Abstract
A poor start in life owing to a restricted diet can have readily detectable detrimental consequences for many adult life-history traits. However, some costs such as smaller adult body size are potentially eliminated when individuals modify their development. For example, male mosquitofish (Gambusia holbrooki) that have reduced early food intake undergo compensatory growth and delay maturation so that they eventually mature at the same size as males that develop normally. But do subtle effects of a poor start persist? Specifically, does a male's developmental history affect his subsequent attractiveness to females? Females prefer to associate with larger males but, controlling for body length, we show that females spent less time in association with males that underwent compensatory growth than with males that developed normally.
Keywords: condition-dependent, compensatory growth, mate choice, sexual selection, Gambusia
1. Introduction
Restricted access to food early in life can affect normal development and negatively influence many adult life-history traits (e.g. adult size, lifespan and fecundity) [1–3]. If feeding conditions later improve, however, then many species compensate for a slow start by accelerated, compensatory growth and/or delaying sexual maturation [4]. This can result in adults that are superficially identical despite having very different developmental histories. Developmental phenotypic plasticity is favoured if it allows individuals to ameliorate reproductive fitness losses incurred owing to an initial period of poor nutrition.
While these compensatory responses confer a net benefit, they might still impose specific costs [4]. Some costs are immediate and obvious. For example, delayed maturation increases the risk of mortality prior to breeding. Long-term costs of compensatory growth are more subtle. Compensatory growth has been shown to correlate negatively with key traits such as survival rates [5], adult longevity [6], reproductive output [7], locomotor performance [8] and social dominance [9]. A potentially widespread cost that has received relatively little attention is the effect of compensatory growth on the expression of sexually selected traits that influence mating and fertilization success. Only two published studies have compared the sexual attractiveness of males undergoing compensatory and normal growth. No effect of compensatory growth on female mate choice was detected in zebra finches, Taeniopygia guttata [10] or green swordtails, Xiphophorus helleri [11].
Here, we investigated the effect of an initial period of dietary restriction and subsequent compensatory growth on male sexual attractiveness in the mosquitofish, Gambusia holbrooki (Family: Poeciliidae). After a three-week period of highly restricted food intake, males returned to a normal diet displayed a compensatory response of both immediate accelerated growth and delayed sexual maturation. Consequently, males that were food limited during their early development attained the same average body size at maturity as males that were not food limited (Livingston et al. 2011, unpublished data). In G. holbrooki, females prefer to associate with larger males [12,13]. Given that males incessantly attempt to mate females, greater association time increases the probability that a male will gain paternity [14] (see electronic supplementary material). After controlling for adult size, we tested whether a poor start in life followed by compensatory growth imposes a ‘hidden’ cost on males by reducing their likely mating success.
2. Material and methods
The males we tested were a sub-sample of offspring from a large-scale full/half-sib study of compensatory growth in G. holbrooki (20 sires, 4 dams per sire). They were the F2 generation of feral fish caught in Canberra, Australia (March 2010). We individually raised 6–10 fry from each of 69 dams in 1 l tanks. All newborn fry were fed ad libitum for 7 days and then randomly assigned to either a control or treatment group (n = 324, 311, respectively). Control fry remained on an ad libitum diet, whereas treatment fry were placed on a severely restricted diet for 21 days then returned to ad libitum feeding (see electronic supplementary material). Fish were inspected every second day to determine age at maturity based on hardening and hook formation at the tip of the gonopodium (a modified anal fin used to transfer sperm). At maturity, we anaesthetised males in iced water, photographed them under a stereomicroscope and measured their standard length (snout to posterior of last vertebrae) using ImageJ software. We then re-measured all males just prior to using them in female association preference trials (mean: 64 days after maturation, range: 21–89).
We created 47 pairs of full-sibling brothers (one control and one treatment group). Each pair was tested with a different control virgin female. Females were housed in groups of approximately 25 in 120 l aquaria for approximately one month prior to choice trials. Trials were conducted in a test aquarium with three compartments (figure 1). One male was placed at each end of the aquarium and the female was placed in a transparent cylinder in the central compartment. After a 5 min acclimation period, the cylinder was lifted via a pulley. We then made focal samples every 10 s for 10 min to measure female association preferences by remotely photographing the trials with a Canon 450D camera. A female was defined as associating with a male if she faced him and was less than 4 cm from the front of his compartment. After the trial, males were returned to their individual tanks and the female placed individually in a 1 l tank overnight. The next day, we repeated the trial after swapping the males between end compartments to control for any side bias. Trials were conducted in April–May 2011. Paired males were brothers from the same brood and therefore have an identical date of birth.
