Abstract
The majority of bird taxa perform water bathing, but little is known about the adaptive value of this behaviour. If bathing is important for feather maintenance then birds that have not bathed should have poorer feather condition, compromised escape ability and therefore increased responsiveness to cues of predation. We conducted two experiments examining the behaviour of captive starlings responding to conspecific alarm calls. Birds that had no access to bathing water showed a decreased willingness to feed and increased their vigilance behaviour following an alarm call. We argue that birds denied access to bathing water interpreted an ambiguous cue of threat as requiring more caution than birds that had access, consistent with higher levels of anxiety. Our results support the provision of bathing water for captive birds as an important welfare measure.
Keywords: bathing, European starling, Sturnus vulgaris, threat perception, animal welfare
1. Introduction
Bathing in water is a trait common to the majority of bird taxa [1,2], but little research has been conducted into its adaptive value [1–6]. If bathing is essential for the maintenance of plumage condition, then we can derive some predictions. Birds that have not bathed should have impaired flight performance, their escape ability should be compromised and consequently, they should be more responsive to signals of predation threat.
Captive European starlings (Sturnus vulgaris) denied access to bathing water collide with more objects but fly more quickly during escape flights [7]. Separate experiments have shown that starlings housed in cages without environmental enrichments (including bathing water) are more likely to interpret ambiguous stimuli as indicating a negative future outcome [8,9]. These findings suggest that lack of access to bathing water may alter threat perception in starlings. To test this hypothesis more directly, we examined the behaviour of caged starlings responding to a conspecific alarm call [10]. This call signals that a predator may be present but it is ambiguous as to the predator's location or identity. We predicted that starlings previously denied access to bathing water should take longer to begin feeding and have elevated vigilance levels on hearing a conspecific alarm call.
2. Material and methods
We used 20 starlings for experiment 1 and 24 for experiment 2. In both experiments, replicates of four birds were housed individually in visually isolated cages. Bark-covered kitten food and drinking water from wall-mounted drinkers were provided ad libitum [10]. All birds were given a large plastic tray; for half of them this was filled daily with clean water. Bathing was not directly observed, but was evinced by wet cage papers and reduced water levels.
Birds were given 3 days to settle and then, on a test day, deprived of food for 2 h. The laboratory lights were subsequently switched off and a bark-filled food bowl containing 10 mealworms (a preferred food) was placed in each cage. The bark increased the difficulty of the foraging task to induce a foraging-vigilance trade-off. The experimenter left the room and after 5 min an acoustic stimulus was played; on its completion, the lights were switched on and the birds’ behaviour recorded on digital video.
For both experiments, the acoustic stimulus comprised a starling alarm call [10] played using an Apple Nano ipod (frequency response: 20 Hz to 20 kHz ± 3 dB) and Yamaha YST-M20DSP active speakers (frequency response: 70 Hz to 20 kHz ± 3 dB). The sound pressure level was standardized to a peak amplitude of 75 dB, measured at the perch in each cage that was furthest from the speakers (all were equidistant from the speakers). Birds in experiment 2 were also subjected to a control starling ‘threat’ call, a signal given in mild agonistic conspecific encounters. Call types were presented individually on consecutive days in a counterbalanced repeated-measures design. Additionally, in experiment 2, all baths were removed prior to the lights being switched off to ensure that there was no motivation for birds to move in order to bathe.
We scored the following behaviours using The Observer (XT v. 8.0, Noldus): latency to move; latency to begin feeding; duration of the first feeding bout; duration of each period spent with the bill continuously below horizontal during this bout (head-down bout duration); the duration of each period spent with the bill continuously above horizontal during the first feeding bout (head-up bout duration); the frequency of transition of the bill from below to above horizontal during the first feeding bout (head-up rate).
