Abstract
Environmental changes currently pose severe threats to biodiversity, and reintroductions and translocations are increasingly used to protect declining populations and species from extinction. Theory predicts that establishment success should be higher for more variable groups of dissimilar individuals. To test this ‘diversity promotes establishment’ hypothesis, we introduced colour polymorphic pygmy grasshoppers (Tetrix subulata) to different sites in the wild. The number of descendants found at the release sites the subsequent year increased with increasing number of colour morphs in the founder group, and variation in founder groups also positively affected colour morph diversity in the established populations. Since colour morphs differ in morphology, physiology, behaviour, reproductive life history and types of niche used, these findings demonstrate that variation among individuals in functionally important traits promotes establishment success under natural conditions, and further indicate that founder diversity may contribute to evolutionary rescue and increased population persistence.
Keywords: biodiversity, conservation biology, evolution, polymorphism, population establishment, Tetrix subulata
1. Introduction
Larger founder groups [1] and repeated introductions [2] are known to increase the odds of successful establishment. Theory predicts that establishment success should also be higher for more variable groups [3–6]. Different mechanisms, operating on different time scales, may contribute to positive effects of genetic diversity. Highly diverse groups may be more successful because they are more likely, for purely statistical reasons, than less diverse groups to harbour productive or pre-adapted genotypes (i.e. a sampling effect), and also because standing genetic variance increases the likelihood of evolutionary rescue [7]. The increased predictability and decreased variance in number of survivors in more diverse groups of Gammarus duebeni amphipods [8] and Tetrix subulata pygmy grasshoppers [9] is consistent with such a sampling effect. If different phenotypes occupy different niches or facilitate the success of other phenotypes, this may lead to more efficient utilization of a broader range of available resource space (i.e. niche complementarity) [10]. In a longer perspective, selection may increase the frequency of specific alleles and high-fitness genotypes present among founding individuals, and sexual recombination may generate novel and more advantageous allele combinations, and thereby allow for continued selection and evolutionary refinement [5,11].
Despite a growing interest among ecologists and conservation biologists in population-level consequences of phenotypic variation and polymorphism [3–6,10,12–14], little experimental evidence is available to confirm that establishment success is higher for groups of dissimilar individuals [15,16], and the role of variation in functionally important phenotypic traits for establishment has never been examined under natural conditions in the wild. Tetrix subulata pygmy grasshoppers (Orthoptera: Tetrigidae) are well suited for such a study. They are small (body length up to 15 mm, average dry body mass approx. 0.07 g), diurnal insects that inhabit biomes ranging from tropical rainforests to arctic regions of Europe, Asia and North to South America (Mexico) [17,18]. Pygmy grasshoppers provide one of the classic examples of colour polymorphism, and intraspecific variation has been documented and analysed for almost a century [19,20]. Colour and pattern in T. subulata are genetically influenced and not affected by developmental plasticity (see Forsman et al. [19] and references therein). Alternative colour variants of Tetrix differ also in temperature preferences and physiology, body size, reproductive life history, predator-avoidance behaviours, microhabitat utilization and diet [21–24]. It has also been demonstrated that colour patterns affect the susceptibility of pygmy grasshoppers to predators, and that relative crypsis of alternative morphs depends on the visual properties of their microhabitats [23,25]. These colour variants thus represent different eco-morphs that vary in ability to cope with and exploit environmental resources, such that they occupy different niches [4]. Differences among populations and shifts of colour morph frequencies within populations demonstrate that polymorphic pygmy grasshoppers may undergo rapid evolutionary modifications in response to environmental changes [19], but whether more variable groups are better able to colonize and establish in novel habitats has not been previously investigated.
Pygmy grasshoppers are well suited for introduction experiments. Tetrix subulata has a high reproductive capacity; each female may produce several egg pods per season, consisting up to 35 eggs per pod. They have a polygynandrous mating system and females mated to several males may produce half-sibling offspring that are more colour morph diverse [9,20]. Like many other insects, T. subulata are wing dimorphic [18,20], and macropterous individuals with functional wings are capable of active flights of up to 100 m. However, mark–recapture data of free-ranging individuals indicate that T. subulata are sedentary and normally move only a few metres per day [23]. They live on the soil's surface where they feed mainly on algae, mosses, and dead, partly decayed plant and animal matter in the litter [24], and are easy to find and capture.
To explore if among individual variation promotes establishment success, we combined T. subulata pygmy grasshopper individuals that belonged to the same or to alternative colour morphs into founder groups, introduced them to different sites in the field, and then tested for an effect of number of alternative colour morphs included in the founder group on number of individuals present at the introduction sites of the next generation.
