Abstract
The hormone melatonin is known to play an important role in regulating many seasonal changes in physiology, morphology and behaviour. In birds, unlike in mammals, melatonin has thus far been thought to play little role in timing seasonal reproductive processes. This view is mainly derived from laboratory experiments on male birds. This study tests whether melatonin is capable of influencing the timing of clutch initiation in wild female songbirds. Free-living female great tits (Parus major) treated with melatonin-filled implants prior to the breeding season initiated their first clutch of the season significantly later than females carrying an empty implant. Melatonin treatment did not affect clutch size. Further, melatonin treatment did not delay the onset of daily activity in the wild nor adversely affect body mass in captivity compared with controls. These data suggest a previously unknown role for this hormone in regulating the timing of clutch initiation in the wild.
Keywords: reproductive timing, songbird, pineal
1. Introduction
The daily rhythm of circulating melatonin concentrations provides vertebrates with a reliable physiological cue of day and night: melatonin is secreted by the pineal gland at night, while it is suppressed by daylight. The duration of the nocturnal melatonin secretion changes seasonally, being extended during the long nights of winter and compressed during the short nights of summer. This seasonal change in the melatonin profile is involved in orchestrating seasonal changes in physiology, morphology and behaviour in many species. For example, in seasonally breeding mammals, melatonin is a potent regulator of reproductive physiology and behaviour [1].
In contrast to mammals, in birds melatonin has been thought to play little role in regulating primary seasonal reproductive processes [2–4]. However, melatonin has been found to influence other seasonal processes, including changes in brain nuclei associated with bird song and in immunity [5]. Sporadic and often conflicting evidence has left open the possibility that melatonin may be capable of modulating seasonal reproductive responses in birds. For example, in Japanese quail (Coturnix coturnix japonica)—housed under inhibitory short-day photoperiods—daily injections with antibody against melatonin just prior to darkness led to enhanced gonad growth compared with control-injected quail [6]. Interestingly, the opposite treatment, injection of melatonin four hours prior to the onset of darkness in quail transferred to stimulatory photoperiods failed to block gonadal recrudescence [7], whereas in castrated white leghorn roosters injection of melatonin reduced luteinizing hormone [8].
Previous investigations of melatonin's role in regulating seasonal reproduction in birds have focused on males, recording gonad growth or transient changes in hormone concentrations as responses [2–4]. Further, studies seeking to address whether melatonin plays a role in reproductive timing in birds have focused on captive birds, which may behave and respond differently than they would in the natural environment [9]. Therefore, although the above-mentioned findings suggest the possibility for melatonin to modulate seasonal reproductive responses in birds, a functional role of melatonin in regulating seasonal reproductive timing in the wild has remained unclear.
This study asks whether experimental manipulation of the melatonin signal affects clutch initiation date in free-living female great tits (Parus major).
2. Material and methods
Adult female great tits were captured between 16 March and 2 April 2010, and 15 March and 30 March 2011 outside Radolfzell, Germany (47°44′24″ N, 8°58′48″). These dates correspond to 9–26 days prior to the first egg laid by the earliest female included in this study. Upon capture, all females received a combination of a numbered metal band as well as two to three colour bands to allow future identification. Each female then received a 10 mm long silastic implant placed subcutaneously on the flank (see electronic supplementary material). Implants were either filled with melatonin or left empty. Implants of this type mask circadian rhythms of melatonin secretion, leading to increased day- and night-time melatonin concentrations in this (see electronic supplementary material) and other songbird species [10,11]. Finally, all birds also received a small (0.51 g or approx. 3% of their body weight) radio transmitter (BD-2N; Holohil Systems, Ltd., Ontario, Canada) attached on the back as previously described [12]. Females were then released at the site of capture. Using a stationary automated recording unit capable of recording changes in signal strength of the transmitters [13], we recorded the onset of daily activity, and activity levels following dawn (see electronic supplementary material).