Figure 1.
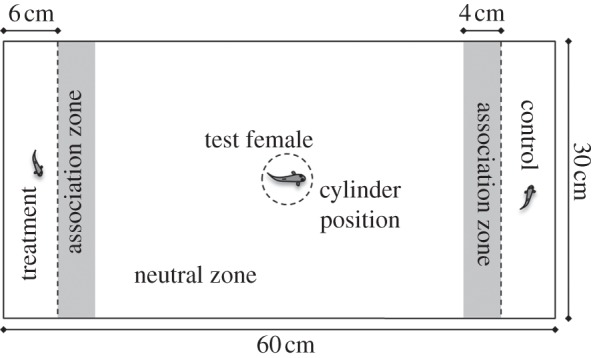
Trial aquarium set-up showing sealed compartments. Solid lines, opaque walls; dashed lines, transparent barriers.
We used a paired t-test to test for significant post-maturation male growth. We report repeatability for relative association time with the control male (focal samples with control/focal samples with either) and total time in association with males using the intraclass correlation coefficient [15]. Repeatability can be low owing to high variation within females and/or low variation among females. To test whether females preferentially associated with control or treatment males, we ran a logistic regression model using a logit link with the proportion of total association time spent with the control male as the dependent variable and the length difference between the control and treatment male as a predictor variable. The trials were summed and each data point was weighted by the total time the female spent associating with either male. To determine whether females preferred to associate with treatment or control males, we tested whether the intercept (i.e. when both males are the same size) differed significantly from 0.5. Likewise, to determine if females preferred larger males, we tested whether the coefficient for the effect of male size difference differed significantly from zero. p-Values were derived from Wald's tests (corrected with the overdispersion parameter set to residual deviance/degrees of freedom).
3. Results
Mean male body length did not increase significantly between sexual maturity and the measurement just prior to trials (22.45 ± 1.36 versus 22.48 ± 1.45 mm; t93 = 0.221, p = 0.826), so we used the second measurement in our analyses. The total time spent in association with males was significantly repeatable among females (rI = 0.406, F46,47 = 2.370, p < 0.01). Female preference based on male type was not repeatable (rI = 0.168, F46,47 = 1.403, p = 0.125), presumably owing to modest statistical power and higher variation within, rather than low variation among, females. For further analysis, the data from the two trials were summed for each female. The same general patterns emerge if we analyse the data separately for first and second trials.
There was a clear effect of relative male size on female association time. The larger the size difference between the males the more time the female spent with the larger male (Z = 2.032, p = 0.042; figure 2). In addition, however, there was an effect of male type because the predicted time with the control male was significantly more than 50 per cent when the control and treatment male were the same size (Z = 2.593, p = 0.0095).
Figure 2.
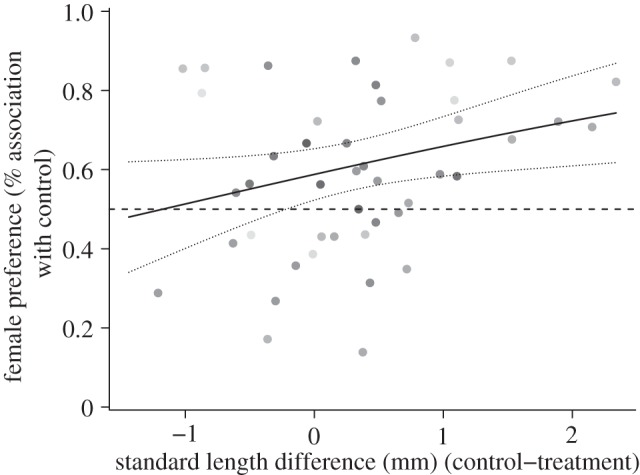
The relationship between the length difference between the control and treatment male and the proportion of time spent with the control male (n = 47). Dot shading is proportional to weighting (i.e. total association time). The solid line shows the fitted model and dotted lines are the 95% CI.