2. Material and methods
(a). Experimental design and source populations
We introduced 396 adult Tetrix subulata pygmy grasshoppers distributed among 61 experimental founder groups to different natural sites in southeast Sweden. We used the entire range of colour morph variation in the founder groups, from one single morph to either six (distributed among six individuals in 2008 and 2009) or seven (distributed among seven individuals in 2007) different morphs. In our experiment, we did not opt for complete combinatorial replication in pure and mixed groups of only a small subset of morphs available in natural populations to specifically disentangle the contribution of selection versus complementarity to effects of variation on establishment [10]. Instead, we used different levels of variation, and a range of morphs that matched approximately the relative frequencies encountered in the natural source populations. In the least diverse groups, all six (in 2008 and 2009) or seven (in 2007) individuals within a group belonged to a single colour morph. To avoid any differences in viability or reproductive capacity among colour morphs per se being mistakenly interpreted as an effect of level of variation, we did not use the same colour morphs in all homogeneous groups. In the most variable groups, all individuals belonged to six (in 2008 and 2009) or seven (in 2007) different colour morphs. We prioritized replicates with either low or high variation, at the expense of founder groups with an intermediate number of morphs. The exact combination of colour morphs included within each group was chosen at random, but subject to the constraint imposed by their relative availability in the source populations.
The experiment was replicated in 3 years. In 25–28 April 2007, founder groups (n = 30) consisted of seven (four females and three males) individuals that originated from a burnt clearcut area near Kosta (N 56°51′37′′, E 15°35′ 64′′) and were released in clearcut, meadows and pastures. In May 2008, founder groups (n = 15) consisted of six (four females and two males) individuals from Bredsättra, a pasture on the island of Öland (N 56°50′96′′, E 16°47′37′′), and were released in coastal meadows. In May 2009, founder groups (n = 16) consisted of six (four females and two males) individuals originating either from Bredsättra or Jordtorpsåsen on Öland (N 56°40′61′′, E 16°33′34′′), and were released in pastures and coastal meadows. Because all habitat types were not represented all years, we cannot separate effects of habitat type on establishment from effects of year of release. Since founder groups were released in random order among sites (see below), any effect on establishment success of habitat type per se would increase the level of variation in our data, but not systematically bias our results and conclusions.
All individuals within a founder group always originated from the same source population. Prior to release, two persons carefully searched each potential release site for grasshoppers during 10 min under sunny weather conditions with a temperature of at least 15°C. The size of searched area was about 50 × 50 m, with some variation among sites depending on the characteristics and spatial scale of habitat heterogeneity. If no T. subulata was observed, the site was either used as an unmanipulated reference site (see below) or as an experimental site, in which case a randomly assigned founder group was released together with the soil in the bucket, since this could contain egg pods, and the position was registered with a GPS receiver. The individuals were released together at the same spot, in the centre of the area that had been searched. If T. subulata were observed during the survey, the area was used as a reference site. Release sites were separated by approximately 1 km or more.
To estimate establishment success, we revisited all release sites 1 year after the introductions. Two persons (A.F. and L.W.) searched for T. subulata for 10 min at each site (during 6–9 May 2008, 25–26 May 2009, and 18, 20, 24 and 28 May 2010), and counted and classified all individuals by sex and colour morph.
To quantify the abundance and estimate colour morph diversity in unmanipulated natural T. subulata populations, we surveyed 36 reference sites, each in two consecutive years, using the same procedure as for experimental release sites.
(b). Statistical analyses
We used generalized linear mixed models (GLMMs) implemented using procedure GLIMMIX in SAS [26] to test for effects of year of introduction (2007, 2008 or 2009) and diversity (number of colour morphs, 1–7) within the founder group on establishment success (number of individuals present at the release site 1 year after the introductions of founder groups). We modelled establishment success using a Poisson distribution, suitable for analysing data in the form of counts. We hypothesized that, within the range of diversity levels used in our experiment, establishment success should increase with increasing number of morphs in the founder groups. Since our treatment included all possible levels of colour morph diversity (given the size of founder groups), diversity was treated as a fixed effect. Year was treated as a random effect. We report the results from the saturated most complex model that included the two explanatory effects and the interaction. In addition, we report the effect of diversity as estimated from the simplest possible model that included only number of colour morphs released. We used the Kenward–Roger method to approximate degrees of freedom [26].
The average number of grasshoppers caught varied among the 4 years of study, both at experimental release sites and at unmanipulated reference sites. For a visual representation of the relationship linking establishment success to colour morph diversity in founder groups, we therefore used standardized values and expressed the number of individuals captured at each site as deviations from the mean number of captured individuals in that year (figure 1b).
Figure 1.
