Abstract
The ability to recognize close relatives in order to cooperate or to avoid inbreeding is widespread across all taxa. One accepted mechanism for kin recognition in birds is associative learning of visual or acoustic cues. However, how could individuals ever learn to recognize unfamiliar kin? Here, we provide the first evidence for a novel mechanism of kin recognition in birds. Zebra finch (Taeniopygia guttata) fledglings are able to distinguish between kin and non-kin based on olfactory cues alone. Since olfactory cues are likely to be genetically based, this finding establishes a neglected mechanism of kin recognition in birds, particularly in songbirds, with potentially far-reaching consequences for both kin selection and inbreeding avoidance.
Keywords: avian olfaction, kin recognition, kin selection, mate choice, olfactory communication
1. Introduction
Distinguishing related from unrelated individuals is a crucial ability for animals, facilitating cooperation among relatives and avoidance of excessive kin competition or inbreeding [1–4]. Helping relatives and avoiding mating with them are two very different fitness-enhancing behaviours, which share an important feature: relatives must be somehow identified first. Recognition of kin can be based on (i) spatial distribution, (ii) familiarity or association, (iii) phenotype matching, and/or (iv) recognition alleles; however, these are not mutually exclusive mechanisms [4,5]. The first two mechanisms are relatively imprecise, as the assessment is not directly based on genetic relatedness, but is indirectly estimated from cues that are likely to be correlated with kinship [5]. The latter two mechanisms, in contrast, refer more directly to the underlying genetic similarity of kin and are independent of experience [5]. There is a large body of evidence for kin recognition in birds and especially songbirds [4,6–12]. Songbirds are primarily known for their visual and acoustic discrimination abilities, and most research on kin recognition cues has focused on songs or calls [7,10,11]. Acoustic patterns learned during the nestling phase from a parental template facilitate kin recognition based on such learned cues [11,13]. Associative learning can thereby explain how individuals recognize parents and siblings of the same brood. However, these mechanisms fail to explain how other, unfamiliar kin, which are not present during the association period, are recognized. For example, Petrie et al. [8] have shown that peacock (Pavo cristatus) males prefer to lek together with unfamiliar relatives and that this case of kin recognition is not based on social and environmental cues. Furthermore, during associative learning recognition errors can occur if traits from other non-related individuals are learned, which would lead to the acceptance of non-kin as kin.
The recognition of relatives, even if they are unfamiliar, in most other animals such as mammals, amphibians, fish and insects, is based on olfactory cues [14–16]. Most birds were long thought to be anosmic [17]. Hence, olfactory-based kin recognition and the possibility of olfactory phenotype matching have so far been largely neglected. Zebra finches, however, have been shown to possess large numbers of olfactory receptor gene-like sequences in their genome [18,19], and the actual use of olfaction for purposes of nest recognition has been recently discovered [20]. Furthermore, zebra finches are ideal model organisms to test for olfactory kin recognition in birds, since they are altricial, highly social and colonial songbirds [21]. Here, we asked whether olfactory cues might provide a mechanism for kin recognition [12]. In a foster experiment, we tested whether zebra finches can distinguish the nest of kin from that of non-kin, based on olfactory cues alone.
