Abstract
Individually distinct vocalizations are widespread among social animals, presumably caused by variation in vocal tract anatomy. A less-explored source of individual variation is due to learned movement patterns of the vocal tract, which can lead to vocal convergence or divergence in social groups. We studied patterns of acoustic similarity in a social call produced by 14 female Diana monkeys (Cercopithecus diana) in two free-ranging groups. Calls showed variability in fundamental frequency contours owing to individual identity and external context. Vocal divergence increased significantly between females during poor visibility and tended to increase in the presence of neighbours. In contrast, vocal convergence increased significantly between females during vocal interactions, because females matched the frequency contour of their own call with another female's preceding call. Our findings demonstrate that these primates have some control over the acoustic fine structure of their most important social vocalization. Vocal convergence and divergence are two opposing processes that enable callers to ensure spatial proximity and social cohesion with other group members.
Keywords: vocal flexibility, individual signatures, call matching, non-human primates
1. Introduction
In human communication, acoustic features of voice and speech signals serve as reliable indicators of individual identity and other important social variables [1]. However, these markers are not fixed but vary with social context and composition of the audience [2]. In socio-linguistics, the ‘communication accommodation theory’ describes the ability of humans to adjust social distance during interactions through a process of vocal convergence and divergence [3]. Although animal communication plays an important role in understanding the evolution of human communication, such processes of vocal accommodation have not received much empirical attention.
This is despite the fact that some animal species possess highly flexible vocal abilities. One frequent consequence is vocal convergence, a process during which individually distinct acoustic features are reduced. The phenomenon is typically observed during group formation, reproductive pair bonding and non-reproductive social bonding [4–7]. In contrast, vocal divergence, a process during which individually distinct acoustic features are highlighted, has been observed in group-living species after separation and during inter-group encounters [8,9]. Thus, callers appear to alternate between the need to differ from others by advertising individual identity and the need to conform to others to secure social bonding.
Non-human primates are famously limited in the amount of control they have over their vocal output, with only limited degrees of plasticity (see Hammerschmidt & Fischer [10] for review). Temporary acoustic modification has been documented in reaction to ambient noise, social isolation and habitat [11–13]. Acoustic convergence has been documented in terms of callers matching some of each others’ acoustic features during vocal exchanges or as part of social bonding [14,15]. Vocal divergence has been documented between neighbouring groups of chimpanzees (Pan troglodytes) [16].
We investigated the presence of vocal convergence and divergence in free-ranging Diana monkeys (Cercopithecus diana), an arboreal, forest-dwelling West African primate species. Females regularly emit close-range vocalizations (‘contact calls’) that function in maintaining social and spatial cohesion and callers often, but not always, respond to each other's calls. We investigated acoustic variability within and between females of two free-ranging groups. In general, we predicted lower acoustic similarity between than within females’ calls. More specifically, we predicted increased divergence when individual identification is crucial, such as during low visibility, travel episodes, presence of neighbours and high group dispersion, while we expected increased convergence during social interactions, such as call exchanges.
2. Material and methods
Data were collected from February 2009 to May 2009 and from January 2010 to June 2010 from two groups of Diana monkeys in Taï Forest, Ivory Coast (5°50′ N, 7°21′ W). Both groups were fully habituated to human observers, consisting each of one adult male and 9–10 individually identified adult females with their offspring. Groups were followed alternatively with data collection between 07:30 and 17:00 h GMT for 46 and 38 days, respectively. Scan samples [17] were taken every 30 min to score the group's main activity, the degree of group dispersion, the presence of a neighbouring group and general luminosity (see electronic supplementary material for definitions). Individual females were observed and all vocalizations recorded during 10 min focal animal sampling [17]. Females produce various social calls, but the most common one is the ‘contact call’ (‘Af’ call; see electronic supplementary material for spectrogram). Recordings were made with a Sennheiser K6/ME66 directional microphone and Marantz PMD660 recorder (sampling rate 44.1 kHz; resolution 16 bits).
