Abstract
Fusions between individuals are a common feature of organisms with modular, indeterminate life forms, including plants, marine invertebrates and fungi. The consequences of fusion for an individual fungus are poorly understood. We used wild-type and fusion mutant strains of the genetic model Neurospora crassa to chronicle the fitness in two different laboratory habitats, and in each experiment started colonies from multiple different densities of asexual spores. On round Petri dishes, fusion enabled wild-type colonies to grow larger than mutant (soft) colonies; but in linear ‘race tubes’, the soft mutant always grew more quickly than the wild-type. Starting a colony with more spores always provided an advantage to a wild-type colony, but was more often neutral or a cost to the soft mutant. The ability to fuse does not provide a consistent advantage to wild-type colonies; net benefits are shaped by both habitat and initial spore densities.
Keywords: fusion, heterokaryons, life history, non-self recognition, cooperation
1. Introduction
Early life-history stages are critical to the fitness of individual organisms and a rich literature documents the evolution of life-history strategies among determinate animals; in contrast, relatively less thought has been directed at modular or indeterminate species [1–4]. When a fungal spore lands on a substrate, critical events in its early life include germination and contacts with other germinating spores. Asexual propagation is ubiquitous in fungi and spores often land close to genetically identical propagules. Fusions may facilitate the formation of a multi-nucleate, syncytial colony. By fusing and cooperating, colonies potentially capture more resources than any single individual could capture on its own.
Fusions are a common feature of sessile, indeterminate and multi-cellular organisms, including plants and marine invertebrates [2,3]. The genetics underpinning the fusion behaviours of fungi is a target of current research [5], but despite the use of these behaviours to define both species [6] and the structure of populations [7], the consequences of fusion for an individual fungus are poorly understood [8]. When fusion facilitates the looting of resources [9] or invasion by parasites [10,11], it may harm the mycelium; an individual may benefit only when it fuses with itself [12]. Fusion is mediated by self versus other recognition systems, and although fusion between genetically different individuals results in chimeric algae [13], tunicates [3] and corals [14], chimeras may be very rare among the filamentous fungi (but see Peabody et al. [15]).
Our aim was to record the fitness dynamics associated with fusion, and test whether benefits associated with fusion increase as the numbers and densities of fusing individuals increases. We use the fungus Neurospora crassa as a model and manipulated both a wild-type strain (FGSC 2489; [16]) and a fusion mutant, soft (FGSC 11293; [17]). The mutant is isogenic with wild-type, except for a deletion of the soft gene. After germination, each soft spore will form an independent colony that cannot fuse with itself or other germinating spores or colonies. We used the area of a colony or growth rate as fitness parameters [18] and grew individuals on either standard Petri dishes or ‘race tubes’. In Petri dishes, individuals forage in every direction and form circular colonies; but in race tubes, individuals are forced to grow as linear colonies and forage along a single, 1 cm wide path. We hypothesized that the inability of soft germlings to fuse and cooperatively forage or distribute resources would adversely affect fitness.
2. Material and methods
Vogel's minimal medium (VMM) [19] was used to germinate different numbers of asexual spores, or conidia. Each experiment was repeated in its entirety at least three times. Results were nearly identical across the duplicate experiments, and data from a single experiment are used in figures (see electronic supplementary material). Data analyses used the R statistical package (www.r-project.org, v. 2.12.0).
(a). Experiment 1: Fusion in Petri dishes
Conidia were harvested, counted and diluted to five different concentrations: from 5 × 103 to 1.25 × 107 conidia ml−1; 2 μl aliquots were dropped onto the surface of Petri dishes to create cell densities ranging from 250 to 650 000 conidia mm−2. Three replicate blocks were created for each experiment including: (i) 100 plates containing 250 conidia mm−2, (ii) 10 plates containing 2500 conidia mm−2 and (iii) one plate containing a 25 000 conidia mm−2. This protocol kept spore numbers constant; we were initially concerned that including very different numbers of spores in each of the treatments would increase the probability of including a rare, developmentally or physiologically unique mutant spore in treatments with greater densities. As we became familiar with the system, this concern was mitigated, and the race tube experiment focused on having equal numbers of plates in the different treatments. The Petri dish experiment also included two additional treatments: single plates containing either 250 000 or 650 000 conidia mm−2. Colonies were incubated at 20°C under natural light, and the growth of each culture was calculated by measuring colony area at 26 h.