Unfortunately, the birds could not be acoustically isolated and auditory disturbances occurred both outside and within the laboratory (e.g. some birds emitted alarm calls in response to the experimenter). Any birds that experienced such disturbance before trials or during the trials were excluded. The recordings for two birds for one of the call-types in experiment 2 allowed latencies to be scored, but the video quality was not satisfactory for scoring vigilance. Hence, the final sample sizes were 14 birds for experiment 1 and 10 for experiment 2.
To reduce our dependent variables, we dropped latency to move since this was highly correlated with latency to feed (experiment 1: r = 0.530, p = 0.051 (the strength of this correlation was greatly reduced by the data from one subject); experiment 2, alarm call: r = 0.999, p < 0.001; experiment 2, ‘threat’ call: r = 0.978, p < 0.001). We entered the remaining measures (transformed to ensure normality) into a principal component analysis (PCA, using PASW Statistics for Mac v. 18.0.3, SPSS Inc.) assuming no rotation (the results also held under an assumption of orthogonal/oblique relationship between factors).
3. Results
The PCA yielded two factors for both experiments 1 and 2 (table 1). For experiment 1, we employed a multivariate analysis of variance with the two factors as the dependent variables. Bathing had a significant effect on the subjects’ behaviour (F2,11 = 5.503, p = 0.022; figure 1). This was limited to the first factor where bathing had a large effect as judged by the effect size estimate, Hedges’ unbiased estimator d [11] (factor 1: no bath group, 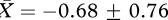 ; bath group,
; bath group, 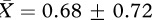 ; F1,12 = 11.565, p = 0.005, d = 1.702; factor 2: no bath group,
; F1,12 = 11.565, p = 0.005, d = 1.702; factor 2: no bath group, 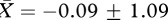 , bath group,
, bath group, 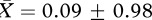 ; F1,12 = 0.113, p = 0.742, d = 0.168). Because of the mixed design in experiment 2, we conducted separate linear mixed model analyses using an unstructured covariance matrix for each (log-transformed) PCA factor (figure 2). For factor 1, the minimal model included significant effects of bathing treatment, acoustic stimulus type and acoustic stimulus presentation order (table 2). For factor 2, there was a significant interaction effect for acoustic stimulus type × acoustic stimulus presentation order (table 2).
; F1,12 = 0.113, p = 0.742, d = 0.168). Because of the mixed design in experiment 2, we conducted separate linear mixed model analyses using an unstructured covariance matrix for each (log-transformed) PCA factor (figure 2). For factor 1, the minimal model included significant effects of bathing treatment, acoustic stimulus type and acoustic stimulus presentation order (table 2). For factor 2, there was a significant interaction effect for acoustic stimulus type × acoustic stimulus presentation order (table 2).
Table 1.
Principal component analysis results for both experiments.
| behavioural measure | experiment 1 |
experiment 2 |
effect of bathing on factor 1 (experiment 1/experiment 2) | ||
|---|---|---|---|---|---|
| factor 1 loading | factor 2 loading | factor 1 loading | factor 2 loading | ||
| latency to feed | −0.642 | −0.205 | 0.772 | −0.155 | ↓/↓ |
| head-up bout duration | −0.688 | 0.696 | 0.917 | −0.077 | ↓/↓ |
| head-up rate | 0.836 | −0.070 | −0.367 | 0.903 | ↑/↑ |
| head-down bout duration | 0.823 | 0.477 | −0.392 | −0.453 | ↑/↑ |
| duration of first feeding visit | 0.014 | 0.918 | 0.745 | 0.462 | ↑/↓ |
Figure 1.
