Abstract
Conodonts have been considered the earliest skeletonizing vertebrates and their mineralized feeding apparatus interpreted as having performed a tooth function. However, the absence of jaws in conodonts and the small size of their oropharyngeal musculature limits the force available for fracturing food items, presenting a challenge to this interpretation. We address this issue quantitatively using engineering approaches previously applied to mammalian dentitions. We show that the morphology of conodont food-processing elements was adapted to overcome size limitations through developing dental tools of unparalleled sharpness that maximize applied pressure. Combined with observations of wear, we also show how this morphology was employed, demonstrating how Wurmiella excavata used rotational kinematics similar to other conodonts, suggesting that this occlusal style is typical for the clade. Our work places conodont elements within a broader dental framework, providing a phylogenetically independent system for examining convergence and scaling in dental tools.
Keywords: conodont, tooth, dental tools, finite-element analysis, Wurmiella excavata
1. Introduction
Conodonts, an extinct clade of jawless vertebrates [1], possessed an oropharyngeal skeleton of elements that formed a feeding apparatus [2,3]. The extensive wear and damage developed on these elements, which could result only from attrition and abrasion of exposed element surfaces [4–6] and clear morphological convergence with other dentitions ([6,7] and references therein), strongly supports the interpretation of conodont elements as functional analogues, if not homologues, of gnathostome teeth.
However, qualitative analyses of conodont elements have revealed that the architecture, growth and basic kinematics of the feeding apparatus differed fundamentally from the teeth of jawed vertebrates [6,8], reflecting independent origin [1,9]. Moreover, at approximately 0.2–2 mm long, conodont elements are at least an order of magnitude smaller than many teeth. Likewise, because conodont oropharyngeal muscles must necessarily have been small, and lacked jaws for anchorage, the absolute force available for fracturing and fragmenting food with these elements is likely to have been miniscule. How then could such structures function effectively as a dentition?
We addressed this issue quantitatively for the first time, using finite-element (FE) analysis, to test loading hypotheses in structures too small and delicate for empirical experimentation [10]. We also analysed elements morphologically, as tools for food fracture, an approach that has formalized the application of engineering principles to teeth and illuminated the influence of shape upon function in mammals [11–16]. Finally, we combine results from these analyses with observations of surface wear to constrain element kinematics and function during food processing.
2. Material and methods
We focus on Wurmiella excavata, which possessed a skeletal morphology common in conodonts [17] and is represented by well-preserved specimens from the Silurian Eramosa Member Lagerstätte, Canada [18]. We analysed eight P1 elements (paired, dedicated food-processing structures at the posterior of the feeding apparatus), using three-dimensional models constructed with synchrotron radiation X-ray computed micro-tomography (SRμCT) on the 20XU beamline of the SPring-8 synchrotron, Japan. Surfaces were extracted from this volume data using Avizo v. 6 (VSG). From these surfaces, three-dimensional sharpness measurements were obtained in Geomagic Studio v. 12 (Geomagic, USA) and two-dimensional angular measurements in ImageJA v. 1.44b [19]. P1 element morphology and the functional variables we measured are illustrated in figure 1. Occlusal models were produced in Blender v. 2.49b (http://www.blender.org). To contextualize our measurements, we compare our data with those acquired from microchiropteran molars [11,14], which overlap the size range of conodont elements and are the only teeth from which comparable data are available. We selected the cusps of a P1 element pair (Royal Ontario Museum 61381) as representative geometries (figure 2) on which to apply FE modelling to test two alternative loading hypotheses: a uniform pressure to either the dorsal cusp edge, simulating a dorsal draw cut, or the ventral cusp edge, simulating a ventral draw cut. Each comprises movement orthogonal to the cusp long axis. These models were created and analysed in Abaqus v. 6.7 (Simulia, USA), based on surfaces imported from Geomagic Studio. See the electronic supplementary material for further details.