4. Discussion
In G. holbrooki, there is a hidden cost to a poor start in life. Males that are similar in body size, but differ in developmental history, are not equally attractive to females. Consistent with previous studies, we found that female G. holbrooki preferentially associate with larger males [12,13]. A greater propensity for females to associate with a male should increase his mating success because males incessantly attempt to mate nearby females. Statistically controlling for this body size effect, however, we found that females also preferred to associate with males that underwent normal, continuous growth over males that suffered a period of poor early growth and subsequent compensatory development (accelerated growth and delayed maturation). There was no detectable negative effect of an early period of low food followed by compensatory growth on male attractiveness in another poecilid fish X. helleri [11], although it did reduce male social dominance, which should also lower male mating success [16].
As with most studies, it is impossible to determine whether compensatory growth per se, or simply an early period of poor nutrition negatively affected male attractiveness to females. It is experimentally challenging to tease these two factors apart (i.e. to undergo compensatory growth, there must be something to compensate for—usually poor nutrition). This does not, however, negate the fact that male developmental history affected female preferences in G. holbrooki. It has been suggested that bird songs and, by extension, other sexually selected traits are honest indicators of male quality because they ‘capture’ the level of stress experienced during development [17,18]. Thus, a poor start in life could reduce male attractiveness independent of his subsequent growth trajectory. To date, however, this hypothesis has been tested only in birds, yielding mixed results. It was supported in starlings [17] but not zebra finches [19].
There are potential direct and indirect benefits to females rejecting males that had a poor start in life. For example, these males might be more susceptible to diseases and parasites that could infect females, or poor early nutrition might be associated with additive genetic variation in parental provisioning ability. It is currently unknown what cues female mosquitofish might use to assess male developmental history. One possibility is that females use relative gonopodium length as a cue. Early food limitation decreases gonopodium length relative to body size (Livingston et al. unpublished data), and female Gambusia spp. seem to prefer males with longer gonopodia [13,20]. Another possibility is that females were able to detect subtle differences in locomotor performance between control and treatment males. Locomotion is affected by developmental history in some poeciliids [16]. Whatever the cue, this study is one of the first to demonstrate that a male's attractiveness can be influenced by his developmental history.
Acknowledgements
This study was conducted under animal ethics permit FBTZ.26.08.
We thank James Davies for assistance, and the Australian Research Council and Australian National University for funding.
References
- 1.Mugabo M., Marquis O., Perret S., Le Galliard J. F. 2010. Immediate and delayed life history effects caused by food deprivation early in life in a short-lived lizard. J. Evol. Biol. 23, 1886–1898 10.1111/j.1420-9101.2010.02052.x (doi:10.1111/j.1420-9101.2010.02052.x) [DOI] [PubMed] [Google Scholar]
- 2.Rickard I. J., Holopainen J., Helama S., Helle S., Russel A. F., Lummaa V. 2010. Food availability at birth limited reproductive success in historical humans. Ecology 91, 3515–3525 10.1890/10-0019.1 (doi:10.1890/10-0019.1) [DOI] [PubMed] [Google Scholar]
- 3.Tschirren B., Rutstein A. N., Postma E., Mariette M., Griffith S. C. 2009. Short- and long-term consequences of early developmental conditions: a case study on wild and domesticated zebra finches. J. Evol. Biol. 22, 387–395 10.1111/j.1420-9101.2008.01656.x (doi:10.1111/j.1420-9101.2008.01656.x) [DOI] [PubMed] [Google Scholar]