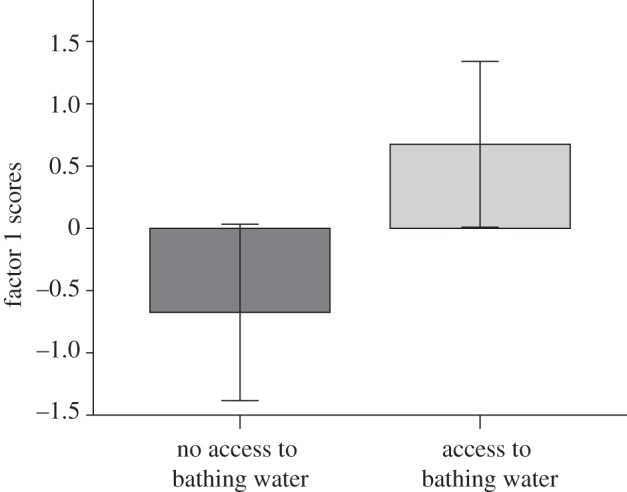
Birds with access to bathing water had significantly higher factor 1 scores indicating reduced vigilance in experiment 1. Error bars represent 95% CI.
Figure 2.
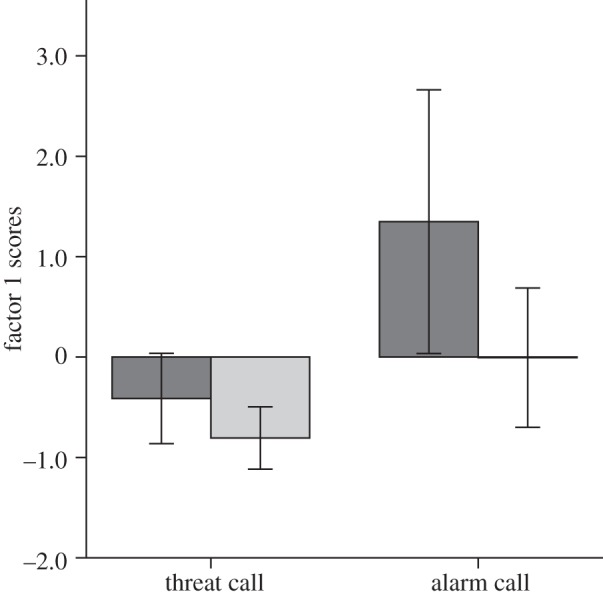
Birds with access to bathing water had significantly lower factor 1 scores indicating reduced vigilance in experiment 2. Note that these are untransformed scores (log-transformed scores were employed for the analysis). Error bars represent 95% CI. Dark grey box represents no access to bathing water; light grey represents access to bathing water.
Table 2.
Linear mixed model analysis results for experiment 2.
| significant model terms (minimal model)a | factor 1 |
factor 2 |
||||
|---|---|---|---|---|---|---|
| F-ratio (d.f.) | coefficient estimateb | p-value | F-ratio (d.f.) | coefficient estimateb | p-value | |
| bathing | 17.0621,7.4 | −0.221 | 0.004c | 0.0041,7 | 0.002 | 0.953 |
| acoustic stimulus type | 24.2961,8 | 0.193 | 0.001c | 5.0891,7 | −0.175 | 0.059 |
| bathing × acoustic stimulus type | 0.7771,8 | 0.084 | 0.404 | 0.0011,7 | 0.001 | 0.978 |
| acoustic stimulus presentation order | 14.3211,7 | −0.147 | 0.007c | 4.4551,7 | −0.234 | 0.073 |
| acoustic stimulus type × acoustic stimulus presentation order | n.s. and excluded from model | 10.2461,7 | −0.205 | 0.015c | ||
aFull model included: acoustic stimulus type, bathing treatment and acoustic stimuli presentation order, and all two-way interactions. Terms were removed sequentially by highest p-value, but the experimental factors and their interaction were retained.
bCoefficient comparisons for main effects are given as: no bathing water versus bathing water; threat call versus alarm call; alarm call heard second versus alarm call heard first.
cIndicates significance at the α = 0.05 level.
Bathing appeared to increase factor 1 scores in experiment 1 and decrease them in experiment 2. However, factor 1 weightings for both experiments were qualitatively equivalent (duration of first feeding bout aside): latency to feed varied positively with the average duration of each head-up bout but varied inversely with the head-up rate per minute and the average duration of each head-down bout (table 1). Hence, bathing had qualitatively the same effect in both experiments.