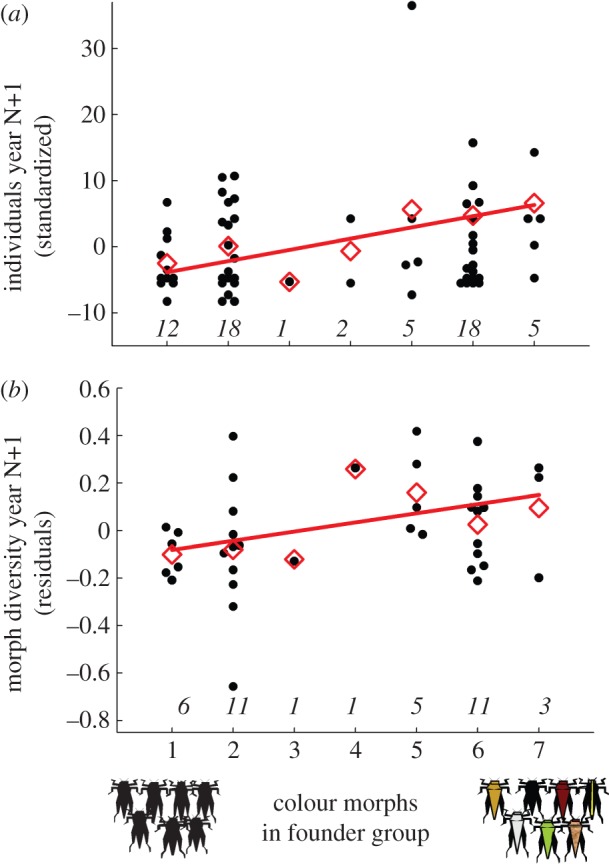
(a) Number of Tetrix subulata pygmy grasshopper individuals captured at experimental introduction sites as a function of number of colour morphs included in founder groups released the previous year. The graph shows pooled data for 3 years, and values are standardized by year. (b) Colour morph diversity in experimental populations as a function of number of colour morphs included in the founder group. Colour morph diversity is expressed as residuals from year-specific least-squares linear regression of log number of colour morphs on log number of individuals. Red diamonds indicate mean values. Numbers in italics above horizontal axes indicate sample sizes (i.e. number of replicates). Overlapping data points are jittered in the x-axis direction.
To test if the number of colour morphs included in founder groups influenced colour morph diversity in established experimental populations, we first estimated colour morph diversity in each sample, expressed as residuals from year-specific least-squares linear regression of log number of colour morphs on log number of individuals. The relationship linking number of colour morphs to number of individuals in experimental populations did not vary among years (as evidenced by a non-significant effect of the interaction between year and number of individuals; F2,32 = 1.77, p = 0.19). In this approach, positive and negative residuals indicate that the sample contained more or fewer colour morphs than expected for the number of individuals. We then evaluated the association between colour morph diversity and number of colour morphs in the founder group using Pearson correlation analysis. The null hypothesis that the relative frequency of different colour morphs in experimentally established populations was independent of the relative frequency of colour morphs among individuals in the founder groups released 1 year earlier and was evaluated using contingency table analysis.
3. Results
(a). Evaluating effects of founder diversity on establishment success
If individuals vary in functionally important traits and use different niches, then more diverse founder groups should be better able to cope with heterogeneous and changing environments and colonize novel habitats. Our results were in agreement with this prediction. When the 61 introduction sites were surveyed after 1 year, we found 354 individuals, yielding an average of 5.8 (range 0–42) individuals per site. The number of grasshoppers captured during revisits to experimental populations increased with increasing colour morph diversity in the introduced founder groups (GLMM, Poisson regression, effect of number of colour morphs included in founder group: F1,57 = 12.47, p < 0.001; figure 1a). The number of grasshoppers in experimental populations also differed between years, in a manner similar to the variation seen in unmanipulated reference areas (figure 2 and below). The relationship linking colour morph diversity in founder groups to establishment success was independent of year of introduction (as evidenced by the non-significant effect of the interaction between year and number of colour morphs released; GLMM: F2, 9.165 = 0.72, p = 0.51), indicating that the positive effect of founder diversity was repeatable in time and across habitat types. The main result was robust and independent of choice of statistical model; the effect of diversity remained highly significant when the interaction and the random effect of year of release were omitted in favour of the simplest possible model that included only number of colour morphs released (F1,59 = 8.16, p = 0.0059).
Figure 2.
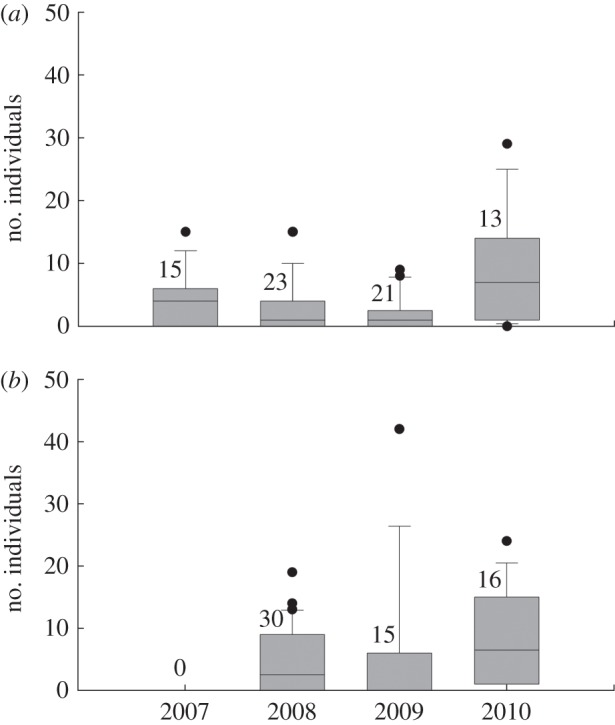
Number of Tetrix subulata pygmy grasshopper individuals captured during standardized searching effort (10 min by two people) (a) in natural populations at unmanipulated references sites in four different years and (b) at experimental sites where founder groups had been introduced the previous year. The figure is based on number of individuals captured. Numbers above boxes indicate sample sizes. Solid lines within boxes indicate median, the boundaries of the box indicate 25th and 75th percentiles, whiskers below and above indicate 10th and 90th percentiles, and dots indicate outlying observations.