(b). Experiment 2: Fusion in race tubes
Conidia were diluted to three different concentrations: from 5 × 103 to 5 × 106 conidia ml−1; 2 μl aliquots were dropped onto the surface of pre-cut 4 mm diameter VMM plugs, and plugs immediately transferred to race tubes [20] made of 25 ml sterile disposable plastic pipettes, to establish conidial densities ranging from 250 to 250 000 conidia mm−2. Three replicate blocks were created for each experiment including: (i) 10 tubes containing 250 conidia mm−2, (ii) 10 tubes containing 2500 conidia mm−2 and (iii) 10 tubes containing 250 000 conidia mm−2. Linear growth rates were measured daily, until colonies reached the ends of the tubes.
3. Results
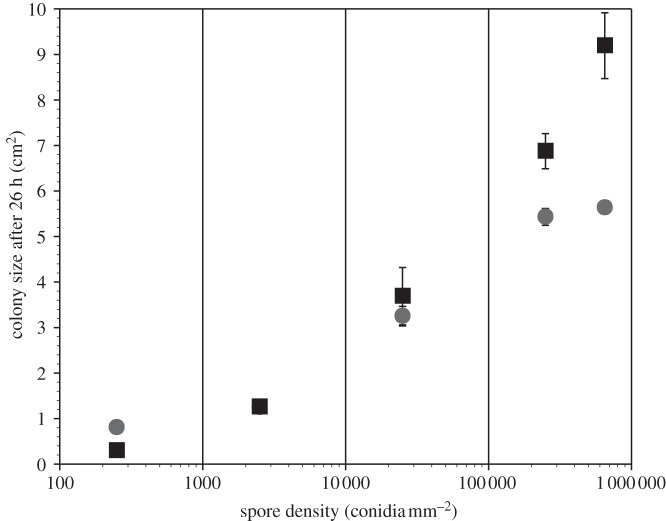
Both wild-type and soft colonies grew larger when Petri dish inoculations included greater densities of spores (figure 1). After 26 h of incubation, wild-type colonies grown from conidial densities of 250, 2500 and 25 000 conidia mm−2 had mean areas of 0.30, 1.26 and 3.69 cm2, respectively. The larger initial densities of 250 000 and 650 000 conidia mm−2 resulted in markedly larger colony areas, 6.87 and 9.19 cm2, respectively. A Kruskal–Wallis test confirmed a significant effect of density on size (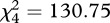 ; p < 0.0001). Dynamics associated with soft mutant colonies were similar but not identical to wild-type (figure 1). Colonies developed from conidial densities of 250, 2500, 25 000 and 250 000 conidia mm−2 had mean areas of 0.81, 1.21, 3.25 and 5.43 cm2, respectively. A Kruskal–Wallis test confirmed a significant and generally positive effect of density on size (
; p < 0.0001). Dynamics associated with soft mutant colonies were similar but not identical to wild-type (figure 1). Colonies developed from conidial densities of 250, 2500, 25 000 and 250 000 conidia mm−2 had mean areas of 0.81, 1.21, 3.25 and 5.43 cm2, respectively. A Kruskal–Wallis test confirmed a significant and generally positive effect of density on size ( ; p < 0.0001). However, colonies started from 650 000 soft conidia mm−2 were smaller (5.63 cm2) than wild-type colonies (9.19 cm2) and were approximately the same size as soft colonies started from 250 000 conidia mm−2.
; p < 0.0001). However, colonies started from 650 000 soft conidia mm−2 were smaller (5.63 cm2) than wild-type colonies (9.19 cm2) and were approximately the same size as soft colonies started from 250 000 conidia mm−2.
Figure 1.
Area covered by wild-type (squares) and fusion mutant (circles) (soft) Neurospora crassa colonies after 26 h, as a function of conidial density. Plates were inoculated with 250 (n = 300), 2500 (n = 30), 25 000 (n = 3), 250 000 (n = 3) or 650 000 (n = 3) conidia mm−2. Bars are s.e.
