Abstract
In many species of animals, individuals advertise their quality with sexual signals to obtain mates. Chemical signals such as volatile pheromones are species specific, and their primary purpose is to influence mate choice by carrying information about the phenotypic and genetic quality of the sender. The deleterious effects of consanguineous mating on individual quality are generally known, whereas the effect of inbreeding on sexual signalling is poorly understood. Here, we tested whether inbreeding reduces the attractiveness of sexual signalling in the mealworm beetle, Tenebrio molitor, by testing the preferences for odours of inbred and outbred (control) individuals of the opposite sex. Females were more attracted to the odours produced by outbred males than the odours produced by inbred males, suggesting that inbreeding reduces the attractiveness of male sexual signalling. However, we did not find any difference between the attractiveness of inbred and outbred female odours, which may indicate that the quality of females is either irrelevant for T. molitor males or quality is not revealed through female odours.
Keywords: preference, pheromones, mate choice, heterozygosity, sexual selection, good-genes
1. Introduction
Females of many species prefer more ornamented males as mates and it is generally considered that choosy females may benefit by having ‘good genes’ for their offspring [1]. According to Hamilton & Zuk [2], mate selection is based on the preference of exaggerated secondary sexual ornaments that are maintained by sexual selection for higher genetic resistance against parasites. Also, heterozygosity may be considered as an indirect genetic benefit or ‘good genes’ [3] because mating with close relatives increases homozygosity. This may increase the probability of expression of recessive disadvantageous alleles resulting in impaired fitness [4]. Inbreeding impairs male courtship behaviour, mating success and the expression of secondary sexual traits [5]. Recent studies suggest that pheromones might function as sexual signals and honest indicators of male quality [6], but to our knowledge, studies testing whether odours transmit information about the genetic quality of a prospective mate are lacking. Especially, male mate choice has received less interest in empirical studies when compared with female choice. Females seem to be more attracted to high quality males, whereas males are found to prefer traits that improve their fertilization prospects [7].
To test whether inbreeding has an effect on sexual signalling, we used the mealworm beetle, Tenebrio molitor (Coleoptera: Tenebrionidae) as a model organism. In T. molitor, sexes are visually monomorphic, but they produce distinct pheromones [8,9]. Attractiveness of the male odours in T. molitor is positively associated with the strength of the immune system [6,10] and body condition [11]. When infected heavily by the tapeworm (Hymenolepis diminuta), T. molitor males produce less attractive pheromones than uninfected males [12]. Because recent studies on T. molitor suggest that attractiveness is closely related to the immune system [6,10], and inbreeding corrupts immunity [13], one could expect that inbreeding might have an effect on the attractiveness of sexual signalling. The aim of this study is to test whether inbreeding reduces the attractiveness of sexual signalling by affecting odours in T. molitor males and females.
2. Material and methods
(a). Insects
The beetles used in the experiment were taken from a laboratory stock population originating from a massive outbreak colony from a commercial stock of Imazon, Sweden. Stocks were kept in a large plastic box containing wheat bran and apple and maintained at a constant temperature of 28 ± 1°C, on a 12 L : 12 D photoperiod at the University of Turku, Finland. Individuals for the experiment were collected from the stocks as pupae and were sorted by sex to ensure virginity (grandparental generation). After hatching, the individuals were placed separately in plastic jars and were fed with fresh apple ad libitum. From these adult male and female beetles, pairs were set up and their offspring (parental generation) were raised up in full-sib families in small plastic boxes (0.5 l) and fed with wheat bran and fresh apple ad libitum.
(b). Families
After the eclosion of parental generation (pupae treated as described above), inbred families were created by mating brothers with sisters, and outbred families by mating randomly chosen unrelated individuals (altogether producing 15 inbred families and 13 outbred families). This method enables an equal representation of alleles within each group, only their combinations changing [14]. From each family, we randomly chose 40 small larvae for the experiment that were split into four (0.25 l) cages.
To ensure virginity, adult beetles were removed from their families daily and kept individually in plastic jars and were fed with fresh apple ad libitum. They were included in the experiment for 9–14 days after eclosion. Prior to the experiment, the fresh body mass of each beetle was measured to the nearest 0.1 mg, so that pairs used in the preference trials (see below) could be matched by body weight.
(c). Odour collection and preference tests
First, pairs of outbred and inbred beetles, matched by body weight, were obtained. Odours were collected by placing each beetle in a Petri dish (diameter: 37 mm) containing a new filter paper disc (diameter: 35 mm) for 48 h [15], with no access to food. For the preference test, the odour disc pairs collected were presented to the opposite sex (details given below). The discs for the preference tests were prepared simultaneously for each pair.
