Abstract
Endosymbiotic bacteria of the genus Wolbachia induce diverse reproductive alterations in their insect hosts. Wolbachia (wSca) infecting the moth Ostrinia scapulalis causes unusual male killing, in which males (genotype: ZZ) selectively die during embryonic and larval development, whereas females (genotype: ZW), in turn, selectively die when cured of infection. To gain insight into the interaction between wSca and the host, we analysed phenotypic and genetic sexes of the embryos and larvae of normal, wSca-infected, and infected-and-cured O. scapulalis by diagnosing the sex-specifically spliced transcripts of Osdsx—a homologue of the sex-determining gene doublesex—and sex chromatin in interphase nuclei, respectively. It was observed that the female-type Osdsx was expressed in the infected male (ZZ) progenies destined to die, whereas the male-type Osdsx was expressed in the cured female (ZW) progenies destined to die. These findings suggest that (i) wSca, a male killer, carries a genetic factor that feminizes the male host, (ii) the sex-determining system of the host is degraded, and (iii) a mismatch between the genetic and phenotypic sexes underlies the sex-specific death.
Keywords: Ostrinia scapulalis, male killing, feminization, masculinization, doublesex, Wolbachia
1. Introduction
Wolbachia, a group of endosymbiotic bacteria harboured by a wide range of insects [1], is known for various manipulations of host reproduction to expedite their own propagation [2]. Among the manipulations is male killing, in which males selectively die during embryonic and larval development, giving rise to all-female progeny [3]. Despite the conspicuous effect of the infection, the molecular interactions between Wolbachia and its hosts that may mediate male-specific death have remained unexplored. Wolbachia (wSca) infecting the adzuki bean borer moth (Ostrinia scapulalis) causes male killing, however, the male killing in this Wolbachia–host system is unusual in that females, in turn, selectively died when wSca was eliminated by antibiotic treatment, giving rise to all-male progeny [4]. The sex dependence of death in this system suggests that wSca interferes with the sex-specific gene expression or physiology of the host. Indeed, the occurrence of sexual mosaic individuals with an exclusively male genotype upon incomplete elimination of wSca with antibiotics or heat treatment strongly suggests that wSca has the ability to feminize genetic males [4–6]. Until now, however, molecular interactions between the symbiont and host have not been investigated in this unique system.
In addition to Wolbachia, diverse bacteria from the genera such as Spiroplasma, Rickettsia and Arsenophonus are known to cause male-specific death in their hosts [3]. Although the mechanism of male killing has been studied in detail only in a few bacterium–host systems [7–9], these studies suggested that mechanisms of male killing might be diverse: dosage compensation is suggested to be involved in Spiroplasma-induced male killing in Drosophila [7], whereas Arsenophonus is reported to target maternally inherited centrosomes to kill males in Nasonia [9].
In the present study, to gain insight into the mechanism of male killing by Wolbachia, we focused on a gene working at the bottom of the sex-determination cascade, doublesex (dsx), which is transcribed into either a male or female isoform by sex-specific splicing and serves as a final regulator of sex-specific gene expression in somatic cells of insects [10,11]. We investigated the developmental changes in phenotypic and genetic sex ratios in the broods of normal, wSca-infected and infected-and-cured O. scapulalis by diagnosing the isoforms of a dsx homologue (female-specific OsdsxFL or male-specific OsdsxM [12]), and sex chromatin in interphase nuclei, respectively.
2. Material and methods
(a). Insects
Adult moths of O. scapulalis (Lepidoptera: Crambidae) were captured at Matsudo, Japan (35.8° N, 139.9° E) in the summer of 2008–2009. Females infected with Wolbachia, which produce all-female offspring, were screened by diagnostic polymerase chain reaction (PCR; see §2b), and maintained as matrilines through crosses with uninfected normal males [4]. Three Wolbachia-infected matrilines and three uninfected cultures of O. scapulalis were used for the present study. Insects were reared at 23 ± 1°C with a photoperiod of 16 L : 8 D. The larvae were reared on an artificial diet (Silkmate 2M, Nosan Corp.) [4].