4. Discussion
Access to bathing water had a large and significant effect on a behavioural factor that captures sensitivity to threat in captive starlings. Bathing caused birds to decrease their latency to feed, decrease the average duration of each head-up scanning bout, increase the average duration of each head-down feeding bout and increase their head-up rate. Thus, birds given access to bathing water were more willing to feed in the face of an ambiguous threat performing shorter, albeit more frequent, vigilance bouts. This indicates two possibilities: either birds that bathed interpreted the ambiguous threat signalled by the acoustic stimuli as being less dangerous, or they were more motivated to move/feed. The latter is unlikely as all birds were fed ad libitum until the day of testing. Taking away water baths during testing in experiment 2 also removed any potential confound of motivation to bathe in the group given access to bathing water (though no subjects did so during testing in experiment 1).
We argue that birds denied access to bathing water interpreted an ambiguous cue of threat as requiring more caution than birds that had access because their ability to cope with threats was impaired. This is consistent with flight trials [7], which suggested that birds with no access to bathing water considered escaping from potential threat to be more important than avoiding physical harm from collisions. We tentatively propose that the findings from both studies may be owing to differences in feather condition caused by a combination of bathing and preening. In any case, the effect of bathing must be short-term as bathing water was only removed for 3 days and had previously been provided ad libitum.
The alarm call elicited a greater defensive response than the ‘threat’ call, but the bathing manipulation had a significant effect on the response to both. A priori we predicted that the ‘threat’ call would not be perceived as a sign of imminent physical danger so the bathing manipulation should have had no effect. There are two possibilities: either the ‘threat’ call contains some connotation of potential harm; or the bathing manipulation more generally changed the perception of the experimental context. Previous experiments showed that starlings also respond more defensively to white noise than to the same ‘threat’ call [10]. Thus, it may be the experimental context that the birds perceive as ambiguously threatening, rather than the ‘threat’ call per se. Further experiments are required using no acoustic stimulus to address this possibility.
European Union legislation regarding laboratory birds advises that bathing water should be available either constantly or on a regular basis, depending on the species concerned (revised Appendix A of ETS 123, Council of Europe Convention). More specific guidelines exist suggesting the constant provision of shallow water baths for starlings [12]. This recommendation is based on a notion that bathing is important for feather maintenance and on the anecdotal observation that starlings are enthusiastic bathers. However, of 106 research articles featuring captive starlings, only 15 reported provision of water for bathing [13]. Our findings suggest that when baths are not provided, starlings may have a continual bias in their perception of ambiguously threatening situations (e.g. ambient noises) arising from a perceived increase in their vulnerability to predation. We therefore hypothesize that long-term lack of access to bathing water may be a cause of chronic stress and/or anxiety-like symptoms in captive starlings [14]. However, further experiments are required in order to demonstrate any potential long-term impact (e.g. permanent changes in willingness to alarm call; changes in baseline and stress-induced corticosterone levels). Whatever the long-term consequences, in the short-term at least, the provision of bathing water for starlings (and arguably, other water-bathing bird species) is clearly of welfare importance given the large effect of bathing water availability on threat perception that we have demonstrated.
Acknowledgements
We adhered to the Association for the Study of Animal Behaviour's “Guidelines for the Use of Animals in Research”. The experimental protocol was also subject to internal ethical review by the Named Animal Care Welfare Officer. The birds were inspected, fed, watered and cleaned out on a daily basis by the experimenter. No birds showed long-term alterations in their behaviour after the experimental manipulations and on release back into free-flight aviaries showed no other signs of adverse effects. The subjects were originally captured from the wild under licence from Natural England. At the close of all experiments the birds received a health inspection by a qualified vet and were then released back to the wild at the site of their initial capture.