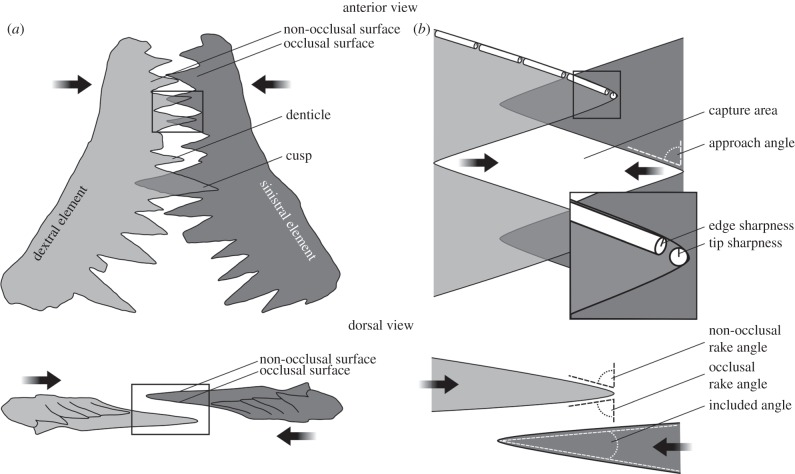
Figure 1.
Functional characteristics of conodont elements, using Wurmiella excavata P1 elements as illustrative example. (a) Occluding element pair, illustrating morphology, with boxed areas enlarged in (b) to show measurements. Arrows indicate probable occlusal vectors for initial occlusal phase (see text). In conodonts, the cusp is defined simply as the structure closest to the point of first growth; the remaining pointed structures are denticles.
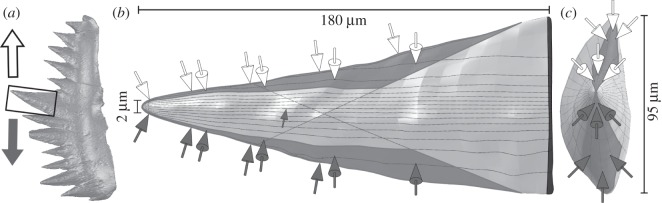
Figure 2.
Model geometry and load parameters for FE analysis of the sinistral Wurmiella excavata P1 element cusp (ROM 61381). (a) Whole element in anterior view, highlighting cusp in boxed area; arrows indicate direction of cusp motion during dorsal (white) and ventral (grey) draw cuts. (a) Anterior and (b) occlusal view of cusp model. Dark grey regions indicate area of applied load and arrows indicate its approximate direction for dorsal (white) and ventral (grey) pressure. Black area in (a) demarcates basal constraints.
3. Results
(a). Element morphology
Our measurements are summarized in table 1 (see the electronic supplementary material for full results). All elements have an included angle of less than 15°; they therefore possess effectively two rake (leading) surfaces, with positive rake angles. The dorsal and ventral surfaces of the cusps and denticles are narrowed to form distinct ridged edges; the element therefore forms a blade. The dorsal edges of the cusp and dorsal process denticles are typically sharper than the ventral edges. The inter-denticle spaces are developed to form sharp, V-shaped notches, sometimes extended into slits. The dorsal denticles and cusp are coaxial, whereas the ventral denticles exhibit a splayed configuration.
Table 1.
Summary values for functional measurements from W. excavata P1 elements from the Eramosa Lagerstätte, Canada. Equivalent data are provided from microchiropteran molars [11,14].