(b). Evaluating effects on diversity in established populations
Variation in founder groups also influenced diversity in established populations, indicating that effects of variation may carry over across generations. Relative colour morph diversity in experimental populations (expressed as residuals from the log–log least-squares linear regression of number of colour morphs on number of captured individuals) increased with increasing number of colour morphs included in the introduced founder groups (r = 0.34, n = 38, p = 0.038; figure 1b).
The distribution of individuals among the 21 different colour morph categories in the sample collected from the 38 established populations 1 year after the introductions was different from the distribution among individuals included in the sample that comprised the 61 founding groups (χ2 = 146.36, d.f. = 20, p < 0.0001), indicating that some colour morphs increased and others decreased in relative frequency between generations. The largest shift in relative frequency was observed for the black colour morph, which declined from 16 per cent in founder groups to 3 per cent in the established populations after 1 year (see the electronic supplementary material, figure S1).
(c). Evaluating effects of among-year variation
The average number of grasshoppers captured at unmanipulated reference areas varied from 2.0 to 8.5 individuals per site among the 4 different years (Kruskal–Wallis test, χ2 = 8.7, d.f. = 3, p = 0.033; figure 2a). Differences among years were evident also in the experimental populations (figure 2b). Overall, the number of grasshoppers increased more between consecutive years in sites where experimental founder groups had been released (mean change ± s.d., 5.9 ± 7.7, range 0–42 individuals, n = 61) than in unmanipulated reference sites (2.9 ± 6.7, range −7 to 22 individuals, n = 36; Wilcoxon two-sample test, Z = 2.28, p = 0.022).
(d). Comparison of morph diversity between experimental and unmanipulated populations
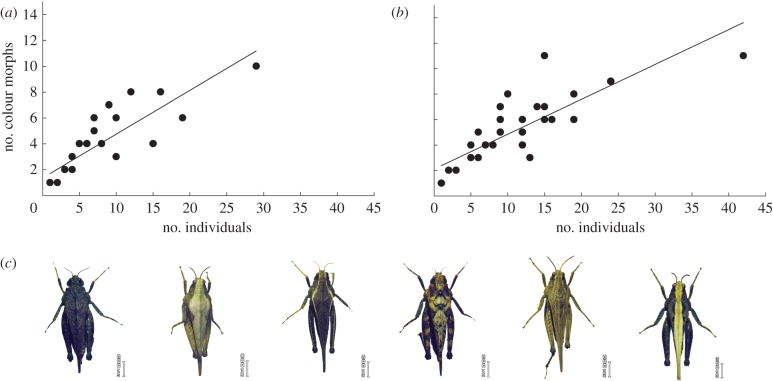
The relationship linking number of colour morphs to number of individuals did not differ between experimental and unmanipulated populations (analysis of covariance on log–log transformed data, effect on number of colour morphs of the interaction between natural versus experimental population category and number of individuals: F1,59 = 1.63, p = 0.21; main effect of population category: F1,59 = 2.29, p = 0.14; figure 3).
Figure 3.
Colour morph diversity in (a) natural and (b) experimentally introduced populations of Tetrix subulata pygmy grasshoppers. The graphs show the relationship between number of different colour morphs and number of T. subulata individuals in samples from natural populations in unmanipulated references sites (n = 27 sites), and experimental populations established by founder groups that were introduced the previous year (n = 38). Lines indicate fitted least-squares linear regressions. (c) Photo showing examples of different pygmy grasshopper colour morphs. Photo: A. Forsman.
4. Discussion
Are more variable groups better able to colonize and establish in novel habitats, as predicted by theory [3–6]? We found that across 61 experimental introduction sites, establishment success, measured as number of descendants found at the points of release the subsequent year, increased with increasing number of colour morphs in the founder group (figure 1a). That the relationship linking colour morph diversity in founder groups to establishment success was independent of year of introduction indicates that the positive effect of founder diversity was repeatable in time and across habitat types. Since pygmy grasshopper colour morphs differ in morphology, physiology, behaviour, reproductive life-history and use different niches [22,23,27], these findings provide rare empirical evidence—in line with predictions [3–6]—that variation among individuals in functionally important traits promotes establishment success under natural conditions in the wild. We also found that variation in founder groups positively affected colour morph diversity in the established populations, indicating that founder diversity may also contribute to increased population persistence [5,7,28]. Association of colour pattern with other kinds of traits owing to genetic, developmental or functional associations are manifest in several species [29,30], and our results for pygmy grasshoppers may therefore be relevant also for other organisms.