Nesting activity was monitored regularly prior to and during egg-laying, with visits to all boxes at least every 1–3 days until clutches were complete. If more than one egg was present in the box at the time of inspection, then the estimate of one egg laid per day was used to establish the clutch initiation date [14]. A total of 14 control (eight in 2010; six in 2011) and 16 melatonin (six in 2010; 10 in 2011) implanted birds initiated nests.
(a). Statistical analysis
Treatment effects on lay date and clutch size were analysed using a generalized-linear model (SPSS Statistics v. 17.0), with treatment and year and their interaction included as fixed factors. To control for any potential effect of date of capture and implantation, implant date was included in the model as a covariate. All data are presented as mean ± 1 s.e.m.
3. Results
Females implanted with melatonin initiated their clutches significantly later in the season than controls (F1,26 = 11.59, p = 0.002; figure 1a). There was also a significant effect of year on the timing of clutch initiation, with the average lay date in both treatment groups occurring earlier in 2011 than in 2010 (F1,26 = 17.108, p < 0.001). However, there was no interaction between treatment and year (p > 0.1), and no effect of the date of implantation on lay date (p > 0.1). Clutch size did not differ between the two treatment groups, and there was no effect of year or implant date on clutch size (p > 0.1; figure 1b). Further, treatment had no effect on the onset of daily activity, or on the amount of activity compared with controls (electronic supplementary material).
Figure 1.
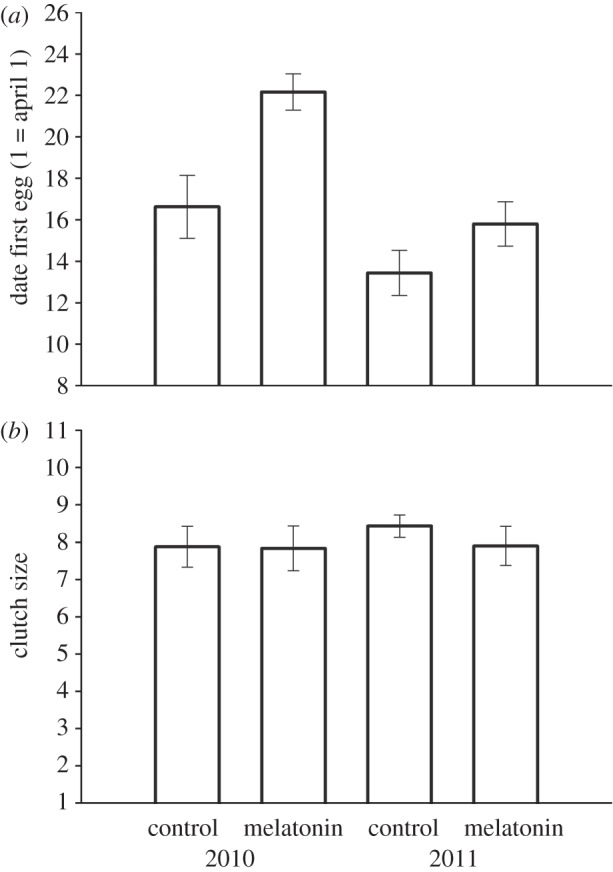
(a) Female great tits (Parus major) that received a melatonin implant initiated their first clutch of the season significantly later than control-implanted females. (b) Clutch size did not differ between treatments, indicating a specific effect of melatonin treatment on the timing of clutch initiation without inhibiting reproduction.
In 2011, a separate group of captive females was exposed to identical treatment as the wild birds to assess the physiological effects of melatonin treatment. Shortly before wild birds initiated clutches, melatonin-treated females displayed elevated day- and night-time melatonin levels compared with controls. Treatment had no adverse effect on body mass (electronic supplementary material).