As observed in Petri dish cultures, wild-type colonies established from more spores had faster growth rates in linear environments (figure 2), at least at early time points. A repeated-measures ANOVA confirmed a general effect of density (F2,48 = 89.38, p < 0.0001) and differences in the effect of density through time (interaction of density and time point F4,96 = 50.95, p < 0.0001). Colonies in race tubes established from 250 conidia mm−2 reached an average growth rate of 0.54 mm h−1 during the first 48 h of growth, when compared with an average growth rate of 1.13 mm h−1 for colonies established from 250 000 conidia mm−2. At 72 h, there is no longer a difference between colonies started from densities of 2500 or 250 000 conidia mm−2. However, colonies established from 250 conidia mm−2 still grow more slowly. By 96 h, the growth rates of wild-type colonies established from the different spore densities were equivalent.
Figure 2.
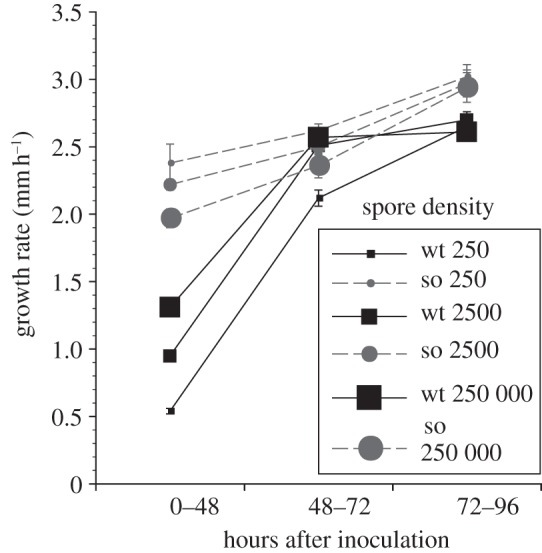
Effect of conidial density (250; 2500; or 25 000 conidia mm−2) on growth rate of Neurospora crassa wild-type and fusion mutant (soft) strains, recorded along a chronosequence. Bars are s.e.
In stark contrast to wild-type data, increasing the density of spores had a negative effect on the growth rate of soft in linear environments (figure 2), suggesting that competitive interactions among soft individuals at higher conidial densities depress the growth rate of the colony. The effect of density is significant (F2,95 = 4.03, p = 0.0209), but the interaction of density and time point is only marginally significant (F4,190 = 2.30, p = 0.0596).
Comparisons between experiments highlight a contrast between the growth of colonies in circular Petri dishes versus linear race tubes: at the lowest spore density (250 conidia mm−2), the soft mutant was a larger colony than the wild-type (0.81 versus 0.30 cm2; figure 1), consistent with observations from race tubes, where the soft growth rate at 26 h is faster than the wild-type growth rate (figure 2). But the size of soft and wild-type colonies started from initial densities of 2500 conidia mm−2 was equivalent (1.25 versus 1.26 cm2; figure 1), and at greater conidial densities, wild-type colonies were significantly larger than soft colonies (figure 1), even though race tube data suggest soft mutants always grow faster than wild-type colonies at 26 h, no matter the conidial density (figure 2). The apparent contradiction is a repeatable result and was consistent across the triplicate experiments.
4. Discussion
Both advantages and disadvantages are associated with the capacity to fuse [12]. Fusion slows the growth of colonies formed from low densities of spores (compare wild-type with soft; figure 1), and colonies growing in spatially constrained environments (figure 2). However, fusion provides a clear advantage to colonies started from greater densities of conidia in open environments (figure 1). The role of density itself changes according and whether or not spores can fuse; in Petri dishes, more spores generally cause both wild-type and soft colonies to grow larger (figure 1), whereas race tube data suggest more spores are an advantage to wild-type colonies and a disadvantage to colonies of the fusion mutant (figure 2).
The differences between experiments probably reflect the forced constraint of foraging along a linear path versus foraging in multiple different directions. In a race tube, competition for a single narrow strip of medium is imposed. Petri dishes facilitate access to more resources, and fusion becomes an advantage because colonies formed from fusing wild-type spores more effectively capture and exploit resources.