(a). Why does diversity promote establishment success?
Several mechanisms may have contributed to the superior establishment of more diverse founder groups. The shifts in relative morph frequencies seen between individuals in founding groups and established populations are indicative of selection, but may also reflect differences among morphs in degree of parent–offspring resemblance. We consider selection for camouflage mediated by differential predation [19,23,31,32] to be one of the most important mechanisms behind the observed pattern. Their small size and locally high population densities render grasshoppers susceptible to visual predators such as birds [31,33] and lizards [34]. Tsurui et al. [25], using humans as dummy predators, demonstrated that differences in relative crypsis among alternative T. japonica pygmy grasshopper colour morphs change across different visual backgrounds (sand versus grass). There are also experimental demonstrations of phenotype-dependent predation in pygmy grasshoppers—including our own previous work based on manipulation of colour patterns and its effect on subsequent survival of free-ranging T. subulata individuals under natural conditions in the wild, as well as staged predator–prey encounters in the laboratory—that colour pattern influences survival [23,34,35]. If more colour variants are introduced in a novel environment, the probability increases that at least one morph will be well protected against predators [36]. The coexistence of two or more colour variants may also reduce predator efficiency and increase survival in polymorphic prey populations [37,38]. We therefore propose that the superior establishment success of more variable founder groups is driven at least in part by a lower rate of predator-induced mortality in more colour-morph-diverse founder groups.
The positive effect of diversity on establishment may have been mediated not only by predation or other factors operating on colour pattern per se. Differential survival or reproductive success owing to selection on traits that are genetically, developmentally or functionally associated with colour pattern [29,30] may also have contributed to our finding. Pygmy grasshopper colour variants differ in a suite of correlated and functionally important morphological, physiological, behavioural and life-history traits [21–23,39]. Superior success of more variable groups is therefore expected also if greater generalism (at the level of the group when summed across different individuals) with regard to thermal requirements, dietary preferences or life-history characteristics promotes establishment [4]. Finally, there is experimental evidence that, at high density, survival of captive pygmy grasshopper individuals is enhanced by high phenotypic diversity, indicating that resource utilization is more efficient and competition less manifest in polymorphic groups [40].
It is possible that inbreeding depression contributed to the reduced success of homogeneous founder groups, under the assumption that individuals in founding groups with few colour morphs were more closely related on average than individuals in groups with many morphs. However, since T. subulata siblings from within a single clutch may represent at least five different colour morphs [9], this seems unlikely.
(b). Founder diversity may contribute to population persistence
Our results show that colour morph diversity in the established experimental populations increased with increasing number of colour morphs included in the introduced founder groups (figure 1b). This result points to the conclusion that the effect of diversity on establishment success was not only owing to greater chances of including more successful genotypes in diverse founder groups, since diversity should decrease as a result of evolutionary rescue. One would expect descendant diversity to be positively related to founder diversity in the complete absence of selection, but such an interpretation is incongruent with the observed shifts in relative morph frequencies between generations. The positive relationship between diversity in founding groups and established populations may also reflect niche complementarity [10] or social heterosis [41], if different morphs occupy different niches or facilitate the success of other morphs. Since phenotypically and genetically more variable populations are hypothesized to be better able to withstand and adapt to changing environmental conditions [3–5,7,12,13], this finding suggests that variation in founder groups not only promotes establishment success; the effect may carry over across generations and contribute to increased population persistence.
(c). It is unlikely that the association between founder diversity and establishment success is compromised by immigration
Since T. subulata live for one reproductive season only, the individuals found in established populations were not survivors from the experimental founder groups, but instead represented their descendants. Founder groups were not introduced outside of the natural distribution range, and we therefore cannot discard the possibility that individuals may have immigrated into the experimental areas after the introductions. The influence of any such immigration is difficult to envisage because genetic admixture resulting from interbreeding between individuals from different populations may have either positive or negative effects [6,42,43].
Comparisons of the number and diversity of grasshoppers found at experimental and unmanipulated reference sites suggest that our results were not compromised by immigration. The reference sites were surveyed during the same time of season, on days with similar weather conditions and using a standardized search effort. That different numbers of grasshoppers were captured in different years (figure 2) therefore probably reflects a combination of spatial variation in abundance and natural fluctuations in population size. Despite uncontrolled variation possibly associated with immigration and fluctuating population sizes, the number of grasshoppers increased more between consecutive years in sites where experimental founder groups had been released than in unmanipulated reference sites. Even if grasshoppers may have immigrated to experimental sites after the introductions, this finding demonstrates that establishment of founder groups positively influenced local population size. Furthermore, if experimental populations established by founder groups had been influenced by immigrants, colour morph diversity should have been greater in experimental populations (because of the additive effect of combining individuals originating from different source populations) than in un-manipulated populations. However, we found that colour morph diversity was similar in experimental and un-manipulated populations (figure 3). Taken together, these results suggest that many of the founding groups resulted in successful establishment of novel populations.