4. Discussion
This study demonstrates that experimentally elevating circulating melatonin concentrations, masking a day–night rhythm of this hormone, leads to a significant delay in clutch initiation date in free-living female great tits. Importantly, melatonin treatment did not affect total number of eggs laid, onset of activity or morning activity levels. Further, melatonin manipulation had no effect on body mass in a set of captive females over the same time course (see electronic supplementary material), indicating a specific effect of melatonin on clutch initiation without generally inhibiting reproduction or impairing the birds' body condition.
An involvement of melatonin in reproductive timing in female birds would be in agreement with recent findings of interactions between melatonin and neuroendocrine mechanisms known to fine tune reproductive processes [15–17]. Neurons in the avian hypothalamus that produce gonadotropin-inhibitory hormone (GnIH)—a neuropeptide capable of downregulating activity of the neuroendocrine reproductive processes—are known to express melatonin receptors [18]. Manipulation of melatonin levels of hamsters and quail in the laboratory alters expression of GnIH in the hypothalamus [18,19] and melatonin stimulates the release of hypothalamic GnIH in vitro in quail [20].
Melatonin may also be capable of altering reproductive processes at the level of the gonad. Melatonin receptors have been identified in the gonads of birds [21–23]. In addition, GnIH peptide and receptor have recently been identified in avian gonads [24], and the addition of melatonin in vitro to testes from European starlings (Sturnus vulgaris) increases the expression of GnIH mRNA within the testes [21], suggesting the possibility for direct actions of melatonin on ovaries as well. In juvenile quail, pinealectomy transiently delayed the onset of oviposition [25]. However, melatonin injections had no effect in juveniles, and in adult quail neither pinealectomy nor melatonin injections altered gonadal status. This suggests the possibility of a sensitive period of the gonads to melatonin.
Disruption of circadian rhythmicity may provide an additional mechanism by which melatonin may delay clutch initiation date. Implants similar to those used in our experiment are known to dampen behavioural circadian rhythmicity in laboratory housed songbirds [4]. In this study, however, melatonin treatment did not alter the timing of daily activity onset (see electronic supplementary material); unlike the delay in activity onset observed in laboratory male songbirds when treated similarly with melatonin [26]. This observation renders it unlikely that the delay of clutch initiation was due to an effect of melatonin on the circadian system, although at present we cannot fully exclude this idea.
The potential mechanisms detailed above are not necessarily mutually exclusive. Future work will be needed to clarify how melatonin acts to alter the timing of clutch initiation.
This study suggests that melatonin can affect primary reproductive processes in a wild female songbird. Our findings of a potential role of melatonin in avian seasonal reproduction also highlight the need to study physiological processes in the natural setting, and in both sexes. Future studies aimed at understanding the neuroendocrine pathways targeted by melatonin, as well as the fitness consequences of an altered melatonin signal will greatly improve our understanding of the physiological mechanisms that underlie seasonal reproductive decisions and the selection pressures that have shaped these systems.
Acknowledgements
All experimental procedures follow NIH guidelines for the Care and Use of Experimental Animals and were approved by the animal ethics committee of the state of Baden-Württemberg.
The authors thank S. Austin, J. Lodde, M. Quetting, H. Schmid and M. van Toor for help in the field, M. Wikelski for help with telemetry and W. Goymann, I. Schwabl and M. Trappschuh for assistance with a pilot study on melatonin manipulation in this species. The melatonin assay was performed by the Animal Endocrinology Laboratory of the University of Ferrara (Italy), by V. Canoine and L. Fusani. K. Delhey, D. Dominoni, J. Partecke and A. Peters provided valuable discussion and feedback. The animal technical staff at the MPIO Radolfzell provided exceptional animal care. Research was funded by an NSF IRFP (no. 0852986) to T.J.G. and by the Max Planck Gesellschaft to M.H.