In these experiments, fitness was measured as either area or growth rate; if fitness had been measured as biomass, then fusion might have been recorded as a benefit even in linear environments. Wild-type colonies in race tubes developed slowly growing, densely reticulated colony fronts, whereas the soft mutant grew in discontinuous and diffuse patterns made up of fast growing, but poorly branched and isolated hyphae.
Our data suggest that fusion imposes a transitory cost to body extension, as measured by colony area and growth rate. Fusion is associated with a complex molecular machinery [5,21–23] and is likely to involve significant energetic expenditures. Within young wild-type colonies, the majority of early acquired resources may be preferentially allocated to convert older parts of the mycelium into a fine-meshed symmetric network of hyphae [24].
Nearly all of the globe's 1.5 million fungi have the capacity to propagate asexually, and fusions among dispersing, asexually derived individuals may facilitate territory and resource capture. Analogous behaviours are found within other microbial groups, including bacteria [25] and social amoeba [26], and elucidating the costs and benefits associated with fusion will be a key to extending life-history theory across the tree of life.
Acknowledgements
We thank Harvard University's Microbial Sciences Initiative, the U.S. NSF and Andrew Murray.
References
- 1.Stearns S. C. 1992. The evolution of life histories. New York, NY: Oxford University Press [Google Scholar]
- 2.Buss L. W. 1982. Somatic-cell parasitism and the evolution of somatic tissue compatability . Proc. Natl Acad. Sci. USA 79, 5337–5341 10.1073/pnas.79.17.5337 (doi:10.1073/pnas.79.17.5337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosberg R. K. 1988. The evolution of allorecognition specificity in clonal invertebrates . Q. Rev. Biol. 63, 377–412 10.1086/416026 (doi:10.1086/416026) [DOI] [Google Scholar]
- 4.Rayner A. D. M., Boddy L. 1988. Fungal decomposition of wood: its biology and ecology. New York, NY: John Wiley & Sons [Google Scholar]
- 5.Fleissner A., Leeder A. C., Roca M. G., Read N. D., Glass N. L. 2009. Oscillatory recruitment of signaling proteins to cell tips promotes coordinated behaviour during cell fusion. Proc. Natl Acad. Sci. USA 106, 19 387–19 392 10.1073/pnas.0907039106 (doi:10.1073/pnas.0907039106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Sierra A., Guillaumin J. J., Spooner B. M., Bridge P. D. 2004. Characterization of Armillaria heimii from Africa. Plant Pathol. 53, 220–230 10.1111/j.0032-0862.2004.00999.x (doi:10.1111/j.0032-0862.2004.00999.x) [DOI] [Google Scholar]
- 7.Boddy L., Rayner A. D. M. 1982. Population-structure, inter-mycelial interactions and infection biology of Stereum gausapatum. Trans. Br. Mycol. Soc. 78, 337–351 10.1016/S0007-1536(82)80018-1 (doi:10.1016/S0007-1536(82)80018-1) [DOI] [Google Scholar]
- 8.Buller A. H. R. 1931. Researches on fungi, vol. 4 London, UK: Longman [Google Scholar]
- 9.Debets A. J. M., Griffiths A. J. F. 1998. Polymorphism of het-genes prevents resource plundering in Neurospora crassa. Mycol. Res. 102, 1343–1349 10.1017/S095375629800639X (doi:10.1017/S095375629800639X) [DOI] [Google Scholar]
- 10.Nauta M. J., Hoekstra R. F. 1994. Evolution of vegetative incompatibility in filamentous ascomycetes. I. Deterministic models. Evolution 48, 979–995 10.2307/2410360 (doi:10.2307/2410360) [DOI] [PubMed] [Google Scholar]
- 11.Brusini J., Robin C., Franc A. 2011. Parasitism and maintenance of diversity in a fungal vegetative incompatibility system: the role of selection by deleterious cytoplasmic elements. Ecol. Lett. 14, 444–452 10.1111/j.1461-0248.2011.01602.x (doi:10.1111/j.1461-0248.2011.01602.x) [DOI] [PubMed] [Google Scholar]