The arena for preference trials consisted of a 20 cm diameter glass dish inverted over a new filter paper on which the disc pair was placed. Preceding the trial, a virgin beetle was placed under a small (diameter: 37 mm) Petri dish in the centre of the circular arena to calm down for 3 min. At the start of the trial, the dish that restricted the beetle was removed and a glass dish was placed over the entire arena. Each trial lasted for 10 min, during which the beetle's movements were recorded. Trials were conducted under a red light. Preference was measured as a total time that the beetle spent on each filter paper disc [6]. We also counted visits per each disc.
PASW Statistics v. 18 (for Windows) was used for analysis. The data were not normally distributed and were transformed. A Student's t-test for paired samples was used to compare the pairs.
3. Results
Females spent more time on the discs collected from outbred males than on the discs from inbred males (t = 2.31; d.f. = 52; p = 0.025; figure 1). Furthermore, there was a tendency that females visited the discs obtained from outbred males more often than the discs from inbred males (t = 1.87; d.f. = 52; p = 0.067; figure 2).
Figure 1.
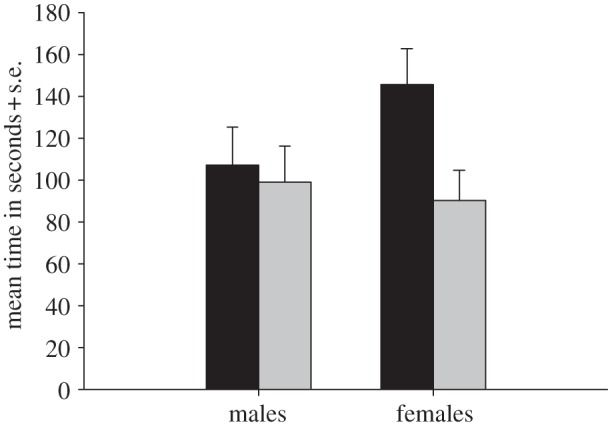
The average time that males (n = 45; outbred: mean = 107.3, s.d. = 121.6; inbred: mean = 99.3, s.d. = 115.6) and females (n = 53; outbred: mean = 145.9, s.d. = 125.5; inbred: mean = 90.5, s.d. = 103.3) spent on odour discs of the opposite sex (grey bar, inbred; black bar, outbred).
Figure 2.
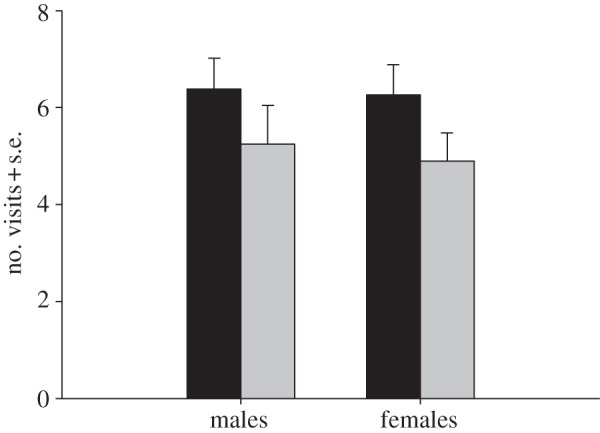
The number of visits that males (n = 41; outbred: mean = 6.4, s.d. = 4.3; inbred: mean = 5.3, s.d. = 5.2) and females (n = 53; outbred: mean = 6.3, s.d. = 4.5; inbred: mean = 4.9, s.d. = 4.2) made on odour discs of the opposite sex (grey bar, inbred; black bar, outbred).
In contrast to the result for females, there were no differences between the time that males spent on the discs collected from outbred females or the discs from inbred females (t = 0.48; d.f. = 45; p = 0.636; figure 1). Likewise, there were no differences between the number of visits that males made on the discs of outbred females and on the discs of inbred females (t = 0.902; d.f. = 40; p = 0.373; figure 2).