We calculated acoustic similarity indices of Af calls based on a procedure used for frequency modulated whistle-like signals in various species [15,18]. We used customized acoustic software ANA [19] to compare the similarity of the arched fundamental frequency contours of pairs of calls within and between females. First, we calculated, for each female, her mean intra-individual similarity index for all her calls. We also calculated the mean similarity index for all calls given by pairs of females. Second, to assess the role of context on call structure, we compared levels of intra-individual acoustic similarity in different contexts for non-responding calls. As responding calls, we considered any Af call given within 3 s of a preceding call by another female [20]. Conversely, non-responding calls were emitted after at least 3 s of silence. Call context was determined by the previous scan sample. We then compared, for each female, her mean intra-individual similarity index within each context. Only females who contributed with at least two focal samples per context of emission with a maximum of five Af calls per sample were included. Third, we compared the acoustic similarity of exchanged (inter-call interval < 3 s) and non-exchanged (inter-call interval > 3 s) calls, by calculating the mean inter-individual similarity indices of pairs of exchanged and not-exchanged calls. Focal females’ calls were compared both with the call to which they responded and to the previous call to which they did not respond. Owing to the small sample size, we used non-parametric Wilcoxon tests to compare similarity indices with SPSS v. 17.0 software. Tests were two-tailed and significance levels set at α = 0.05.
3. Results
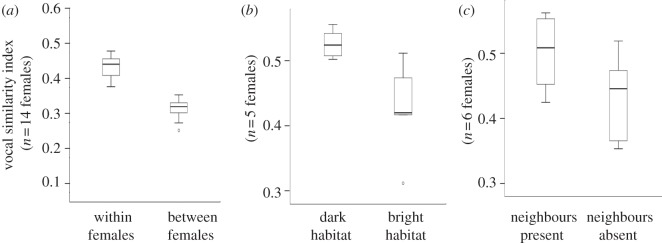
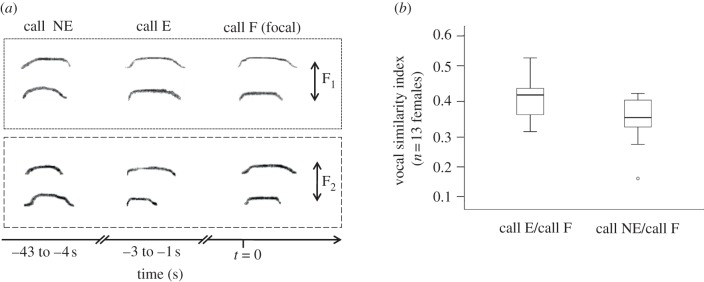
We recorded 1091 Af calls from 14 adult females (group 1: n = 6; group 2: n = 8) during 44 focal observation hours (mean ± s.e. = 3.1 ± 0.5 h per female) and 789 calls were of sufficient quality for acoustic analysis (mean ± s.e. = 56.4 ± 9.5 calls per female). First, we found that Af calls reliably conveyed individual identity, as intra-individual similarity indices were significantly higher than inter-individual ones (n = 14 females, Z = −3.408, p < 0.001; figure 1a). Second, we found that intra-individual acoustic variability varied within some but not all contexts of emission in which we expected increased levels of individual identity. In particular, we found that similarity indices were significantly higher in dark when compared with bright habitats (n = 5 females, Z = −2.023, p = 0.043; figure 1b). Similarity indices were also higher in the presence than in the absence of a neighbouring group, but not significantly so (n = 6 females, Z = −1.782, p = 0.075; figure 1c). We failed to find significant effects between group travel and foraging (n = 5 females, Z = −0.135, p = 0.893) and during high versus low group spread (n = 12 females, Z = −0.941, p = 0.347). Finally, we compared pairs of exchanged and not-exchanged calls, provided they were emitted within the same minute. We found that pairs of exchanged calls resembled each other significantly more than not-exchanged calls (n = 13 females, Z = −2.411, p = 0.006; figure 2).
Figure 1.
(a) Box plots illustrate the intra-individual (within females) and the inter-individual (between females) vocal similarity. Similarity of calls within females varied with the presence of (b) habitat luminosity and (c) neighbouring groups.
Figure 2.
(a) Illustration of exchanged and non-exchanged call sequences from two focal females (F1, F2). Call F (focal) was emitted in response to call E (exchanged) but not to call NE (non-exchanged). (b) Box plots illustrate vocal similarity of F calls with E and NE calls.
4. Discussion
We have documented that the main social call of free-ranging female Diana monkeys contains sufficiently stable acoustic variation across individuals to convey individual identity. However, these individual differences in acoustic structure were not fixed, but varied systematically in relation to a number of external factors including social context. We observed significant vocal divergence when the habitat illumination is low, most probably owing to individuals highlighting individual identity. In contrast, we observed significant vocal convergence during peaceful vocal exchanges. This was due to the fact that exchanged calls were acoustically more similar than non-exchanged calls, i.e. vocalizations given during the same narrow time period that were not part of a vocal exchange. To our knowledge, this study thus presents some of the first evidence of context-specific vocal accommodation, i.e. convergence or divergence, in a non-human primate.