2. Material and methods
We fostered single zebra finch hatchlings between days 1 and 4 of life (on average, day 2 of life ± 1 day s.d.) into unrelated broods of a similar age (average brood size prior to fostering 2.5 ± 1.6 s.d. chicks; for details of the fostering protocol see the electronic supplementary material). We tested the foster chick and a randomly chosen unrelated nest-mate shortly after fledging (days 20–23 of life). In a simultaneous choice situation (see the electronic supplementary material), we presented the familiar nest odour in which both test individuals were raised, and the nest odour of the nest from which the foster chick came and which it had not experienced for 19–22 days. Individuals were tested for 10 min in total. To control for side preferences, we switched odour samples after 5 min [20]. The two stimuli differed in their genetic source and time of exposure to the two test individuals. For each pair of chicks, we used nest material from the nest they were raised in, and nest material from the nest the foster chick hatched in, as odour stimuli. Hence, fostered chicks had a choice between the odour of the nest used by unrelated, but familiar, individuals and the odour of the nest used by closely related individuals, which they had not experienced for the last 20 days. In contrast, non-fostered chicks had a choice between the odour of the nest used by closely related and familiar individuals, and the unfamiliar odour of a nest used by unrelated and unfamiliar individuals. Owing to our unidirectional fostering, the genetic purity of odour stimuli differed. The nest from which the fostered chick originated consisted of one genetic source only, whereas the other consisted of two genetic sources (non-fostered and fostered chicks). We analysed the time the individual spent in each preference zone as the proportion of time spent in the respective zone relative to the time the individual spent in both preference zones (see the electronic supplementary material). The preference strength was calculated as the net difference between the times the individual spent in each preference zone.
3. Results
In total, we performed experiments with 33 individuals, 17 fostered chicks and one of the unrelated nest-mates (non-fostered chicks) each. Since the non-fostered chicks of one brood died, we used 16 non-fostered chicks. Foster chicks significantly preferred the odour of the nest in which they hatched, over the nest odour in which they were raised (Wilcoxon; nfoster chicks = 17, Z =−2.22, p = 0.026; mean preference strength: 121 s ± 141 s.d.; figure 1). Furthermore, foster chicks and their unrelated nest-mates differed significantly in their preference for the nest odour of the nest in which they were raised (χ2-test; χ2 = 6.68; p = 0.009; figure 1).
Figure 1.
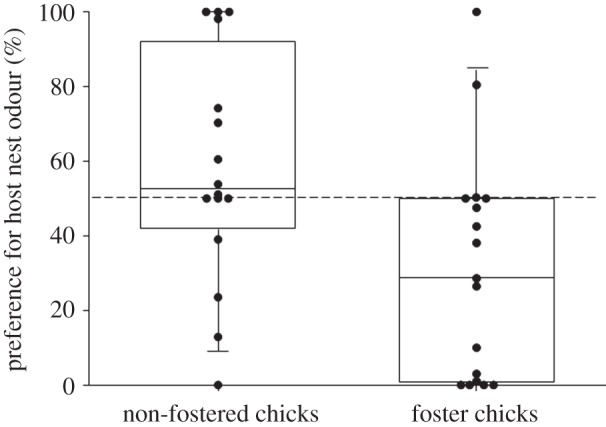
Evidence for olfactory kin recognition in a songbird. Proportion of time that foster (n = 17) and non-fostered chicks (n = 16) spent in the preference zone of the odour of the host nest odour. Foster chicks showed a significant preference for the odour of the nest they originated from, i.e. their genetic nest odour and the preferences from foster and non-fostered chicks were significantly different (central line in the boxes, median; spread of the box, 25 and 75% quartile; whiskers, entire data range; dots, individual data points).
In contrast to the foster chicks, non-fostered chicks did not show a clear preference for the home nest odour which provided a familiar and genetically related stimulus (Wilcoxon; nnon-fostered chicks = 16; Z = −1.05, p = 0.29, mean preference strength: 148 s ± 188 s.d.; figure 1). The preference of non-fostered chicks for the home nest odour was positively correlated with the number of full siblings in the nest (nnon-fostered chicks = 16, rS = 0.55, p = 0.029; figure 2). The number of full siblings is an indirect measure of relative nest odour purity, as the more full siblings are present in the nest, the lower the relative contamination from the non-related fostered chick, and thus the higher is the expected preference for the home nest odour.
Figure 2.
