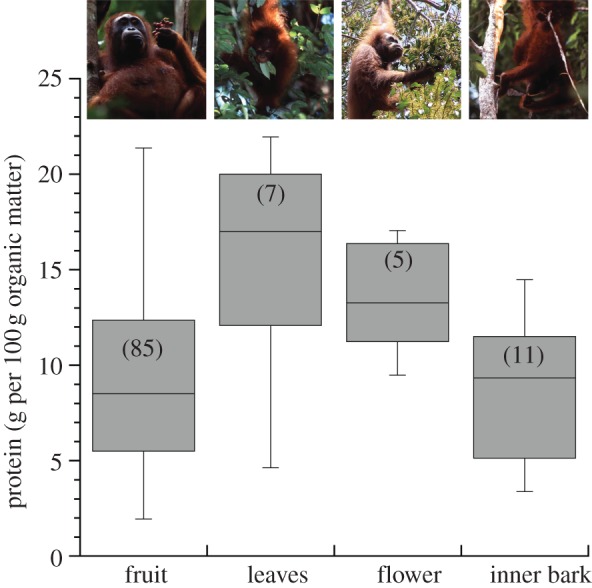
Protein mass (gram per 100 g organic matter) in food items consumed by orangutans. Inner-bark includes both cambium and phloem. Sample sizes are reported in parentheses (photographs ©Tim Laman).
4. Discussion
Orangutans voided surplus N from dietary protein when fruit was widely available and recycled N when fruit was scarce. When fruit was limited, the orangutans consumed more leaves and bark—items high in protein. Yet, despite this dietary shift, adult males and females consumed an average of 1.3 and 1.6 g protein kg−1 metabolic body mass per day, respectively (electronic supplementary material, table S4). Such low values are inadequate for humans [21] and one-tenth the intake of mountain gorillas [22,23]. The lower urinary urea and N excretion during this period could indicate a protein intake that was marginally adequate to cover metabolic requirements or protein recycling and the onset of a negative N balance. The elevated δ15N in urine after several months of low protein intake indicates the commencement of tissue wasting. Taken together, these results suggest that orangutans endure prolonged protein deficits and that protein intake levels are narrowly sufficient to avert a more severe state of tissue wasting.
For orangutans, these findings attest to the importance of assimilating protein from foods with high fracture toughness [24], and highlights the functional and adaptive advantages of crenulated molar teeth, large jaws and chewing muscles, and a proliferation of gene copies that code for protein-digesting enzymes [24,25]. Our characterization of Bornean orangutans as protein recyclers living on the brink of protein bankruptcy may contribute to their vulnerability to anthropogenic or climatic changes that result in more extended periods of low food availability. Such vulnerability could partly explain why population sizes have waned in the past 400 000 years [26]. Our findings also support the view that partially logged forests are better than none at all [27]. The loss of primary habitat in Borneo—50 per cent since 1950 [28]—has pushed many orangutans into forests with a history of selective logging. Such forests have conservation value because they have retained substantial populations of orangutans [27], perhaps because protein is relatively abundant and accessible. Non-dipterocarp trees and lianas flourish when dipterocarps are removed, factors that favour the production of tender young leaves and broaden the protein available to orangutans.
Acknowledgements
We received approval from PHKA, BTNGP, LIPI, RISTEK, PPPB and the IACUC of UCSC (20061056-122204). We thank D. Andreasen, S. Kim, P. L. Koch, R. M. Kudela, T. Laman, R. S. Scott, B. Walker and all field assistants. Funding was received from the Conservation, Food and Health Foundation, George Washington University Selective Excellence Fund, Packard Foundation (2007-31754), Leakey Foundation, National Geographic Society, National Science Foundation (BCS-0643122, -721288 and -9414388), Orangutan Conservancy, US Fish and Wildlife Service and Wenner-Gren Foundation.