We thank Michelle Waddle, Mark Whittingham, Dan Blumstein and an anonymous reviewer. This work was supported by a grant awarded to M.B. by the Biotechnology and Biological Sciences Research Council (BB/E012000/1).
References
- 1.Simmons K. E. L. 1964. Feather maintenance. In A new dictionary of birds (ed. Thomson A. L.), pp. 278–286 New York, NY: McGraw-Hill [Google Scholar]
- 2.Slessers M. 1970. Bathing behavior of land birds. Auk 87, 91–99 [Google Scholar]
- 3.Van Rhijn J. G. 1977. Processes in feathers caused by bathing in water. Ardea 65, 126–147 [Google Scholar]
- 4.Elowson A. M. 1984. Spread-wing postures and the water repellency of feathers: a test of Rijke's hypothesis. Auk 101, 371–383 [Google Scholar]
- 5.Oswald S. A., Bearhop S., Furness R. W., Huntley B., Hamer K. C. 2008. Heat stress in a high-latitude seabird: effects of temperature and food supply on bathing and nest attendance of great skuas Catharacta skua. J. Avian Biol. 39, 163–169 10.1111/j.2008.0908-8857.04187.x (doi:10.1111/j.2008.0908-8857.04187.x) [DOI] [Google Scholar]
- 6.Murphy S. M., Braun J. V., Millama J. R. 2011. Bathing behavior of captive orange-winged Amazon parrots (Amazona amazonica). Appl. Anim. Behav. Sci. 132, 200–210 10.1016/j.applanim.2011.04.010 (doi:10.1016/j.applanim.2011.04.010) [DOI] [Google Scholar]
- 7.Brilot B. O., Asher L., Bateson M. 2009. Water bathing alters the speed-accuracy trade-off of escape flights in European starlings. Anim. Behav. 78, 801–807 10.1016/j.anbehav.2009.07.022 (doi:10.1016/j.anbehav.2009.07.022) [DOI] [Google Scholar]
- 8.Bateson M., Matheson S. M. 2007. Removal of environmental enrichment induces ‘pessimism’ in captive European starlings (Sturnus vulgaris). Anim. Welfare 16, 33–36 [Google Scholar]
- 9.Matheson S. M., Asher L., Bateson M. 2008. Larger, enriched cages are associated with ‘optimistic’ response biases in captive European starlings (Sturnus vulgaris). Appl. Anim. Behav. Sci. 109, 374–383 10.1016/j.applanim.2007.03.007 (doi:10.1016/j.applanim.2007.03.007) [DOI] [Google Scholar]
- 10.Brilot B. O., Normandale C. L., Parkin A., Bateson M. 2009. Can we use starlings’ aversion to eyespots as the basis for a novel ‘cognitive bias’ task? Appl. Anim. Behav. Sci. 118, 182–190 10.1016/j.applanim.2009.02.015 (doi:10.1016/j.applanim.2009.02.015) [DOI] [Google Scholar]
- 11.Nakagawa S., Cuthill I. C. 2007. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605 10.1111/j.1469-185X.2007.00027.x (doi:10.1111/j.1469-185X.2007.00027.x) [DOI] [PubMed] [Google Scholar]
- 12.Hawkins P., et al. 2001. Laboratory birds: refinements in husbandry and procedures. Lab. Anim. 35, S1–S163 [PubMed] [Google Scholar]
- 13.Asher L., Bateson M. 2008. Use and husbandry of captive European starlings (Sturnus vulgaris) in scientific research: a review of current practice. Lab. Anim. 42, 111–126 10.1258/la.2007.007006 (doi:10.1258/la.2007.007006) [DOI] [PubMed] [Google Scholar]
- 14.Bateson M., Brilot B. O., Nettle D. 2011. Anxiety: an evolutionary approach. Can. J. Psychiat. 56(12), 707–715 [DOI] [PubMed] [Google Scholar]