References
- 1.Goldman B. D. 2001. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhythms 16, 283–301 10.1177/074873001129001980 (doi:10.1177/074873001129001980) [DOI] [PubMed] [Google Scholar]
- 2.Dawson A., King V. M., Bentley G. E., Ball G. F. 2001. Photoperiodic control of seasonality in birds. J. Biol. Rhythms 16, 365–380 10.1177/074873001129002079 (doi:10.1177/074873001129002079) [DOI] [PubMed] [Google Scholar]
- 3.Sharp P. J. 2005. Photoperiodic regulation of seasonal breeding in birds. Ann. N.Y. Acad. Sci. 1040, 189–199 10.1196/annals.1327.024 (doi:10.1196/annals.1327.024) [DOI] [PubMed] [Google Scholar]
- 4.Gwinner E., Hau M. 2000. The pineal gland, circadian rhythms and photoperiodism. In Avian physiology (ed. Whittew G. C.), pp. 557–568, 5th edn New York, NY: Academic Press [Google Scholar]
- 5.Bentley G. E. 2001. Unraveling the enigma: the role of melatonin in seasonal processes in birds. Microsc. Res. Tech. 53, 63–71 10.1002/jemt.1069 (doi:10.1002/jemt.1069) [DOI] [PubMed] [Google Scholar]
- 6.Ohta M., Kadota C., Konishi H. 1989. A role of melatonin in the initial stage of photoperiodism in the Japanese quail. Biol. Reprod. 40, 935–941 10.1095/biolreprod40.5.935 (doi:10.1095/biolreprod40.5.935) [DOI] [PubMed] [Google Scholar]
- 7.Juss T. S., Meddle S. L., Servant R. S., King V. M. 1993. Melatonin and photoperiodic time measurement in Japanese quail (Coturnix coturnix japonica). Proc. R. Soc. Lond. B 254, 21–28 10.1098/rspb.1993.0121 (doi:10.1098/rspb.1993.0121) [DOI] [PubMed] [Google Scholar]
- 8.Rozenboim I., Aharony T., Yahav S. 2002. The effect of melatonin administration on circulating plasma luteinizing hormone concentration in castrated white leghorn roosters. Poult. Sci. 81, 1354–1359 [DOI] [PubMed] [Google Scholar]
- 9.Calisi R. M., Bentley G. E. 2009. Lab and field experiments: are they the same animal? Horm. Behav. 56, 1–10 10.1016/j.yhbeh.2009.02.010 (doi:10.1016/j.yhbeh.2009.02.010) [DOI] [PubMed] [Google Scholar]
- 10.Beldhuis H. J. A., Dittami J. P., Gwinner E. 1988. Melatonin and the circadian rhythms of feeding and perch-hopping in the European starling, Sturnus vulgaris. J. Comp. Physiol. A 164, 7–14 10.1007/BF00612712 (doi:10.1007/BF00612712) [DOI] [Google Scholar]
- 11.Hau M., Gwinner E. 1994. Melatonin facilitates synchronization of sparrow circadian-rhythms to light. J. Comp. Physiol. A 175, 343–347 10.1007/BF00192993 (doi:10.1007/BF00192993) [DOI] [Google Scholar]
- 12.Cochran W. W., Wikelski M. 2005. Individual migratory tactics of new world Catharus thrushes: current knowledge and future tracking options from space. In Birds of two worlds: the ecology and evolution of migration (eds Greenberg R., Marra P. P.), pp. 274–289 Baltimore, MD: The John's Hopkins University Press [Google Scholar]
- 13.Bisson I.-A., Butler L. K., Hayden T. J., Romero L. M., Wikelski M. C. 2009. No energetic cost of anthropogenic disturbance in a songbird. Proc. R. Soc. B 276, 961–969 10.1098/rspb.2008.1277 (doi:10.1098/rspb.2008.1277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrins C. 1965. Population fluctuations and clutch-size in the great tit, Parus major L. J. Anim. Ecol. 34, 601–647 10.2307/2453 (doi:10.2307/2453) [DOI] [Google Scholar]