(b). Diagnostic PCR
DNA was extracted from the ovaries of female moths using a DNeasy Tissue Kit (Qiagen), and PCR was performed using Ex Taq DNA polymerase (Takara Bio Inc.) and wsp gene-specific primers, wsp-81F and wsp-691R [13]. The primers used for amplification of the actin gene, as a positive control, were actin-F and actin-R [12].
(c). Tetracycline treatment
Wolbachia was eliminated from the infected matrilines by rearing larvae on an artificial diet containing tetracycline hydrochloride (0.06%, w/w) throughout the entire larval stage. Only female adults were obtained in the generation treated. The absence of Wolbachia in the females was confirmed by diagnostic PCR. These Wolbachia-eliminated females, when crossed with normal males, produce all-male progeny [4,12].
(d). Observation of the sex chromatin for genetic sexing
The genetic sex of individuals can be determined from the presence (female) or absence (male) of sex chromatin in interphase nuclei in the cells of Malpighian tubules or silk glands [4,12]. Malpighian tubules were dissected out from larvae or adults in sterile saline, and fixed with methanol : acetic acid (3 : 1) for approximately 1 min. The preparations were stained with lactic acetic orcein, and examined under a light microscope. In the case of embryos, embryos (pre-hatched larvae) were taken out from the eggs, and tissues including the Malpighian tubules and silk glands were isolated by pulling the abdominal tip with fine forceps. The remainder of the embryo was used for RNA extraction.
(e). Analysis of the type of Osdsx expressed in embryos
Total RNA was isolated from embryos, larvae and adults of Wolbachia-infected, infected-and-cured and uninfected O. scapulalis using RNAiso (Takara Bio Inc.), and treated with RNase-free DNase I (Qiagen). First-strand cDNA was synthesized from the total RNA using a PrimeScript first-strand cDNA Synthesis Kit (Takara Bio Inc.). PCR amplification was performed using KOD FX DNA polymerase (Toyobo) with the primers exon 1-F and exon 5-R under the following conditions: 98°C for 2 min, 30 cycles of 98°C for 15 s, 60°C for 10 s, 68°C for 60 s and a final extension at 72°C for 10 min [12]. The sizes of the PCR products with the above primers were 468 bp for OsdsxM and 725 bp for OsdsxFL.
3. Results
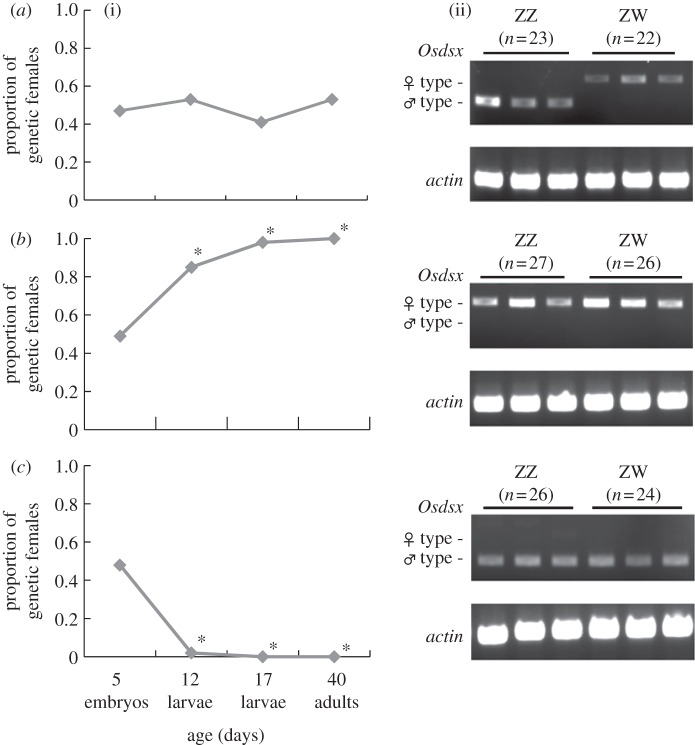
In an uninfected normal strain of O. scapulalis, the sex ratio of individuals did not deviate significantly from 1 : 1 (proportion of females = 0.5) throughout development (figure 1a), and the type of dsx homologue expressed was in accordance with the genetic sex, that is, the male-type OsdsxM was expressed in individuals with the ZZ genotype, and the female-type OsdsxFL was expressed in individuals with the ZW genotype (figure 1a). We confirmed that all genetic males in the wSca-infected strain eventually died during the larval stage (figure 1b), and conversely, all genetic females