References
- 1.MacKinnon K., Hatta G., Halim H., Mangalik A. 1996. The ecology of Kalimantan. Singapore: Periplus [Google Scholar]
- 2.Wich S., Vogel E., Larsen M., Frederiksson G., Leighton M., Yeager C., Brearley F., van Schaik C., Marshall A. 2011. Forest fruit production is higher on Sumatra than on Borneo. PLoS ONE 6, e21278. 10.1371/journal.pone.0021278 (doi:10.1371/journal.pone.0021278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meiri S., Meijaard E., Wich S. A., Groves C. P., Helgen K. M. 2008. Mammals of Borneo: small size on a large island. J. Biogeogr. 35, 1087–1094 10.1111/j.1365-2699.2008.01897.x (doi:10.1111/j.1365-2699.2008.01897.x) [DOI] [Google Scholar]
- 4.Pontzer H., Raichlen D. A., Shumaker R. W., Ocobock C., Wich S. A. 2010. Metabolic adaptation for low energy throughput in orangutans. Proc. Natl Acad. Sci. USA 107, 14 048–14 052 10.1073/pnas.1001031107 (doi:10.1073/pnas.1001031107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knott C. D. 1998. Changes in orangutan caloric intake, energy balance, and ketones in response to fluctuating fruit availability. Int. J. Primatol. 19, 1061–1079 10.1023/A:1020330404983 (doi:10.1023/A:1020330404983) [DOI] [Google Scholar]
- 6.Emery Thompson M., Knott C. D. 2008. Urinary C-peptide of insulin as a non-invasive marker of energy balance in wild orangutans. Horm. Behav. 53, 526–535 10.1016/j.yhbeh.2007.12.005 (doi:10.1016/j.yhbeh.2007.12.005) [DOI] [PubMed] [Google Scholar]
- 7.Knott C. D. 2005. Radioimmunoassay of estrone conjugates from urine dried on filter paper. Am. J. Primatol. 67, 121–135 10.1002/ajp.20173 (doi:10.1002/ajp.20173) [DOI] [PubMed] [Google Scholar]
- 8.White T. C. R. 1993. The inadequate environment: nitrogen and the abundance of animals. Berlin, Germany: Springer [Google Scholar]
- 9.Mattson W. J. 1980. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Syst. 11, 119–161 10.1146/annurev.es.11.110180.001003 (doi:10.1146/annurev.es.11.110180.001003) [DOI] [Google Scholar]
- 10.Ganzhorn J. U., et al. 2009. Possible fruit protein effects on primate communities in Madagascar and the Neotropics. PLoS ONE 4, e8253. 10.1371/journal.pone.0008253 (doi:10.1371/journal.pone.0008253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel E. R., Crowley B. E., Knott C. D., Blakely M. E., Larsen M. D., Dominy N. J. In press A noninvasive method for estimating nitrogen balance in free-ranging primates. Int. J. Primatol. (doi:10.1007/s10764-011-9543-6) [Google Scholar]
- 12.Martínez del Rio C., Wolf B. O. 2005. Mass-balance models for animal isotopic ecology. In Physiological and ecological adaptations to feeding in vertebrates (eds Starck J. M., Wang T.), pp. 141–174 Enfield, NH: Science Publishers [Google Scholar]
- 13.Lechtig A., Martorell R., Yarbrough C., Delgado H., Klein R. E. 1976. The urea/creatinine ratio: is it useful for field studies? J. Trop. Pediatr. 22, 121–128 [DOI] [PubMed] [Google Scholar]
- 14.Parker K. L., Barboza P. S., Stephenson T. R. 2005. Protein conservation in female caribou (Rangifer tarandus): effects of decreasing diet quality during winter. J. Mammal. 86, 610–622 10.1644/1545-1542(2005)86[610:PCIFCR]2.0.CO;2 (doi:10.1644/1545-1542(2005)86[610:PCIFCR]2.0.CO;2) [DOI] [Google Scholar]