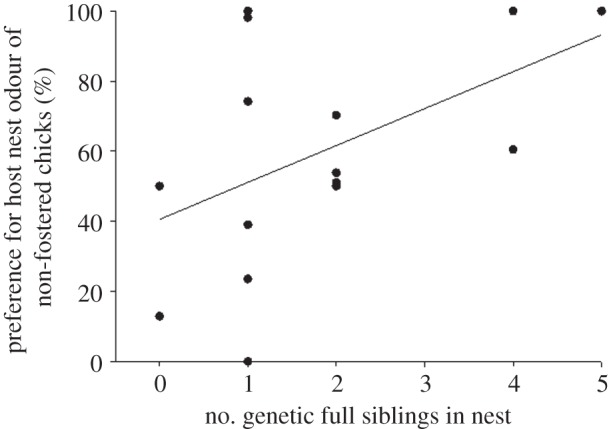
Genetic purity (number of genetic full siblings in the nest) affects odour preference from non-fostered chicks. Preference strength of the non-fostered chicks for the home nest odour, i.e. their familiar and genetically related nest odour is significantly correlated with the number of full-siblings in the nest (some dots overlap).
4. Discussion
Our results show that zebra finch fledglings are able to distinguish between kin and non-kin by using olfactory cues alone. Foster fledglings prefer the nest odour of close relatives, even though they have been fostered into a host nest shortly after hatching.
The preference of non-fostered chicks for their home nest odours is positively linked to the proportion of genetic relatives in the nest, i.e. the stimulus quality, indicating the ability to perceive kin labels from olfactory nest cues.
Since olfactory cues are likely to be genetically based [14–16,22], olfactory kin recognition bears the potential to be a novel mechanism for kin recognition in songbirds and possibly birds in general. A potential olfactory kin label has very recently been documented in petrels (Halobaena caerulea) [23], in which mice were used to demonstrate the similarity of odours from closely related petrel individuals, but no ecologically relevant evidence of actual usage and perception of these cues has been provided. One potential reason for the use of olfaction in zebra finches might be to detect extra-pair paternity or conspecific brood parasitism, but this selection pressure is likely to be negligible, as extra-pair paternity is relatively rare, as is the frequency of egg dumping [24,25]. The ability to distinguish closely related from non-related individuals is crucial, not only for parent–offspring recognition but also for the evolution of cooperative breeding and inbreeding avoidance [1,3]. The use of olfaction in mate recognition has been demonstrated in Antarctic prions (Pachyptila desolata) [26], and zebra finches, which are not explicitly cooperative breeders, might also use olfactory cues during mate choice decisions in terms of inbreeding avoidance [12]. Furthermore, if olfactory cues indeed facilitate kin recognition, this might well explain results that have so far escaped a plausible mechanistic explanation in other birds, as for example the finding that peacocks within a lek are more related than expected by chance [8].
The ability of zebra finches to recognize the nest odour of closely related individuals may result from learning the genetic nest odour within the first few hours after hatching as a type of olfactory familial imprinting [2], or it may be innate and distinguishing kin from non-kin may be based on self-referent phenotype matching [15]. If learning is the underlying mechanism, the acquisition of the nest odour might occur pre-hatching [27] or shortly after hatching [20,28], as is known from other bird species. Nevertheless, if the ability of zebra finches to distinguish the two nest odours results from olfactory imprinting, the sensitive phase during which the olfactory template is acquired is much earlier in life than for visual [21,29] and acoustic cues [13,21]. Thus, olfactory kin recognition decreases the possibility of recognition errors owing to learning kin cues from other than kin.
The finding that even songbirds, and possibly birds in general [18,30], might be able to rely on olfactory-based labels for kin recognition leads to the conclusion that the general mechanism of kin recognition might be based on phenotype matching, for example, by genetically based markers, such as the major histocompatibility complex [2].
Acknowledgements
We thank Fritz Trillmich and Nick Davies, as well as four anonymous reviewers for helpful comments. Funding for this research was provided to B.A.C. by the University of Bielefeld (young researchers' fund). O.K. was funded by the German Science Foundation (DFG) through a Heisenberg-Professorship (KR 2089/2-1).