freed from infection died at the early larval stage (figure 1c). Diagnoses of the Osdsx isoforms in the same individuals showed that the female-type OsdsxFL was expressed in all individuals infected with wSca irrespective of genetic sex (ZZ and ZW; figure 1b), indicating that wSca feminized genetic males (ZZ). By contrast, the male-type OsdsxM was expressed in all individuals freed from infection irrespective of the genetic sex (figure 1c), indicating that elimination of wSca brought about the masculinization of genetic females (ZW). Inviability of embryos/larvae was observed when the genetic sex and phenotypic sex differed (table 1).
Figure 1.
Changes in the genetic sex ratio associated with the development of Ostrinia scapulalis ((i) n = 23–92), and the expression of male and female-type Osdsx transcripts in 5-day-old embryos ((ii) only examples are shown). Age refers to days after oviposition. Genetic sex was determined by the presence/absence of sex chromatin in the cells [4]. The embryos (pre-hatched larvae) were checked for sex chromatin and the type of Osdsx (OsdsxM or OsdsxFL, see the text). The actin gene was used as a reference. ZZ, male genotype; ZW, female genotype. Asterisks represent significantly different from 0.5 by Fisher's exact test (p < 0.01). Wolbachia: (a) uninfected; (b) infected and (c) eliminated.
Table 1.
Viability of progenies produced by normal (uninfected), Wolbachia-infected and infected-and-cured Ostrinia scapulalis female moths with reference to the genotypic and phenotypic sexes of progenies. (W* indicates a W chromosome suggested to have a dysfunctional female-determining factor.)
| infection state of mother | Wolbachiaa | genotypeb | sexual phenotypec | viability |
|---|---|---|---|---|
| uninfected | − | ZW | female | viable |
| ZZ | male | viable | ||
| infected | + | ZW* | female | viable |
| ZZ | female | inviable | ||
| curedd | − | ZW* | male | inviable |
| ZZ | male | viable |
aMinus and plus indicate absence and presence of Wolbachia in progenies, respectively.
bThe genotype of an individual was determined by the presence/absence of sex chromatin in interphase nuclei, which is a condensed heterochromatin formed from the W chromosome.
cThe sexual phenotype of an individual was determined by the sex-specific isoforms of Osdsx, a homologue of the sex-determining gene doublesex (see the text for more details).
dCured of Wolbachia infection by tetracycline treatment.
4. Discussion
In the silkmoth (Bombyx mori), the presence of a single W chromosome ensures female development, whereas in its absence male development takes place [14]. Thus, the W chromosome is believed to carry an epistatic female-determining factor (referred to hereafter as the F factor), although its molecular nature as well as the transcriptional cascade leading to the female-specific splicing of dsx is largely unknown (reviewed in earlier studies [11,15,16]).