| W. excavata | microchiroptera | |
|---|---|---|
| length (μm) | 270–850 | 1087–2110 |
| tip sharpness (radius of curvature; μm) | 1–3 | 15–40 |
| cusp sharpness (103 μm3 at 100 μm from tip) | 3–42 | 600–1700 |
| edge sharpness (radius of curvature; μm) | 1–4 | 10–20 |
| rake angle (°) | 83–85 | −11–40 |
| approach angle (°) | 60–80 | 24–42 |
(b). Constraints on element occlusion
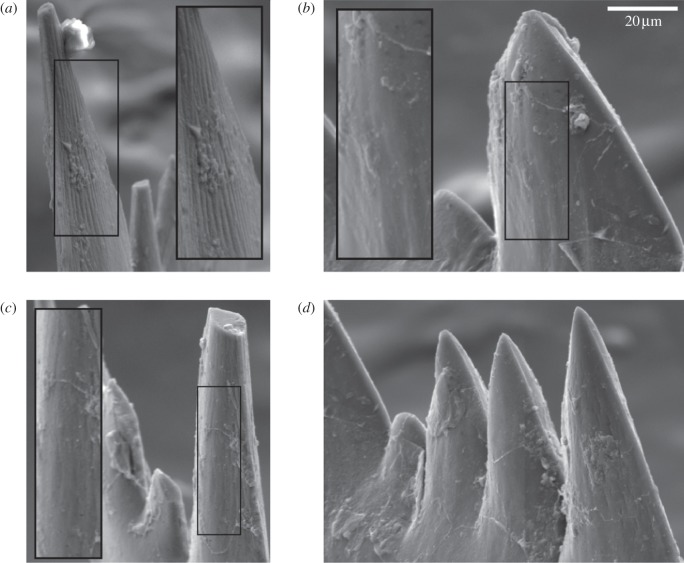
Pristine W. excavata cusps and denticles possess pronounced micro-ornament (figure 3a) that shows frequent evidence of in vivo wear along its entire length (figure 3b,c) on the occlusal surface of the element. This polishing is smooth, exhibiting no microtexture (i.e. pits and scratches). Moreover, wear is present along most of the denticle row on both processes, although its extent diminishes away from the cusp (figure 3d). Lighter wear is also present along both denticle rows on the non-occlusal surface of the cusp and denticles.
Figure 3.
Scanning electron micrographs of W. excavata elements (Silurian, UK). (a) Unworn occlusal surface of cusp; (b,c) occlusal surfaces of two cusps, illustrating worn state; (d) ventral denticle row, showing progressive decrease in extent of wear from proximal to distal (left to right). Scale applies to non-inset images.
The results of the FE analysis of P1 element cusps (figure 4 and table 2) reveal that mean maximum principal stresses are lower when cusps are loaded on the dorsal edge compared to when loaded on the ventral edge.
Figure 4.
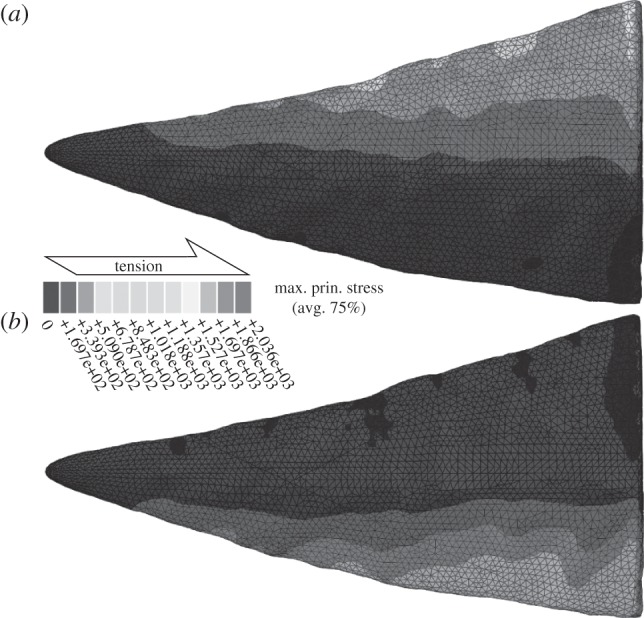
Maximum principal stress patterns in the FE model of the sinistral W. excavata P1 element cusp (ROM 61381) under (a) dorsal and (b) ventral loading. Black areas indicate negative values (compression).
Table 2.
Mean maximum principal stress in the model of the sinistral W. excavata P1 element cusp (ROM 61381) in the FE analyses.