5. Conclusions
Our findings provide rare experimental evidence, in line with predictions from theory [3–6], that genetic diversity and variation in functionally important phenotypic traits among individuals promotes establishment success of founder groups under natural conditions in the wild. Furthermore, we found that colour morph diversity was larger in populations established by more variable groups, indicating that the effects of founder variation may carry over across generations and also influence long-term persistence. These findings may help explain why, despite the eroding effects of founder events and drift in small peripheral populations, the amount of genetic variation in marginal populations of some species is surprisingly high when compared with central parts of the distribution range [6,15]. Our results also have implications for conservation biology, since taking variation into consideration may improve the success of re-introduction and translocation projects aiming to protect endangered populations, species and communities [4,5,14]. In this context, it is interesting that species with variable colour patterns have larger geographical range distributions and occupy more diverse habitat types compared with less variable species [44,45]. Finally, that some of the most well-known invasive species (e.g. the harlequin ladybird Harmonia axyridis, the zebra mussel Dreissena polymorpha, the Spanish slug Arion vulgaris and the lizard Anolis sagrei) are characterized by high levels of intraspecific (colour pattern) variation [13,46,47] indicates that information on the level of variation may help identify potential invading species before they become invasive.
Acknowledgements
We are grateful to Sara Forsman for assistance in the laboratory and field. J. Höglund, J. Merilä and two anonymous reviewers commented on the manuscript. This work was supported by the Linnaeus University Centre for Ecology and Evolution in Microbial model Systems (EEMiS). The study was funded by The Swedish Research Council, The Swedish Research Council Formas (grants to A.F.) and Linnaeus University.
References
- 1.Drake J. M., Baggenstos P., Lodge D. M. 2005. Propagule pressure and persistence in experimental populations. Biol. Lett. 22, 480–483 10.1098/rsbl.2005.0375 (doi:10.1098/rsbl.2005.0375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lockwood J. L., Cassey P., Blackburn T. 2005. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 20, 223–228 10.1016/j.tree.2005.02.004 (doi:10.1016/j.tree.2005.02.004) [DOI] [PubMed] [Google Scholar]
- 3.Bolnick D. I., Svanbäck R., Fordyce J. A., Yang L. H., Davis J. M., Hulsey C. D., Forister M. L. 2003. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28 10.1086/343878 (doi:10.1086/343878) [DOI] [PubMed] [Google Scholar]
- 4.Forsman A., Ahnesjö J., Caesar S., Karlsson M. 2008. A model of ecological and evolutionary consequences of color polymorphism. Ecology 89, 34–40 10.1890/07-0572.1 (doi:10.1890/07-0572.1) [DOI] [PubMed] [Google Scholar]
- 5.Hughes A. R., Inouye B. D., Johnson M. T. J., Underwood N., Vellend M. 2008. Ecological consequences of genetic diversity. Ecol. Lett. 11, 609–623 10.1111/j.1461-0248.2008.01179.x (doi:10.1111/j.1461-0248.2008.01179.x) [DOI] [PubMed] [Google Scholar]
- 6.Simberloff D. 2009. The role of propagule pressure in biological invasions. Ann. Rev. Ecol. Evol. Syst. 40, 81–102 10.1146/annurev.ecolsys.110308.120304 (doi:10.1146/annurev.ecolsys.110308.120304) [DOI] [Google Scholar]
- 7.Bell G., Gonzalez A. 2009. Evolutionary rescue can prevent extinction following environmental change. Ecol. Lett. 12, 942–948 10.1111/j.1461-0248.2009.01350.x (doi:10.1111/j.1461-0248.2009.01350.x) [DOI] [PubMed] [Google Scholar]
- 8.Gamfeldt L., Kallstrom B. 2007. Increasing intraspecific diversity increases predictability in population survival in the face of perturbations. Oikos 116, 700–705 10.1111/j.0030-1299.2007.15382.x (doi:10.1111/j.0030-1299.2007.15382.x) [DOI] [Google Scholar]
- 9.Forsman A., Ahnesjö J., Caesar S. 2007. Fitness benefits of diverse offspring in pygmy grasshoppers. Evol. Ecol. Res. 9, 1305–1318 [Google Scholar]