4. Discussion
We found that females were more attracted to the odours of outbred males than those of inbred males. Our results are consistent with the study of Ilmonen et al. [16], which showed that house mice (Mus musculus musculus) females prefer the odours of outbred males. More generally, studies on birds, mammals and fish have revealed that inbreeding severely reduces sexual signalling [5]. In contrast to vertebrates, experimental tests of the effect of inbreeding and heterozygosity on sexual signalling in insects are scarce. However, Drayton et al. [17] found that inbreeding reduced the calling effort of the cricket, Teleogryllus commodus, affecting the mating success of inbred males negatively. Similarly, inbreeding in Drosophila montana decreased the frequency of courtship song frequency associated with male's courtship success [18]. Thus, this is the first study showing that inbreeding has a negative effect on odour communication in insects. Volatile male-specific compounds that attract females have previously been identified to be a single compound [9] so, rather than changing the quality of pheromones, inbreeding might cause production of a lower quantity of pheromones as a result of exposure to recessive mutations that interfere with their production or reduced male condition [11].
Only high quality individuals in good condition are thought to be able to withstand the costs associated with the production of elaborated ornaments and maintenance of an effective immune system preferred by females [19]. Previously, in T. molitor starvation was found to reduce the attractiveness of male pheromones, indicating that pheromonal signalling might be condition related [11]. Although theoretical models suggest that mating with heterozygous males may provide no genetic benefits for females [20], it still may offer direct benefits. In the study of Rantala et al. [13], no effect on the encapsulation response was found after one generation of inbreeding, while it reduced resistance against entomopathogenic fungi [13,21], suggesting that mating with inbred individuals might increase the risk of contagion.
Studies concerning the evolution of sexual ornaments and mate choice have generally emphasized mate choice by females, whereas male choice has been relatively neglected, especially in insects [7]. In T. molitor, males have been found to prefer the pheromones of virgin females to non-virgin, and odours of mature females to odours of non-mature females [22]. In our study, inbreeding reduced the attractiveness of odours in males, but there was no similar effect on females. This suggests that the genetic quality of their mates may not be as important for males as it is for females. When choosing mates, males may be selected to be more interested in number and fecundity of their sexual partners, whereas females choose with respect to the phenotypic and genetic quality of their mates. On the other hand, it may be possible that the female odours do not signal the individual's quality or males do not rely on quality at all, which should be tested in the future studies.
To conclude, although previous studies suggest that the sexual signalling is not always honest in T. molitor [23], our results suggest that male odours reveal the quality of males to the choosy females. In contrast, T. molitor males are not able to discriminate between odours of outbred and inbred females or quality is not revealed through female pheromones at all. Thus, odours may provide different kinds of information to different sexes in T. molitor.
Acknowledgements
This study was supported by the Turku University Foundation to M.P. and by the Academy of Finland to M.J.R.
References
- 1.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Hamilton W. D., Zuk M. 1982. Heritable true fitness and bright birds: a role for parasites. Science 218, 384–387 10.1126/science.7123238 (doi:10.1126/science.7123238) [DOI] [PubMed] [Google Scholar]
- 3.Brown J. L. 1997. A theory of mate choice based on heterozygosity. Behav. Ecol. 8, 60–65 [Google Scholar]
- 4.Charlesworth D., Charlesworth B. 1987. Inbreeding depression and its evolutionary consequences. Ann. Rev. Ecol. Syst. 18, 237–268 [Google Scholar]
- 5.Kempenaers B. 2007. Mate choice and genetic quality: a review of the heterozygosity theory. Adv. Stud. Behav. 37, 189–278 10.1016/S0065-3454(07)37005-8 (doi:10.1016/S0065-3454(07)37005-8) [DOI] [Google Scholar]