- 15.Greives T. J., Kriegsfeld L. J., Bentley G. E., Tsutsui K., Demas G. E. 2008. Recent advances in reproductive neuroendocrinology: a role for RFamide peptides in seasonal reproduction? Proc. R. Soc. B 275, 1943–1951 10.1098/rspb.2008.0433 (doi:10.1098/rspb.2008.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bentley G. E., Kriegsfeld L. J., Ukena T., Tsutsui K., Wingfield J. C. 2006. Interactions of gonadotropin-releasing hormone (GnRH) and gonadotropin-inhibitory hormone (GnIH) in birds and mammals. J. Exp. Zool. A Ecol. Genet. Physiol. 305, 807–814 10.1002/jez.a.306 (doi:10.1002/jez.a.306) [DOI] [PubMed] [Google Scholar]
- 17.Kriegsfeld L. J. 2006. Driving reproduction: RFamide peptides behind the wheel. Horm. Behav. 50, 655–666 10.1016/j.yhbeh.2006.06.004 (doi:10.1016/j.yhbeh.2006.06.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ubuka T., Bentley G. E., Ukena K., Wingfield J. C., Tsutsui K. 2005. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc. Natl Acad. Sci. USA 102, 3052–3057 10.1073/pnas.0403840102 (doi:10.1073/pnas.0403840102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Revel F. G., Saboureau M., Pevet P., Simonneaux V., Mikkelsen J. D. 2008. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology 149, 902–912 10.1210/en.2007-0848 (doi:10.1210/en.2007-0848) [DOI] [PubMed] [Google Scholar]
- 20.Chowdhury V. S., Yamamoto K., Ubuka T., Bentley G. E., Hattori A., Tsutsui K. 2010. Melatonin stimulates the release of gonadotropin-inhibitory hormone by the avian hypothalamus. Endocrinology 151, 271–280 10.1210/en.2009-0908 (doi:10.1210/en.2009-0908) [DOI] [PubMed] [Google Scholar]
- 21.McGuire N. L., Kangas K., Bentley G. E. 2011. Effects of melatonin on peripheral reproductive function: regulation of testicular GnIH and testosterone. Endocrinology 152, 3461–3470 10.1210/en.2011-1053 (doi:10.1210/en.2011-1053) [DOI] [PubMed] [Google Scholar]
- 22.Ayre E. A., Pang S. F. 1994. 2-(125I) Iodomelatonin binding sites in the testis and ovary: putative melatonin receptors in the gonads. Neurosignals 3, 71–84 10.1159/000109528 (doi:10.1159/000109528) [DOI] [PubMed] [Google Scholar]
- 23.Sundaresan N. R., et al. 2009. Expression analysis of melatonin receptor subtypes in the ovary of domestic chicken. Vet. Res. Commun. 33, 49–56 10.1007/s11259-008-9071-9 (doi:10.1007/s11259-008-9071-9) [DOI] [PubMed] [Google Scholar]
- 24.McGuire N. L., Bentley G. E. 2010. A functional neuropeptide system in vertebrate gonads: gonadotropin-inhibitory hormone and its receptor in testes of field-caught house sparrow (Passer domesticus). Gen. Comp. Endocrinol. 166, 565–572 10.1016/j.ygcen.2010.01.010 (doi:10.1016/j.ygcen.2010.01.010) [DOI] [PubMed] [Google Scholar]
- 25.Sayler A., Wolfson A. 1968. Influence of the pineal gland on gonadal maturation in the Japanese quail. Endocrinology 83, 1237–1246 10.1210/endo-83-6-1237 (doi:10.1210/endo-83-6-1237) [DOI] [PubMed] [Google Scholar]
- 26.Hau M., Gwinner E. 1995. Continuous melatonin administration accelerates resynchronization following phase-shifts of a light–dark cycle. Physiol. Behav. 58, 89–95 10.1016/0031-9384(95)00002-Z (doi:10.1016/0031-9384(95)00002-Z) [DOI] [PubMed] [Google Scholar]