The expression of the female-type OsdsxFL in genetic males (ZZ) of the wSca-infected O. scapulalis clearly indicates that wSca, a male killer, carries a feminizing factor that interferes with upstream sex-determination processes, or possibly the sex-specific splicing of Osdsx itself. Meanwhile, the masculinization of genetic females (ZW) freed from infection indicates that a factor in the female-determining cascade is degraded in the wSca-infected strains. Here, it should be noted that wSca-infected strains, which comprise females only, have been maintained by crossing with males from normal strains. Given that the degraded factor has been stably transmitted from mother to daughter, it is most likely to be located on the recombinationally isolated W chromosome, and it is possible that the F factor itself is degraded.
Although discordance of the genetic and phenotypic sexes is suggested to underlie the sex-specific death (table 1), the mechanism of death is unresolved. In the male killing, caused by wSca, (i) the growth of embryo/larvae destined to die was generally retarded, (ii) no discernible abnormalities were found in their morphology, and (iii) the occurrence of death was irregular. Therefore, the mechanism of death is not likely to be linked to a specific developmental process or event. One plausible explanation for growth retardation and eventual death may be discordance in dosage compensation, i.e. sex-dependent adjustment of the expression levels of genes on the sex chromosomes. Indeed, involvement of dosage compensation is suggested in the Spiroplasma-induced male killing in Drosophila [7]. In moths, however, the presence of dosage compensation is itself currently under discussion [17,18]. Studies on the expression levels of Z-linked genes in wSca-infected and infected-and-cured individuals may shed light on the dosage compensation in moths.
Our recent identification of sex-specific isoforms of Osdsx [12] paved the way for the simultaneous analysis of genetic and phenotypic sexes. It was hitherto difficult to determine the phenotypic sex of embryos and young larvae, because they do not show any sexual difference in morphology. Discordance between the genetic and phenotypic sexes in embryos/larvae destined to die was uncovered for the first time, to our knowledge, by this simultaneous analysis.
In addition to male killing, reproductive alterations performed by Wolbachia include, among others, feminization [2]. It is intriguing that a feminizing effect underlies the male killing, because feminized individuals are viable when produced by a ‘true’ feminizer in the butterfly, Eurema hecabe [19]. Comparison of the male killer in O. scapulalis and the feminizer in E. hecabe might shed light on how Wolbachia developed their repertoire of reproductive manipulations.
Acknowledgements
We thank Drs T. Matsuo, S. Hoshizaki, D. Kageyama, T. Kayukawa, E. Sunamura, H. Sakamoto and T. Fujii for advice.
References
- 1.Hilgenboecker K., Hammerstein P., Schlattmann P., Telschow A., Werren J. H. 2008. How many species are infected with Wolbachia? A statistical analysis of current data. FEMS Microbiol. Lett. 281, 215–220 10.1111/j.1574-6968.2008.01110.x (doi:10.1111/j.1574-6968.2008.01110.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werren J. H., Baldo L., Clark M. E. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6, 741–751 10.1038/nrmicro1969 (doi:10.1038/nrmicro1969) [DOI] [PubMed] [Google Scholar]