The research was carried out according to the German laws for experimentation with animals.
References
- 1.Hamilton W. D. 1964. The genetical evolution of social behaviour. I. J. Theor. Biol. 7, 1–16 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 2.Penn D., Potts W. 1998. MHC-disassortative mating preferences reversed by cross-fostering. Proc. R. Soc. Lond. B 265, 1299–1306 10.1098/rspb.1998.0433 (doi:10.1098/rspb.1998.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldman B. 1988. The ecology of kin recognition. Annu. Rev. Ecol. Syst. 19, 543–571 10.1146/annurev.es.19.110188.002551 (doi:10.1146/annurev.es.19.110188.002551) [DOI] [Google Scholar]
- 4.Nakagawa S., Waas J. R. 2004. O sibling, where art thou?': a review of avian sibling recognition with respect to the mammalian literature. Biol. Rev. 79, 101–119 10.1017/S1464793103006249 (doi:10.1017/S1464793103006249) [DOI] [PubMed] [Google Scholar]
- 5.Blaustein A. R. 1983. Kin recognition mechanisms: phenotype matching or recognition alleles? Am. Nat. 121, 749–754 10.1086/284101 (doi:10.1086/284101) [DOI] [Google Scholar]
- 6.Bateson P. 1982. Preferences for cousins in Japanese quails. Nature 295, 236–237 10.1038/295236a0 (doi:10.1038/295236a0) [DOI] [Google Scholar]
- 7.Beecher M. D. 1988. Kin recognition in birds. Behav. Genet. 18, 465–482 10.1007/BF01065515 (doi:10.1007/BF01065515) [DOI] [PubMed] [Google Scholar]
- 8.Petrie M., Krupa A., Burke T. 1999. Peacocks lek with relatives even in the absence of social and environmental cues. Nature 401, 155–157 10.1038/43651 (doi:10.1038/43651) [DOI] [Google Scholar]
- 9.Komdeur J., Hatchwell B. J. 1999. Kin recognition: function and mechanism in avian societies. Trends Ecol. Evol. 14, 237–241 10.1016/S0169-5347(98)01573-0 (doi:10.1016/S0169-5347(98)01573-0) [DOI] [PubMed] [Google Scholar]
- 10.Halpin Z. T. 1991. Kin recognition cues of vertebrates In Kin recognition (ed. Hepper P. G.), pp. 220–258 Cambridge, UK: Cambridge University Press [Google Scholar]
- 11.Sharp S., McGowan A., Wood M. J., Hatchwell B. J. 2005. Learned kin recognition cues in a social bird. Nature 434, 1127–1130 10.1038/nature03522 (doi:10.1038/nature03522) [DOI] [PubMed] [Google Scholar]
- 12.Arct A., Rutkowska J., Martyka R., Drobniak S. M., Cichon M. 2010. Kin recognition and adjustment of reproductive effort in zebra finches. Biol. Lett. 6, 762–764 10.1098/rsbl.2010.0417 (doi:10.1098/rsbl.2010.0417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brainard M. S., Doupe A. J. 2002. What songbirds teach us about learning. Nature 417, 351–358 10.1038/417351a (doi:10.1038/417351a) [DOI] [PubMed] [Google Scholar]
- 14.Busquet N., Baudoin C. 2005. Odour similarities as a basis for discriminating degrees of kinship in rodents: evidence from Mus spicilegus. Anim. Behav. 70, 997–1002 10.1016/j.anbehav.2004.12.023 (doi:10.1016/j.anbehav.2004.12.023) [DOI] [Google Scholar]
- 15.Mateo J. M., Johnston R. E. 2000. Kin recognition and the ‘armpit effect’: evidence of self-referent phenotype matching. Proc. R. Soc. Lond. B 267, 695–700 10.1098/rspb.2000.1058 (doi:10.1098/rspb.2000.1058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurst J. L., Beynon R. J. 2010. Making progress in genetic kin recognition among vertebrates. J. Biol. 9, 13. 10.1186/jbiol221 (doi:10.1186/jbiol221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bang B. G., Cobb S. 1968. Size of olfactory bulb in 108 species of birds. Auk 85, 55–61 [Google Scholar]