- 6.Rantala M. J., Jokinen I., Kortet R., Vainikka A., Suhonen J. 2002. Do pheromones reveal male immunocompetence? Proc. R. Soc. Lond. B 269, 1681–1685 10.1098/rspb.2002.2056 (doi:10.1098/rspb.2002.2056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonduriansky R. 2001. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. 76, 305–339 10.1017/S1464793101005693 (doi:10.1017/S1464793101005693) [DOI] [PubMed] [Google Scholar]
- 8.Tanaka Y., Honda H., Ohsawa K., Yamamoto I. 1986. A sex attractant of the yellow mealworm, Tenebrio molitor L, and its role in the mating behavior. J. Pestic. Sci. 11, 49–55 [Google Scholar]
- 9.Bryning G. P., Chambers J., Wakefield M. E. 2005. Identification of a sex pheromone from male yellow mealworm beetles, Tenebrio molitor. J. Chem. Ecol. 31, 2721–2730 [DOI] [PubMed] [Google Scholar]
- 10.Rantala M. J., Vainikka A., Kortet R. 2003. The role of juvenile hormone in immune function and pheromone production trade-offs: a test of the immunocompetence handicap principle. Proc. R. Soc. Lond. B 270, 2257–2261 10.1098/rspb.2003.2472 (doi:10.1098/rspb.2003.2472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rantala M., Kortet R., Kotiaho J., Vainikka A., Suhonen J. 2003. Condition dependence of pheromones and immune function in the grain beetle Tenebrio molitor. Funct. Ecol. 17, 534–540 [Google Scholar]
- 12.Worden B. D., Parker P. C., Pappas P. W. 2000. Parasites reduce attractiveness and reproductive success in male grain beetles. Anim. Behav. 59, 543–550 10.1006/anbe.1999.1368 (doi:10.1006/anbe.1999.1368) [DOI] [PubMed] [Google Scholar]
- 13.Rantala M. J., Viitaniemi H., Roff D. A. 2011. Effects of inbreeding on potential and realized immune responses in Tenebrio molitor. Parasitology 138, 906–912 10.1017/s0031182011000473 (doi:10.1017/s0031182011000473) [DOI] [PubMed] [Google Scholar]
- 14.Roff D. A. 1998. Effects of inbreeding on morphological and life history traits of the sand cricket, Gryllus firmus. Heredity 81, 28–37 10.1046/j.1365-2540.1998.00363.x (doi:10.1046/j.1365-2540.1998.00363.x) [DOI] [Google Scholar]
- 15.Vainikka A., Rantala M. J., Seppälä O., Suhonen J. 2007. Do male mealworm beetles, Tenebrio molitor, sustain the honesty of pheromone signals under immune challenge? Acta Ethol. 10, 63–72 10.1007/s10211-007-0031-0 (doi:10.1007/s10211-007-0031-0) [DOI] [Google Scholar]
- 16.Ilmonen P., Stundner G., Thoss M., Penn D. J. 2009. Females prefer the scent of outbred males: good-genes-as-heterozygosity? BMC Evol. Biol. 9, 104. 10.1186/1471-2148-9-104 (doi:10.1186/1471-2148-9-104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drayton J. M., Milner R. N. C., Hunt J., Jennions M. D. 2010. Inbreeding and advertisement calling in the cricket Teleogryllus commodus: laboratory and field experiments. Evolution 64, 3069–3083 10.1111/j.1558-5646.2010.01053.x (doi:10.1111/j.1558-5646.2010.01053.x) [DOI] [PubMed] [Google Scholar]
- 18.Aspi J. 2000. Inbreeding and outbreeding depression in male courtship song characters in Drosophila montana. Heredity 84, 273–282 10.1046/j.1365-2540.2000.00655.x (doi:10.1046/j.1365-2540.2000.00655.x) [DOI] [PubMed] [Google Scholar]
- 19.Qvarnström A., Forsgren E. 1998. Should females prefer dominant males? Trends Ecol. Evol. 13, 498–501 10.1016/S0169-5347(98)01513-4 (doi:10.1016/S0169-5347(98)01513-4) [DOI] [PubMed] [Google Scholar]
- 20.Lehmann L., Keller L. F., Kokko H. 2007. Mate choice evolution, dominance effects, and the maintenance of genetic variation. J. Theor. Biol. 244, 282–295 10.1016/j.jtbi.2006.07.033 (doi:10.1016/j.jtbi.2006.07.033) [DOI] [PubMed] [Google Scholar]
- 21.Rantala M., Roff D. 2007. Inbreeding and extreme outbreeding cause sex differences in immune defence and life history traits in Epirrita autumnata. Heredity 98, 329–336 10.1038/sj.hdy.6800945 (doi:10.1038/sj.hdy.6800945) [DOI] [PubMed] [Google Scholar]
- 22.Carazo P., Sanchez E., Font E., Desfilis E. 2004. Chemosensory cues allow male Tenebrio molitor beetles to assess the reproductive status of potential mates. Anim. Behav. 68, 123–129 10.1016/j.anbehav.2003.10.014 (doi:10.1016/j.anbehav.2003.10.014) [DOI] [Google Scholar]
- 23.Kivleniece I., Krams I., Daukste J., Krama T., Rantala M. J. 2010. Sexual attractiveness of immune-challenged male mealworm beetles suggests terminal investment in reproduction. Anim. Behav. 80, 1015–1021 10.1016/j.anbehav.2010.09.004 (doi:10.1016/j.anbehav.2010.09.004) [DOI] [Google Scholar]