- 12.Aanen D. K., Debets A. J. M., de Visser A. G. M., Hoekstra R. F. 2008. The social evolution of somatic fusion. BioEssays 30, 1193–1203 10.1002/bies.20840 (doi:10.1002/bies.20840) [DOI] [PubMed] [Google Scholar]
- 13.Santelices B., Correa J. A., Aedo D., Flores V., Hormazabal M., Sanchez P. 1999. Convergent biological processes in coalescing rhodophyta. J. Phycol. 35, 1127–1149 10.1046/j.1529-8817.1999.3561127.x (doi:10.1046/j.1529-8817.1999.3561127.x) [DOI] [Google Scholar]
- 14.Puill-Stephan E., Willis B. L., van Herwerden L., van Oppen M. J. H. 2009. Chimerism in wild adult populations of the broadcast spawning coral Acropora millepora on the great barrier reef. PLoS ONE 4, e7751. 10.1371/journal.pone.0007751 (doi:10.1371/journal.pone.0007751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peabody R. B., Peabody D. C., Sicard K. M. 2000. A genetic mosaic in the fruiting stage of Armillaria gallica. Fungal Genet. Biol. 29, 72–80 10.1006/fgbi.2000.1187 (doi:10.1006/fgbi.2000.1187) [DOI] [PubMed] [Google Scholar]
- 16.Galagan J. E., et al. 2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature 422, 859–868 10.1038/nature01554 (doi:10.1038/nature01554) [DOI] [PubMed] [Google Scholar]
- 17.Fleissner A., Sarkar S., Jacobson D. J., Roca M. G., Read N. D., Glass N. L. 2005. The so locus is required for vegetative cell fusion and postfertilization events in Neurospora crassa. Eukaryot. Cell 4, 920–930 10.1128/EC.4.5.920-930.2005 (doi:10.1128/EC.4.5.920-930.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pringle A., Taylor J. W. 2002. The fitness of filamentous fungi. Trends Microbiol. 10, 474–481 10.1016/S0966-842X(02)02447-2 (doi:10.1016/S0966-842X(02)02447-2) [DOI] [PubMed] [Google Scholar]
- 19.Vogel H. J. 1964. Distribution of lysine pathways among fungi: evolutionary implications. Am. Nat. 98, 435–446 10.1086/282338 (doi:10.1086/282338) [DOI] [Google Scholar]
- 20.Ryan F. J., Beadle G. W., Tatum E. L. 1943. The tube method of measuring the growth rate of Neurospora. Am. J. Bot. 30, 784–799 10.2307/2437554 (doi:10.2307/2437554) [DOI] [Google Scholar]
- 21.Hickey P. C., Jacobson D. J., Read N. D., Glass N. L. 2002. Live-cell imaging of vegetative hyphal fusion in Neurospora crassa. Fungal Genet. Biol. 37, 109–119 10.1016/S1087-1845(02)00035-X (doi:10.1016/S1087-1845(02)00035-X) [DOI] [PubMed] [Google Scholar]
- 22.Araujo-Palomares C. L., Riquelme M., Castro-Longoria E. 2009. The polarisome component SPA-2 localizes at the apex of Neurospora crassa and partially colocalizes with the Spitzenkorper. Fungal Genet. Biol. 46, 551–563 10.1016/j.fgb.2009.02.009 (doi:10.1016/j.fgb.2009.02.009) [DOI] [PubMed] [Google Scholar]
- 23.Brand A., Gow N. A. R. 2009. Mechanisms of hypha orientation of fungi. Curr. Opin. Microbiol. 12, 350–357 10.1016/j.mib.2009.05.007 (doi:10.1016/j.mib.2009.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rayner A. D. M. 1991. The challenge of individualistic mycelium. Mycologia 83, 48–71 10.2307/3759832 (doi:10.2307/3759832) [DOI] [Google Scholar]
- 25.Diggle S. P., Griffin A. S., Campbell G. S., West S. A. 2007. Cooperation and conflict in quorum-sensing bacterial populations. Nature 450, 411–414. (doi:10.1038/nature06279) [DOI] [PubMed] [Google Scholar]
- 26.Strassmann J. E., Queller D. C. 2011. How social evolution theory impacts our understanding of development in the social amoeba Dictyostelium . Dev. Growth Differ. 53, 597–607 10.1111/j.1440-169X.2011.01272.x (doi:10.1111/j.1440-169X.2011.01272.x) [DOI] [PubMed] [Google Scholar]