- 10.Reusch T. B. H., Ehlers A., Hämmerli A., Worm B. 2005. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc. Natl Acad. Sci. USA 102, 2826–2831 10.1073/pnas.0500008102 (doi:10.1073/pnas.0500008102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agashe D., Falk J. J., Bolnick D. I. 2011. Effects of founding genetic variation on adaptation to a novel resource. Evolution 65, 2481–2491 10.1111/j.1558-5646.2011.01307.x (doi:10.1111/j.1558-5646.2011.01307.x) [DOI] [PubMed] [Google Scholar]
- 12.Chevin L.-M., Lande R., Mace G. M. 2010. Adaptation, plasticity and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357. 10.1371/journal.pbio.1000357 (doi:10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolbe J. J., Glor R. E., Schettino L. R. G., Lara A. C., Larson A., Losos J. B. 2004. Genetic variation increases during biological invasion by a Cuban lizard. Nature 431, 177–181 10.1038/nature02807 (doi:10.1038/nature02807) [DOI] [PubMed] [Google Scholar]
- 14.Naeem S., Bunker D. E., Hector A., Loreau M., Perrings C. (ed.) 2009. Biodiversity, ecosystem functioning, and human wellbeing: an ecological and economic perspective. Oxford, UK: Oxford University Press [Google Scholar]
- 15.Crawford K. M., Whitney K. D. 2010. Population genetic diversity influences colonization success. Mol. Ecol. 19, 1253–1263 10.1111/j.1365-294X.2010.04550.x (doi:10.1111/j.1365-294X.2010.04550.x) [DOI] [PubMed] [Google Scholar]
- 16.Gamfeldt L., Wallen J., Jonsson P. R., Berntsson K. M., Havenhand J. N. 2005. Increasing intraspecific diversity enhances settling success in a marine invertebrate. Ecology 86, 3219–3224 10.1890/05-0377 (doi:10.1890/05-0377) [DOI] [Google Scholar]
- 17.Holst K. T. 1986. The Saltatoria of northern Europe. Fauna Entomol. Scand. 16, 1–127 [Google Scholar]
- 18.Rehn J. A. G., Grant H. J. J. 1955. Tetrix subulata (Orthoptera; Acridoidea; Tetrigidae) as occurring in North America. Proc. Acad. Nat. Sci. Phila. 107, 145–165 [Google Scholar]
- 19.Forsman A., Karlsson M., Wennersten L., Johansson J., Lapid E. 2011. Rapid evolution of fire melanism in replicated populations of pygmy grasshoppers. Evolution 65, 2530–2540 10.1111/j.1558-5646.2011.01324.x (doi:10.1111/j.1558-5646.2011.01324.x) [DOI] [PubMed] [Google Scholar]
- 20.Nabours R. K. 1929. The genetics of the Tettigidae. Bib. Genet. 5, 27–104 [Google Scholar]
- 21.Ahnesjö J., Forsman A. 2006. Differential habitat selection by pygmy grasshopper color morphs; interactive effects of temperature and predator avoidance. Evol. Ecol. 20, 235–257 10.1007/s10682-006-6178-8 (doi:10.1007/s10682-006-6178-8) [DOI] [Google Scholar]
- 22.Forsman A. 1999. Reproductive life history variation among colour morphs of the pygmy grasshopper Tetrix subulata. Biol. J. Linn. Soc. 67, 247–261 10.1111/j.1095-8312.1999.tb01863.x (doi:10.1111/j.1095-8312.1999.tb01863.x) [DOI] [Google Scholar]
- 23.Forsman A., Appelqvist S. 1999. Experimental manipulation reveals differential effects of colour pattern on survival in male and female pygmy grasshoppers. J. Evol. Biol. 12, 391–401 10.1046/j.1420-9101.1999.00041.x (doi:10.1046/j.1420-9101.1999.00041.x) [DOI] [Google Scholar]
- 24.Karpestam E., Forsman A. 2011. Dietary differences among colour morphs of pygmy grasshoppers revealed by behavioural experiments and stable isotopes. Evol. Ecol. Res. 13, 461–477 [Google Scholar]
- 25.Tsurui K., Honma A., Nishida T. 2010. Camouflage effects of various colour-marking morphs against different microhabitat backgrounds in a polymorphic pygmy grasshopper Tetrix japonica. PLoS ONE 5, e11446. 10.1371/journal.pone.0011446 (doi:10.1371/journal.pone.0011446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Littell R. C., Milliken G. A., Stroup W. W., Wolfinger R. D., Schabenberger O. 2006. SAS for mixed models. Cary, NC: SAS Institute Inc [Google Scholar]
- 27.Forsman A. 2000. Some like it hot: intra-population variation in behavioral thermoregulation in color-polymorphic pygmy grasshoppers. Evol. Ecol. 14, 25–38 10.1023/A:1011024320725 (doi:10.1023/A:1011024320725) [DOI] [Google Scholar]
- 28.Agashe D. 2009. The stabilizing effect of intraspecific genetic variation on population dynamics in novel and ancestral habitats. Am. Nat. 174, 255–267 10.1086/600085 (doi:10.1086/600085) [DOI] [PubMed] [Google Scholar]
- 29.McKinnon J. S., Pierotti M. E. R. 2010. Colour polymorphism and correlated characters: genetic mechanisms and evolution. Mol. Ecol. 19, 5101–5125 10.1111/j.1365-294X.2010.04846.x (doi:10.1111/j.1365-294X.2010.04846.x) [DOI] [PubMed] [Google Scholar]