- 4.Metcalfe N. B., Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260 10.1016/S0169-5347(01)02124-3 (doi:10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 5.Dmitriew C., Rowe L. 2007. Effects of early resource limitation and compensatory growth on lifetime fitness in the ladybird beetle (Harmonia axyridis). J. Evol. Biol. 20, 1298–1310 10.1111/j.1420-9101.2007.01349.x (doi:10.1111/j.1420-9101.2007.01349.x) [DOI] [PubMed] [Google Scholar]
- 6.Birkhead T. R., Fletcher F., Pellatt E. J. 1999. Nestling diet, secondary sexual traits and fitness in the zebra finch. Proc. R. Soc. Lond. B 266, 385–390 10.1098/rspb.1999.0649 (doi:10.1098/rspb.1999.0649) [DOI] [Google Scholar]
- 7.Auer S. K., Arendt J. D., Chandramouli R., Reznick D. N. 2010. Juvenile compensatory growth has negative consequences for reproduction in Trinidadian guppies (Poecilia reticulata). Ecol. Lett. 13, 998–1007 [DOI] [PubMed] [Google Scholar]
- 8.Billerbeck J. M., Lankford T. E., Conover D. O. 2001. Evolution of intrinsic growth and energy acquisition rates. I. Trade-offs with swimming performance in Menidia menidia. Evolution 55, 1863–1872 [DOI] [PubMed] [Google Scholar]
- 9.Royle N. J., Lindstrom J., Metcalfe N. B. 2005. A poor start in life negatively affects dominance status in adulthood independent of body size in green swordtails Xiphophorus helleri. Proc. R. Soc. B 272, 1917–1922 10.1098/rspb.2005.3190 (doi:10.1098/rspb.2005.3190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blount J. D., Metcalfe N. B., Arnold K. E., Surai P. F., Devevey G. L., Monaghan P. 2003. Neonatal nutrition, adult antioxidant defences and sexual attractiveness in the zebra finch. Proc. R. Soc. Lond. B 270, 1691–1696 10.1098/rspb.2003.2411 (doi:10.1098/rspb.2003.2411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walling C. A., Royle N. J., Metcalfe N. B., Lindstrom J. 2007. Early nutritional conditions, growth trajectories and mate choice: does compensatory growth lead to a reduction in sexual attractiveness? Behav. Ecol. Sociobiol. 7, 1007–1014 10.1007/s00265-006-0333-7 (doi:10.1007/s00265-006-0333-7) [DOI] [Google Scholar]
- 12.Bisazza A., Vaccari G., Pilastro A. 2001. Female mate choice in a mating system dominated by sexual coercion. Behav. Ecol. 12, 59–64 [Google Scholar]
- 13.Kahn A. T., Mautz B., Jennions M. D. 2010. Females prefer to associate with males with longer intromittent organs in mosquitofish. Biol. Lett. 6, 55–58 10.1098/rsbl.2009.0637 (doi:10.1098/rsbl.2009.0637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mautz B. S., Jennions M. D. 2011. The effect of competitor presence and relative competitive ability on male mate choice. Behav. Ecol. 22, 769–775 10.1093/beheco/arr048 (doi:10.1093/beheco/arr048) [DOI] [Google Scholar]
- 15.Lessells C. M., Boag P. T. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104, 116–122 [Google Scholar]
- 16.Royle N. J., Lindstrom J., Metcalfe N. B. 2006. Effect of growth compensation on subsequent physical fitness in green swordtails Xiphophorus helleri. Biol. Lett. 2, 39–42 10.1098/rsbl.2005.0414 (doi:10.1098/rsbl.2005.0414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchanan K. L., Spencer K. A., Goldsmith A. R., Catchpole C. K. 2003. Song as an honest signal of past developmental stress in the European starling (Sturnus vulgaris). Proc. R. Soc. Lond. B 270, 1149–1156 10.1098/rspb.2003.2330 (doi:10.1098/rspb.2003.2330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spencer K. A., MacDougall-Shackleton S. A. 2011. Indicators of development as sexually selected traits: the developmental stress hypothesis in context. Behav. Ecol. 22, 1–9 10.1093/beheco/arq068 (doi:10.1093/beheco/arq068) [DOI] [Google Scholar]
- 19.Bolund E., Schielzeth H., Forstmeier W. 2010. No heightened condition dependence of zebra finch ornaments—a quantitative genetic approach. J. Evol. Biol. 23, 586–597 10.1111/j.1420-9101.2009.01927.x (doi:10.1111/j.1420-9101.2009.01927.x) [DOI] [PubMed] [Google Scholar]
- 20.Langerhans R. B., Layman C. A., DeWitt T. J. 2005. Male genital size reflects a tradeoff between attracting mates and avoiding predators in two live-bearing fish species. Proc. Natl Acad. Sci. USA 102, 7618–7623 10.1073/pnas.0500935102 (doi:10.1073/pnas.0500935102) [DOI] [PMC free article] [PubMed] [Google Scholar]