Individual vocal signatures seem to be particularly important in species where individuals depend on each other socially. Diana monkeys form tightly bonded social groups, with individuals cooperating during inter- and intra-group conflicts [21]. In forest primates, signalling individual identity by acoustic means is crucial, owing to the danger of losing contact with others, especially during periods of darkness or during conflicts. Further research, including playback experiments, will be required to confirm that listeners are able to infer individual identity from these calls, although this has partly been demonstrated with the homologous call type of female Campbell's monkeys (Cercopithecus campbelli) [22].
In many animal species, including non-human primates, call exchanges show a number of properties that resemble aspects of human communication [23]. For example, callers adhere to rules that determine the patterns of turn-taking [24]. Another phenomenon found in humans is acoustic convergence during conversations, an effect that is particularly common among closely bonded individuals. In non-human primates, similar effects appear to exist, both long-term (months and years) [15,25] and short-term, as shown by patterns of coo call exchanges in Japanese macaques (Macaca fuscata) [14]. In our study, females produced calls that were more similar to calls they responded than to calls they did not respond to, suggesting that females were not just similarly motivated but adjusted the acoustic structure of their calls in relation to specific social motivations. As we only considered calls given within the same very narrow time limit (less than 60 s), it is not likely that response patterns could be explained with general states of arousal. To conclude, some non-human primates can temporarily alter the acoustic fine structure of their social calls, to both increase or decrease individual distinctiveness, depending on whether highlighting individual identity or social affiliation takes communicative priority.
Acknowledgements
We thank the Ivorian Ministry of Scientific Research and the ‘Office Ivoirien des Parcs et Réserves’ for permission to conduct research, the ‘Centre Suisse de Recherches Scientifiques en Côte d'Ivoire’ for logistic support, F. Belé and F. Gnepa for help with data collection. We thank Robert Seyfarth and an anonymous referee for helpful comments on the manuscript. Funding was from the French Ministry of Research, PICS-CNRS, ANR ‘Orilang’, Wissenschaftskolleg zu Berlin, Leverhulme Trust and Institut Universitaire de France.
References
- 1.Milroy L. 1987. Language and social networks. Oxford, UK: /New York, NY: B Blackwell [Google Scholar]
- 2.Bell A. 1984. Language style as audience design. Lang. Soc. 13, 145–204 10.1017/S004740450001037X (doi:10.1017/S004740450001037X) [DOI] [Google Scholar]
- 3.Giles H., Coupland N., Coupland J. 1991. Accommodation theory: communication, context, and consequence. In Contexts of accommodation: developments in applied sociolinguistics (eds Giles H., Coupland N., Coupland J.), pp. 1–68 Cambridge, UK: Cambridge University Press [Google Scholar]
- 4.Tyack P. L. 2008. Convergence of calls as animals form social bonds, active compensation for noisy communication channels, and the evolution of vocal learning in mammals. J. Comp. Psych. 122, 319–331 10.1037/a0013087 (doi:10.1037/a0013087) [DOI] [PubMed] [Google Scholar]
- 5.Hile A. G., Plummer T. K., Striedter G. F. 2000. Male vocal imitation produces call convergence during pair bonding in budgerigars, Melopsittacus undulatus. Anim. Behav. 59, 1209–1218 10.1006/anbe.1999.1438 (doi:10.1006/anbe.1999.1438) [DOI] [PubMed] [Google Scholar]
- 6.Boughman J. W. 1998. Vocal learning by greater spear-nosed bats. Proc. R. Soc. Lond. B 265, 227–233 10.1098/rspb.1998.0286 (doi:10.1098/rspb.1998.0286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitani J. C., Brandt K. L. 1994. Social factors influence the acoustic variability in the long-distance calls of male chimpanzees. Ethology 96, 233–252 10.1111/j.1439-0310.1994.tb01012.x (doi:10.1111/j.1439-0310.1994.tb01012.x) [DOI] [Google Scholar]