| cusp | element no. | force direction | force (Pa) | mean maximum principal tensile stress (Pa) |
|---|---|---|---|---|
| dextral | 175 156 | dorsal | 101 | 195 |
| dextral | 175 156 | ventral | 100 | 237 |
| sinistral | 205 859 | dorsal | 76 | 84 |
| sinistral | 205 859 | ventral | 100 | 133 |
4. Interpretation of the occlusal cycle
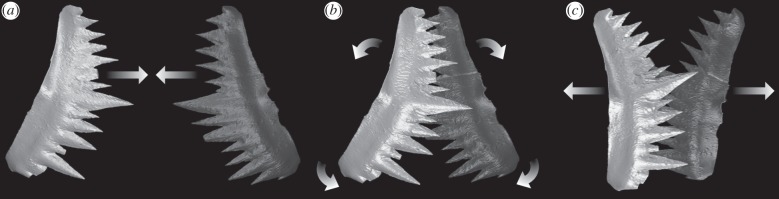
Figure 5 illustrates our occlusal model. Articulated W. excavata P1 elements reveal that the dextral element was positioned anterior to the sinistral [20], as in other conodonts [2]. However, smoothly polished wear is characteristic of element–element attrition [4]. Its presence on the non-occlusal surface suggests that elements sometimes occluded dextral caudal to sinistral and so must have separated during the occlusal cycle (figure 5a). This allows food to move between the elements, which would otherwise be difficult. The development of occlusal wear along the length of both denticle rows requires a rotational component to occlusion, because element curvature would prevent such wear occurring were movement purely orthogonal to the element long axis. The sharper dorsal edges of the cusp and denticles (electronic supplementary material, table S1) indicate that the direction of rotation for the primary power stroke was dorsal. FE analysis tests and supports this conclusion: cusp morphology is better able to resist tensile stress during a dorsally directed draw cut (table 2). This suggests that the elements initially occluded with the dorsal process long axis of each element vertical and parallel. Rotation then separated the dorsal processes, moving the dorsal denticles and cusp upward and outward, and bringing the ventral processes and their denticles into occlusion (figure 5b,c). The extent of occlusal wear reveals that occlusion was deep; i.e. the cusp and denticles overlapped almost completely.
Figure 5.
(a–c) Occlusal cycle in W. excavata P1 elements in anterior view, based on SRμCT models of P1 element pair ROM 61381. Dextral element in light grey, sinistral element in dark grey, arrows indicate movement. See the electronic supplementary material for animation.
Cusp tip spalling in P1 elements with W. excavata morphology further suggests that these elements separated during the occlusal cycle; by analogy with teeth [21], this would also have resulted from element–element contact. Cusp size can estimate the separation distance: dental tools are of similar dimensions or smaller than the food being fractured [22]; using a large cusp to fracture much smaller food items is ineffective. Consistent with this are the typically larger cusps of the P2 elements in W. excavata [20], which are anterior to the P1, encountered food first and, thus, larger fragments. A similar pattern of relative P element cusp sizes is observed in many conodonts.
Initial occlusion in W. excavata takes advantage of the sharp tips of cusps and dorsal denticles (table 1), which reduce the contact area between element and food, concentrating force and increasing penetration efficiency [12,23,24]. The sharp, bladed edges of the cusp and denticles (table 1) functioned similarly. Their influence becomes increasingly important as they enter and so displace a progressively larger volume of the food, thus requiring higher force to maintain penetration, because the edges direct force towards crack propagation rather than plastic deformation [13,24,25]. Likewise, the high cusp sharpness (table 1) reduces the energy required for the cusp or denticle to drive through tough food, needing fewer bonds in the material to be broken or strained, reducing the force required for crack propagation [13]. The high approach angles of the cusp and denticle edges (table 1) increase the elements' mechanical advantage [13] by decreasing the area of contact between the two elements, increasing pressure and decreasing friction. Deep occlusion exploits the acute inter-denticle notches, further concentrating forces [13,26,27], and enclosing the food before it is fully divided, making it more likely to be fractured. Subsequent rotation exploits the sharp tips (table 1) of the splayed ventral denticles.