- 3.Hurst G. D. D., Jiggins F. M. 2000. Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg. Infect. Dis. 6, 329–336 10.3201/eid0604.000402 (doi:10.3201/eid0604.000402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kageyama D., Traut W. 2004. Opposite sex-specific effects of Wolbachia and inference with the sex determination of its host Ostrinia scapulalis. Proc. R. Soc. Lond. B 271, 251–258 10.1098/rspb.2003.2604 (doi:10.1098/rspb.2003.2604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kageyama D., Ohno S., Hoshizaki S., Ishikawa Y. 2003. Sexual mosaics induced by tetracycline treatment in the Wolbachia-infected adzuki bean borer, Ostrinia scapulalis. Genome 46, 983–989 10.1139/g03-082 (doi:10.1139/g03-082) [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto H., Kageyama D., Hoshizaki S., Ishikawa Y. 2008. Heat treatment of the Adzuki bean borer, Ostrinia scapulalis infected with Wolbachia gives rise to sexually mosaic offspring. J. Insect Sci. 8, 1–5 10.1673/031.008.6701 (doi:10.1673/031.008.6701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veneti Z., Bentley J. K., Koana T., Braig H. R., Hurst G. D. D. 2005. A functional dosage compensation complex required for male killing in Drosophila. Science 307, 1461–1463 10.1126/science.1107182 (doi:10.1126/science.1107182) [DOI] [PubMed] [Google Scholar]
- 8.Bentley J. K., Veneti Z., Heraty J., Hurst G. D. D. 2007. The pathology of embryo death caused by the male-killing Spiroplasma bacterium in Drosophila nebulosa. BMC Biol. 5, 9. 10.1186/1741-7007-5-9 (doi:10.1186/1741-7007-5-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferree P. M., Avery A., Azpurua J., Wilkes T., Werren J. H. 2008. A bacterium targets maternally inherited centrosomes to kill males in Nasonia. Curr. Biol. 18, 1409–1414 10.1016/j.cub.2008.07.093 (doi:10.1016/j.cub.2008.07.093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshijima K., Inoue K., Higuchi I., Sakamoto H., Shimura Y. 1991. Control of doublesex alternative splicing by transformer and transformer-2 in Drosophila. Science 252, 833–836 10.1126/science.1902987 (doi:10.1126/science.1902987) [DOI] [PubMed] [Google Scholar]
- 11.Shukla J. N., Nagaraju J. 2010. Doublesex: a conserved downstream gene controlled by diverse upstream regulators. J. Genet. 89, 341–356 10.1007/s12041-010-0046-6 (doi:10.1007/s12041-010-0046-6) [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto T. N., Fujii T., Kayukawa T., Sakamoto H., Ishikawa Y. 2010. Expression of a doublesex homologue is altered in sexual mosaics of Ostrinia scapulalis moths infected with Wolbachia. Insect Biochem. Mol. Biol. 40, 847–854 10.1016/j.ibmb.2010.08.004 (doi:10.1016/j.ibmb.2010.08.004) [DOI] [PubMed] [Google Scholar]
- 13.Zhou W., Rousset F., O'Neill S. 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B 265, 509–515 10.1098/rspb.1998.0324 (doi:10.1098/rspb.1998.0324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tazima Y. 1964. The genetics of the silkworm. London, UK: Academic Press [Google Scholar]
- 15.Traut W., Sahara K., Marec F. 2007. Sex chromosomes and sex determination in Lepidoptera. Sex. Dev. 1, 332–346 10.1159/000111765 (doi:10.1159/000111765) [DOI] [PubMed] [Google Scholar]
- 16.Fujii T., Shimada T. 2007. Sex determination in the silkworm, Bombyx mori: a female determinant on the W chromosome and the sex-determining gene cascade. Semin. Cell Dev. Biol. 18, 379–388 10.1016/j.semcdb.2007.02.008 (doi:10.1016/j.semcdb.2007.02.008) [DOI] [PubMed] [Google Scholar]
- 17.Suzuki M. G., Shimada T., Kobayashi M. 1998. Absence of dosage compensation at the transcription level of a sex-linked gene in a female heterogametic insect, Bombyx mori. Heredity 81, 275–283 10.1046/j.1365-2540.1998.00356.x (doi:10.1046/j.1365-2540.1998.00356.x) [DOI] [PubMed] [Google Scholar]
- 18.Walters J. R., Hardcastle T. J. 2011. Getting a full dose? Reconsidering sex chromosome dosage compensation in the silkworm, Bombyx mori. Genome Biol. Evol. 3, 491–504 10.1093/gbe/evr036 (doi:10.1093/gbe/evr036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiroki M., Kato Y., Kamito T., Miura K. 2002. Feminization of genetic males by a symbiotic bacterium in a butterfly, Eurema hecabe (Lepidoptera: Pieridae). Naturwissenschaften 89, 167–170 10.1007/s00114-002-0303-5 (doi:10.1007/s00114-002-0303-5) [DOI] [PubMed] [Google Scholar]