- 8.Charrier I., Pitcher B. J., Harcourt R. G. 2009. Vocal recognition of mothers by Australian sea lion pups: individual signature and environmental constraints. Anim. Behav. 78, 1127–1134 10.1016/j.anbehav.2009.07.032 (doi:10.1016/j.anbehav.2009.07.032) [DOI] [Google Scholar]
- 9.Ford J. K., Fisher H. D. 1983. Group-specific dialects of killer whales (Orcinus orca) in British Columbia. In Communication and behavior of whales (ed. Payn R.), pp. 129–161 Boulder, CO: Westview Press [Google Scholar]
- 10.Hammerschmidt K., Fischer J. 2008. Constraints in primate vocal production. In The evolution of communicative creativity: from fixed signals to contextual flexibility (eds Griebel U., Oller D. K.), pp. 93–119 Cambridge, MA: MIT Press [Google Scholar]
- 11.Brumm H., Voss K., Köllmer I., Todt D. 2004. Acoustic communication in noise: regulation of call characteristics in a New World monkey. J. Exp. Biol. 207, 443–448 10.1242/jeb.00768 (doi:10.1242/jeb.00768) [DOI] [PubMed] [Google Scholar]
- 12.Masataka N., Symmes D. 1986. Effect of separation distance on isolation call structure in squirrel monkeys (Saimiri sciureus). Am. J. Primatol. 10, 271–278 10.1002/ajp.1350100307 (doi:10.1002/ajp.1350100307) [DOI] [PubMed] [Google Scholar]
- 13.Ey E., Rahn C., Hammerschmidt K., Fischer J. 2009. Wild female olive baboons adapt their grunt vocalizations to environmental conditions. Ethology 115, 493–503 10.1111/j.1439-0310.2009.01638.x (doi:10.1111/j.1439-0310.2009.01638.x) [DOI] [Google Scholar]
- 14.Sugiura H. 1998. Matching of acoustic features during the vocal exchange of coo calls by Japanese macaques. Anim. Behav. 55, 673–687 10.1006/anbe.1997.0602 (doi:10.1006/anbe.1997.0602) [DOI] [PubMed] [Google Scholar]
- 15.Lemasson A., Hausberger M. 2004. Patterns of vocal sharing and social dynamics in a captive group of Campbell's monkeys (Cercopithecus campbelli campbelli). J. Comp. Psychol. 118, 347–359 10.1037/0735-7036.118.3.347 (doi:10.1037/0735-7036.118.3.347) [DOI] [PubMed] [Google Scholar]
- 16.Crockford C., Herbinger I., Vigilant L., Boesch C. 2004. Wild chimpanzees produce group-specific calls: a case for vocal learning? Ethology 110, 221–243 10.1111/j.1439-0310.2004.00968.x (doi:10.1111/j.1439-0310.2004.00968.x) [DOI] [Google Scholar]
- 17.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–267 10.1163/156853974X00534 (doi:10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 18.Janik V. M., Todt D., Dehnhardt G. 1994. Signature whistle variations in a bottlenosed dolphin, Tursiops truncatus. Behav. Ecol. Sociob. 35, 243–248 10.1007/BF00170704 (doi:10.1007/BF00170704) [DOI] [Google Scholar]
- 19.Richard J. P. 1991. Sound analysis and synthesis using an amiga micro-computer. Bioacoustics 3, 45–60 [Google Scholar]
- 20.Candiotti A., Zuberbühler K., Lemasson A. In press. Context-related call combinations in female Diana monkeys. Anim. Cogn. 10.1007/s10071-011-0456-8 (doi:10.1007/s10071-011-0456-8) [DOI] [PubMed] [Google Scholar]
- 21.Buzzard P., Eckardt W. 2007. The social systems of the guenons. In Monkeys of the Taï Forest: an African monkey community (eds McGraw W. S., Zuberbühler K., Noë R.), pp. 51–71 Cambridge, UK: Cambridge University Press [Google Scholar]
- 22.Lemasson A., Hausberger M., Zuberbühler K. 2005. Socially meaningful vocal plasticity in adult Campbell's monkeys (Cercopithecus campbelli). J. Comp. Psychol. 119, 220–229 10.1037/0735-7036.119.2.220 (doi:10.1037/0735-7036.119.2.220) [DOI] [PubMed] [Google Scholar]
- 23.Lemasson A. 2011. What can forest guenons ‘tell’ us about the origin of language? In Primate communication and human language: vocalisation, gestures, imitation and deixis in humans and non-humans (eds Vilain A., Schwartz J-L., Abry C., Vauclair J.), pp. 39–70 Amsterdam, The Netherlands: John Benjamins Publishing Company [Google Scholar]
- 24.Hauser M. D. 1992. A mechanism guiding conversational turn-taking in vervet monkeys and rhesus macaques. In Topics in primatology. Human origins, vol. 1 (eds Nishida T., McGrew W., Marler P., Pickford M., De Waal F. B. M.), pp. 235–248 Tokyo, Japan: Tokyo University Press [Google Scholar]
- 25.Snowdon C. T., Elowson A. M. 1999. Pygmy marmosets modify call structure when paired. Ethology, 105, 893–908 10.1046/j.1439-0310.1999.00483.x (doi:10.1046/j.1439-0310.1999.00483.x) [DOI] [Google Scholar]