- 18.Steiger S. S., Kuryshev V. Y., Stensmyr M. C., Kempenaers B., Mueller J. C. 2009. A comparison of reptilian and avian olfactory receptor gene repertoires: species-specific expansion of group gamma genes in birds. BMC Genomics 10, 446. 10.1186/1471-2164-10-446 (doi:10.1186/1471-2164-10-446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren W. C., et al. 2010. The genome of a songbird. Nature 464, 757–762 10.1038/nature08819 (doi:10.1038/nature08819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caspers B. A., Krause E. T. 2011. Odour-based natal nest recognition in the zebra finch (Taeniopygia guttata), a colony-breeding songbird. Biol. Lett. 7, 184–186 10.1098/rsbl.2010.0775 (doi:10.1098/rsbl.2010.0775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zann R. A. 1996. The zebra finch: a synthesis of field and laboratory studies. Oxford, UK: Oxford University Press [Google Scholar]
- 22.Singh P. B., Brown R. E., Roser B. 1987. MHC antigens in urine as olfactory recognition cues. Nature 327, 161–164 10.1038/327161a0 (doi:10.1038/327161a0) [DOI] [PubMed] [Google Scholar]
- 23.Celerier A., Bon C., Malapert A., Palmas P., Bonadonna F. 2011. Chemical kin label in seabirds. Biol. Lett. 7, 807–810 10.1098/rsbl.2011.0340 (doi:10.1098/rsbl.2011.0340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffith S. C., Holleley C. E., Mariette M. M., Pryke S. R., Svedin N. 2010. Low level of extrapair parentage in wild zebra finches. Anim. Behav. 79, 261–264 10.1016/j.anbehav.2009.11.031 (doi:10.1016/j.anbehav.2009.11.031) [DOI] [Google Scholar]
- 25.Schielzeth H., Bolund E. 2010. Patterns of conspecific brood parasitism in zebra finches. Anim. Behav. 79, 1329–1337 10.1016/j.anbehav.2010.03.006 (doi:10.1016/j.anbehav.2010.03.006) [DOI] [Google Scholar]
- 26.Bonadonna F., Nevitt G. A. 2004. Partner specific odor recognition in an Antarctic seabird. Science 306, 835. 10.1126/science.1103001 (doi:10.1126/science.1103001) [DOI] [PubMed] [Google Scholar]
- 27.Sneddon H., Hadden R., Hepper P. G. 1998. Chemosensory learning in the chicken embryo. Physiol. Behav. 64, 133–139 10.1016/S0031-9384(98)00037-7 (doi:10.1016/S0031-9384(98)00037-7) [DOI] [PubMed] [Google Scholar]
- 28.Cunningham G. B., Nevitt G. A. 2011. Evidence for olfactory learning in procellariiorm seabird chicks. J. Avian. Biol. 4, 85–88 10.1111/j.1600-048X.2010.05184.x (doi:10.1111/j.1600-048X.2010.05184.x) [DOI] [Google Scholar]
- 29.Witte K., Caspers B. 2006. Sexual imprinting on a novel blue ornament in zebra finches. Behaviour 143, 969–991 10.1163/156853906778623626 (doi:10.1163/156853906778623626) [DOI] [Google Scholar]
- 30.Steiger S. S., Fidler A. E., Valcu M., Kempenaers B. 2008. Avian olfactory receptor gene. repertoires: evidence for a well-developed sense of smell in birds? Proc. R. Soc. B 275, 2309–2317 10.1098/rspb.2008.0607 (doi:10.1098/rspb.2008.0607) [DOI] [PMC free article] [PubMed] [Google Scholar]