- 30.True J. R. 2003. Insect melanism: the molecules matter. Trends Ecol. Evol. 18, 640–647 10.1016/j.tree.2003.09.006 (doi:10.1016/j.tree.2003.09.006) [DOI] [Google Scholar]
- 31.Isley F. B. 1938. Survival value of acridian protective coloration. Ecology 19, 370–389 10.2307/1930592 (doi:10.2307/1930592) [DOI] [PubMed] [Google Scholar]
- 32.Mullen L. M., Vignieri S. N., Gore J. A., Hoekstra H. 2009. Adaptive basis of geographic variation: genetic, phenotypic and environmental differences among beach mouse populations. Proc. R. Soc. B 276, 3809–3818 10.1098/rspb.2009.1146 (doi:10.1098/rspb.2009.1146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bock C. E., Bock J. H., Grant M. 1992. Effects of bird predation on grasshopper densities in an Arizona grassland. Ecology 73, 1706–1717 10.2307/1940022 (doi:10.2307/1940022) [DOI] [Google Scholar]
- 34.Civantos E., Ahnesjö J., Forsman A., Martin J., Lopez P. 2004. Indirect effects of prey coloration on predation risk: pygmy grasshoppers versus lizards. Evol. Ecol. Res. 6, 201–213 [Google Scholar]
- 35.Forsman A., Appelqvist S. 1998. Visual predators impose correlational selection on prey color pattern and behavior. Behav. Ecol. 9, 409–413 10.1093/beheco/9.4.409 (doi:10.1093/beheco/9.4.409) [DOI] [Google Scholar]
- 36.Bond A. B. 2007. The evolution of color polymorphism: crypticity, searching images, and apostatic selection. Ann. Rev. Ecol. Syst. 38, 489–514 10.1146/annurev.ecolsys.38.091206.095728 (doi:10.1146/annurev.ecolsys.38.091206.095728) [DOI] [Google Scholar]
- 37.Glanville P. W., Allen J. A. 1997. Protective polymorphism in populations of computer-simulated moth-like prey. Oikos 80, 565–571 10.2307/3546630 (doi:10.2307/3546630) [DOI] [Google Scholar]
- 38.Wennersten L., Forsman A. 2009. Does colour polymorphism enhance survival of prey populations? Proc. R. Soc. B 276, 2187–2194 10.1098/rspb.2009.0252 (doi:10.1098/rspb.2009.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forsman A., Ringblom K., Civantos E., Ahnesjö J. 2002. Coevolution of color pattern and thermoregulatory behavior in polymorphic pygmy grasshoppers Tetrix undulata. Evolution 56, 349–360 [DOI] [PubMed] [Google Scholar]
- 40.Caesar S., Karlsson M., Forsman A. 2010. Diversity and relatedness enhance survival in colour polymorphic grasshoppers. PLoS ONE 5, e10880. 10.1371/journal.pone.0010880 (doi:10.1371/journal.pone.0010880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nonacs P., Kapheim K. M. 2008. Social heterosis and the maintenance of genetic diversity at the genome level. J. Evol. Biol. 21, 631–635 10.1111/j.1420-9101.2007.01489.x (doi:10.1111/j.1420-9101.2007.01489.x) [DOI] [PubMed] [Google Scholar]
- 42.McClelland E., Naish K. 2007. What is the fitness outcome of crossing unrelated fish populations? A meta-analysis and an evaluation of future research directions. Conserv. Genet. 8, 397–416 10.1007/s10592-006-9178-x (doi:10.1007/s10592-006-9178-x) [DOI] [Google Scholar]
- 43.Verhoeven K. J. F., Macel M., Wolfe L. M., Biere A. 2011. Population admixture, biological invasions and the balance between local adaptation and inbreeding depression. Proc. R. Soc. B 278, 2–8 10.1098/rspb.2010.1272 (doi:10.1098/rspb.2010.1272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forsman A., Hagman M. 2009. Association of coloration mode with population declines and endangerment in Australian frogs. Conserv. Biol. 23, 1535–1543 10.1111/j.1523-1739.2009.01244.x (doi:10.1111/j.1523-1739.2009.01244.x) [DOI] [PubMed] [Google Scholar]
- 45.Forsman A., Åberg V. 2008. Associations of variable coloration with niche breadth and conservation status among Australian reptiles. Ecology 89, 1201–1207 10.1890/07-1670.1 (doi:10.1890/07-1670.1) [DOI] [PubMed] [Google Scholar]
- 46.Johnson L. E., Carlton J. T. 1996. Post-establishment spread in large-scale invasions: dispersal mechanisms of the zebra mussel Dreissena polymorpha. Ecology 77, 1686–1690 10.2307/2265774 (doi:10.2307/2265774) [DOI] [Google Scholar]
- 47.Majerus M., Strawson V., Roy H. 2006. The potential impacts of the arrival of the harlequin ladybird, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), in Britain. Ecol. Entomol. 31, 207–215 10.1111/j.1365-2311.2006.00734.x (doi:10.1111/j.1365-2311.2006.00734.x) [DOI] [Google Scholar]