- 15.Barboza P. S., Parker K. L. 2006. Body protein stores and isotopic indicators of N balance in female reindeer (Rangifer tarandus) during winter. Physiol. Biochem. Zool. 79, 628–644 10.1086/502811 (doi:10.1086/502811) [DOI] [PubMed] [Google Scholar]
- 16.Barboza P. S., Parker K. L. 2008. Allocating protein to reproduction in Arctic reindeer and caribou. Physiol. Biochem. Zool. 81, 835–855 10.1086/590414 (doi:10.1086/590414) [DOI] [PubMed] [Google Scholar]
- 17.Cannon C. H., Curran L. M., Marshall A. J., Leighton M. 2007. Long-term reproductive behaviour of woody plants across seven Bornean forest types in the Gunung Palung National Park (Indonesia): suprannual synchrony, temporal productivity and fruiting diversity. Ecol. Lett. 10, 956–969 10.1111/j.1461-0248.2007.01089.x (doi:10.1111/j.1461-0248.2007.01089.x) [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro J., Bates D., DebRoy S., Sarkar D. and the R Core Development Team 2011. nlme: linear and nonlinear mixed effects models. R package version 3, pp. 1–100 [Google Scholar]
- 19.Zar J. H. 1999. Biostatistical analysis, 4th edn Upper Saddle River, NJ: Prentice Hall [Google Scholar]
- 20.Marshall A. J., Boyko C. M., Feilen K. L., Boyko R. H., Leighton M. 2009. Defining fallback foods and assessing their importance in primate ecology and evolution. Am. J. Phys. Anthopol. 140, 603–614 10.1002/ajpa.21082 (doi:10.1002/ajpa.21082) [DOI] [PubMed] [Google Scholar]
- 21.Leonard W. R. 2000. Human nutritional evolution. In Human biology: an evolutionary and biocultural perspective (eds Stinson S., Bogin B., Huss-Ashmore R., O'Rourke D.), pp. 295–343 New York, NY: John Wiley [Google Scholar]
- 22.Rothman J. M., Chapman C. A., Pell A. N. 2008. Fiber-bound nitrogen in gorilla diets: implications for estimating dietary protein intake of primates. Am. J. Primatol. 70, 690–694 10.1002/ajp.20540 (doi:10.1002/ajp.20540) [DOI] [PubMed] [Google Scholar]
- 23.Rothman J. M., Raubenheimer D., Chapman C. A. In press Nutritional geometry: gorillas prioritize non-protein energy while consuming surplus protein. Biol. Lett. 7 10.1098/rsbl.2011.0321 (doi:10.1098/rsbl.2011.0321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogel E. R., van Woerden J. T., Lucas P. W., Atmoko S. S. U., van Schaik C. P., Dominy N. J. 2008. Functional ecology and evolution of hominoid molar enamel thickness: Pan troglodytes schweinfurthii and Pongo pygmaeus wurmbii. J. Hum. Evol. 55, 60–74 10.1016/j.jhevol.2007.12.005 (doi:10.1016/j.jhevol.2007.12.005) [DOI] [PubMed] [Google Scholar]
- 25.Narita Y., Oda S., Takensaka O., Kageyama T. 2010. Lineage-specific duplication and loss of pepsinogen genes in hominoid evolution. J. Mol. Evol. 70, 313–324 10.1007/s00239-010-9320-8 (doi:10.1007/s00239-010-9320-8) [DOI] [PubMed] [Google Scholar]
- 26.Locke D. P., et al. 2011. Comparative and demographic analysis of orang-utan genomes. Nature 469, 529–533 10.1038/nature09687 (doi:10.1038/nature09687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meijaard E., Sheil D. 2007. A logged forest in Borneo is better than none at all. Nature 446, 974. 10.1038/446974a (doi:10.1038/446974a) [DOI] [PubMed] [Google Scholar]
- 28.UNEP. 2007. The last stand of the orangutan: state of emergency: illegal logging, fire, and palm oil in Indonesia's national parks. (eds Nellemann C., Miles L., Kaltenborn B. P., Virtue M., Ahlenium H.), pp. 1–52 Cambridge, UK: UNEP [Google Scholar]