5. Discussion
(a). Comparison to other conodonts
The P1 element morphology of W. excavata is common in conodonts [17], and so insights derived here can be generalized to furnish functional hypotheses for many species. Similar rotational kinematics have also been proposed for two taxa with morphologically different P1 elements. The ventral processes in Idiognathodus, which form denticulated blades, are interpreted principally to have guided the alignment of the dorsal processes during occlusion, which are expanded into food processing platforms [6]. Nodes on the occlusal surfaces of the ventral processes of P1 elements of Vogelgnathus campbelli were argued to improve food processing, through slicing and cutting food, with rotation about the dorsal processes [28]. In both interpretative models, elements remain in contact throughout the occlusal cycle. However, non-occlusal wear and tip spalling in Idiognathodus suggests separation occurred [5]; the same is probable and testable for V. campbelli. Therefore, our occlusal model of separation and rotation may be general for conodonts. However, whereas the power stoke in V. campbelli was dorsal to ventral, as the evidence suggests for W. excavata, in Idiognathodus it was opposite in sense: ventral to dorsal. It may be that the evolution of platforms was associated with a reversal of initial rotation direction; this is also testable using our approach.
The development of sharp, bladed cusps and denticles suggests facultative consumption of tough, viscoelastic food. To fragment this food, deep occlusion would be required to drive the elements completely through it, as cracks would not self-propagate [22,29]. However, conodonts were not constrained to produce elements with sharp, bladed cusps and denticles: the morphology and orientation of these structures varies greatly, even between processes on a single element. The absence of sharp blades in many conodont species that lack platforms like W. excavata, suggests a reliance on puncture rather than draw cutting in these taxa, and concomitantly orthogonal occlusion with little rotational movement. Our approach provides a means of understanding and testing these scenarios.
(b). Comparison to other vertebrate dentitions
Our analytical approach places conodont elements within a broader dental framework. Convergence with the bladed edges of conodont element cusps and denticles is evident in chiropteran canines [30] and various marine predators [7], and convergence with the inter-denticle notches is apparent in chondrichthyan, theropod dinosaur and mammalian carnassial teeth [31,32]. Although high sharpness renders the elements demonstrably vulnerable to wear and breakage [5], the external appositional growth of elements meant that conodonts could re-sharpen worn tips and edges, and replace damaged cusps and denticles through life. Although this largely releases the constraint on sharpness in conodonts, many gnathostomes could achieve the same result by replacing teeth. The larger absolute muscular force available to gnathostomes for fracturing food probably allows them to apply the same effective pressure to food with the blunter teeth that higher dental stresses necessitate to minimize tooth fracture [24,33].
6. Conclusions
Using quantitative analyses based on engineering approaches applied to conodont food-processing elements, we show that their morphology is adapted to overcome size limitations by developing dental tools of unparalleled sharpness which maximize applied stress. We also show how this morphology is employed in W. excavata using rotational kinematics similar to other conodonts, suggesting that this occlusal style is typical, though not universal, for the clade. Using these techniques to improve our understanding of conodont element function has broad implications. Conodonts provide a resource for establishing the generality of functional principles derived from teeth, and for exploring convergence in food-processing structures from different groups. Their small size places them in a position to elucidate the little-studied effects of scaling in dental morphology [22,34]. The key position they occupied within marine ecosystems means that increased constraints on their diet will allow more comprehensive reconstruction of trophic structure in ancient oceans.
Acknowledgements
We extend grateful thanks to Peter von Bitter and Mark Purnell for specimen loan, and thank Ian Stewart for specimen mounting materials and the two reviewers who provided helpful comments on an earlier version of the manuscript. Work was funded by Marie Curie PIOF-GA-2009-235868 (to D.J.) and the Australian Research Council DP0880120 (to A.R.E.). Travel to Japan was funded by the International Synchrotron Access Programme of the Australian Government, managed by the Australian Synchrotron. Synchrotron Proposal no. 2010A1047 was performed with the approval of the Japan Synchrotron Radiation Research Institute.
References
- 1.Donoghue P. C. J., Forey P. L., Aldridge R. J. 2000. Conodont affinity and chordate phylogeny. Biol. Rev. 75, 191–251 10.1017/s0006323199005472 (doi:10.1017/s0006323199005472) [DOI] [PubMed] [Google Scholar]
- 2.Purnell M. A., Donoghue P. C. J. 1997. Architecture and functional morphology of the skeletal apparatus of ozarkodinid conodonts. Phil. Trans. R. Soc. Lond. B 352, 1545–1564 10.1098/rstb.1997.0141 (doi:10.1098/rstb.1997.0141) [DOI] [Google Scholar]
- 3.Purnell M. A., Donoghue P. C. J. 1998. Skeletal architecture, homologies and taphonomy of ozarkodinid conodonts. Palaeontology 41, 57–102 [Google Scholar]
- 4.Purnell M. A. 1995. Microwear on conodont elements and macrophagy in the first vertebrates. Nature 374, 798–800 10.1038/374798a0 (doi:10.1038/374798a0) [DOI] [Google Scholar]
- 5.Purnell M. A., Jones D. Submitted Quantitative analysis of conodont tooth wear and damage as a test of ecological and functional hypotheses. Paleobiology. [Google Scholar]
- 6.Donoghue P. C. J., Purnell M. A. 1999. Mammal-like occlusion in conodonts. Paleobiology 25, 58–74 [Google Scholar]
- 7.Jeppsson L. 1979. Conodont element function. Lethaia 12, 153–170 10.1111/j.1502-3931.1979.tb00994.x (doi:10.1111/j.1502-3931.1979.tb00994.x) [DOI] [Google Scholar]
- 8.Donoghue P. C. J., Purnell M. A. 1999. Growth, function, and the conodont fossil record. Geology 27, 251–254 (doi:10.1130/0091-7613(1999)027<0251:gfatcf>2.3.co;2) [DOI] [Google Scholar]
- 9.Donoghue P. C. J., Sansom I. J. 2002. Origin and early evolution of vertebrate skeletonization. Microsc. Res. Tech. 59, 352–372 10.1002/jemt.10217 (doi:10.1002/jemt.10217) [DOI] [PubMed] [Google Scholar]
- 10.Rayfield E. J. 2007. Biomechanics and evolution of living and fossil organisms. Annu. Rev. Earth Planet. Sci. 35, 541–576 [Google Scholar]
- 11.Evans A. R. 2005. Connecting morphology, function and tooth wear in microchiropterans. Biol. J. Linn. Soc. 85, 81–96 10.1111/j.1095-8312.2005.00474.x (doi:10.1111/j.1095-8312.2005.00474.x) [DOI] [Google Scholar]
- 12.Evans A. R., Sanson G. D. 1998. The effect of tooth shape on the breakdown of insects. J. Zool. 246, 391–400 10.1017/s0952836998009832 (doi:10.1017/s0952836998009832) [DOI] [Google Scholar]
- 13.Evans A. R., Sanson G. D. 2003. The tooth of perfection: functional and spatial constraints on mammalian tooth shape. Biol. J. Linn. Soc. 78, 173–191 10.1046/j.1095-8312.2003.00146.x (doi:10.1046/j.1095-8312.2003.00146.x) [DOI] [Google Scholar]
- 14.Evans A. R., Sanson G. D. 2005. Correspondence between tooth shape and dietary biomechanical properties in insectivorous microchiropterans. Evol. Ecol. Res. 7, 453–478 [Google Scholar]
- 15.Evans A. R., Sanson G.D. 2005. Biomechanical properties of insects in relation to insectivory: cuticle thickness as an indicator of insect ‘hardness’ and ‘intractability’. Aust. J. Zool. 53, 9–19 10.1071/zo04018 (doi:10.1071/zo04018) [DOI] [Google Scholar]
- 16.Evans A. R., Sanson G. D. 2006. Spatial and functional modeling of carnivore and insectivore molariform teeth. J. Morphol. 267, 649–662 10.1002/jmor.10285 (doi:10.1002/jmor.10285) [DOI] [PubMed] [Google Scholar]
- 17.Sweet W. C. 1988. The conodonta: morphology, taxonomy, paleoecology, and evolutionary history of a long-extinct animal phylum 212 p Oxford, UK: Clarendon Press [Google Scholar]
- 18.von Bitter P. H., Purnell M. A., Tetreault D. K., Stott C. A. 2007. Eramosa Lagerstatte—exceptionally preserved soft-bodied biotas with shallow-marine shelly and bioturbating organisms (Silurian, Ontario, Canada). Geology 35, 879–882 10.1130/g23894a.1 (doi:10.1130/g23894a.1) [DOI] [Google Scholar]
- 19.Rasband W. S. 1997–2011. ImageJ. Bethesda, MD: U.S. National Institute of Health; See http://imagej.nih.gov/ij/. [Google Scholar]
- 20.Jones D., Purnell M. A., Von Bitter P. H. 2009. Morphological criteria for recognising homology in isolated skeletal elements: comparison of traditional and morphometric approaches in conodonts. Palaeontology 52, 1243–1256 10.1111/j.1475-4983.2009.00915.x (doi:10.1111/j.1475-4983.2009.00915.x) [DOI] [Google Scholar]
- 21.Schubert B. W., Ungar P. S. 2005. Wear facets and enamel spalling in tyrannosaurid dinosaurs. Acta Palaeontol. Pol. 50, 93–99 [Google Scholar]
- 22.Popowics T. E., Fortelius M. 1997. On the cutting edge: tooth blade sharpness in herbivorous and faunivorous mammals. Ann. Zool. Fennici 34, 73–88 [Google Scholar]
- 23.Freeman P. W., Weins W.N. 1997. Puncturing ability of bat canine teeth: the tip. In Life among the muses: papers in honor of J. S. Findley (eds Yates T. L., Gannon W. L., Wilson D. E.), pp. 151–157 Albuquerque, NM: Museum of Southwestern Biology [Google Scholar]
- 24.Rensberger J. M. 1995. Determination of stresses in mammalian dental enamel and their relevance to the interpretation of feeding behaviors in extinct taxa. In Functional morphology in vertebrate paleontology (ed. Thomason J. J.), pp. 151–172 Cambridge, UK: Cambridge University Press [Google Scholar]
- 25.Freeman P. W., Lemen C. 2006. Puncturing ability of idealized canine teeth: edged and non-edged shanks. J. Zool. 269, 51–56 10.1111/j.1469-7998.2006.00049.x (doi:10.1111/j.1469-7998.2006.00049.x) [DOI] [Google Scholar]
- 26.Anderson P. S. L. 2009. The effects of trapping and blade angle of notched dentitions on fracture of biological tissues. J. Exp. Biol. 212, 3627–3632 10.1242/jeb.033712 (doi:10.1242/jeb.033712) [DOI] [PubMed] [Google Scholar]
- 27.Anderson P. S. L., LaBarbera M. 2008. Functional consequences of tooth design: effects of blade shape on energetics of cutting. J. Exp. Biol. 211, 3619–3626 10.1242/jeb.020586 (doi:10.1242/jeb.020586) [DOI] [PubMed] [Google Scholar]
- 28.Purnell M. A., von Bitter P. H. 1992. Blade-shaped conodont elements functioned as cutting teeth. Nature 359, 629–631 10.1038/359629a0 (doi:10.1038/359629a0) [DOI] [Google Scholar]
- 29.Lucas P. W. 2004. Dental functional morphology: how teeth work, 355 p Cambridge, UK: Cambridge University Press [Google Scholar]
- 30.Freeman P. W. 1992. Canine teeth of bats (Microchiroptera): size, shape and role in crack-propagation. Biol. J. Linn. Soc. 45, 97–115 10.1111/j.1095-8312.1992.tb00634.x (doi:10.1111/j.1095-8312.1992.tb00634.x) [DOI] [Google Scholar]
- 31.Abler W. L. 1992. The serrated teeth of tyrannosaurid dinosaurs, and biting structures in other animals. Paleobiology 18, 161–183 [Google Scholar]
- 32.Frazzetta T. H. 1988. The mechanics of cutting and the form of shark teeth (Chondrichthyes, Elasmobranchii). Zoomorphology 108, 93–107 10.1007/bf00539785 (doi:10.1007/bf00539785) [DOI] [Google Scholar]
- 33.Rensberger J. M. 2000. Pathways to functional differentiation in mammalian enamel. In Development, function and evolution of teeth (eds Teaford M. F., Smith M. M., Ferguson M. W. J.), pp. 252–268 Cambridge, UK: Cambridge University Press [Google Scholar]
- 34.Evans A. R., Hunter J., Fortelius M., Sanson G. D. 2005. The scaling of tooth sharpness in mammals. Ann. Zool. Fennici 42, 603–613 [Google Scholar]