Abstract
In numerous species, egg chemoattractants play a critical role in guiding sperm towards unfertilized eggs (sperm chemotaxis). Until now, the known functions of sperm chemotaxis include increasing the effective target size of eggs, thereby promoting sperm–egg encounters, and facilitating species recognition. Here, we report that in the broadcast spawning mussel, Mytilus galloprovincialis, egg chemoattractants may play an unforeseen role in sexual selection by enabling sperm to effectively ‘choose’ between the eggs of different conspecific females. In an initial experiment, we confirmed that sperm chemotaxis occurs in M. galloprovincialis by showing that sperm are attracted towards unfertilized eggs when given the choice of eggs or no eggs in a dichotomous chamber. We then conducted two cross-classified mating experiments, each comprising the same individual males and females crossed in identical male × female combinations, but under experimental conditions that offered sperm ‘no-choice’ (each fertilization trial took place in a Petri dish and involved a single male and female) or a ‘choice’ of a female's eggs (sperm were placed in the centre of a dichotomous choice chamber and allowed to choose eggs from different females). We show that male-by-female interactions characterized fertilization rates in both experiments, and that there was remarkable consistency between patterns of sperm migration in the egg-choice experiment and fertilization rates in the no-choice experiment. Thus, sperm appear to exploit chemical cues to preferentially swim towards eggs with which they are most compatible during direct sperm-to-egg encounters. These results reveal that sperm differentially select eggs on the basis of chemical cues, thus exposing the potential for egg chemoattractants to mediate mate choice for genetically compatible partners. Given the prevalence of sperm chemotaxis across diverse taxa, our findings may have broad implications for sexual selection in other mating systems.
Keywords: genetic compatibility, gamete proteins, sperm choice, cryptic female choice
1. Introduction
Since the discovery that sperm are guided towards eggs via chemoattractants released from unfertilized ova more than a century ago, sperm chemotaxis has been documented in numerous taxa, including marine species with external fertilization and terrestrial animals with internal fertilization (including humans) [1,2]. Although prevalent across most metazoans [3], sperm chemotaxis is best documented in broadcast spawning marine invertebrates, where males and females release gametes into the external environment. In these taxa, the evolutionary and ecological functions of sperm chemotaxis include increasing the effective target size of eggs, thereby enabling sperm to remotely orient towards fertile eggs via chemical signals [4–7], and maintaining species integrity by facilitating species recognition [8,9]. Although these naturally selected functions of sperm chemotaxis are highly relevant to broadcast spawning marine invertebrates, particularly in species with overlapping distributions and breeding seasons [4], the potential for chemoattractants to also mediate sperm–egg interactions within species remains enigmatic [10].
An intriguing question that has yet to be addressed in any system is whether sperm respond differentially to the chemoattractants released from eggs of different females of the same species. Such a selective mechanism would be highly relevant to broadcast spawning sessile marine animals, as reproductive incompatibilities between males and females are known to limit fertilization rates in these taxa [11,12]. Moreover, the limited opportunity for behavioural interactions among adults to facilitate mate choice prior to mating means that mechanisms that promote assortative combinations of compatible gametes after they are spawned are likely to be key targets of sexual selection in these taxa. The only known mechanism for avoiding such incompatibilities occurs at the moment of sperm–egg contact and involves structurally diverse gamete-recognition proteins, which can be highly polymorphic [13,14], leading to gamete-level differentiation among individuals of the same species [15,16]. These cell-surface proteins are typically invoked to account for the male-by-female interactions at fertilization frequently reported in sea urchins, molluscs and other marine invertebrates, thus supporting their role in facilitating the selection of genetically compatible mates [11,12,16]. However, the prevalence of sperm chemotaxis in marine invertebrates [1,3] means that signalling between female and male gametes commences upstream of cell-surface proteins (i.e. prior to gamete contact). Consequently, egg-derived chemoattractants have the potential to serve a similar role in gamete-recognition proteins in mediating contact rates between fertilizable sperm and eggs [10]. If so, chemoattractants may serve as an arbiter of mate choice for compatible partners. Surprisingly, the potential for chemoattractants to play such a role has not been investigated in marine invertebrates or any other taxa.
To explore the potential role that egg chemoattractants play in mediating intraspecific patterns of fertilization, we focused on the blue mussel, Mytilus galloprovincialis, a cosmopolitan broadcast spawning sessile invertebrate that dominates rocky intertidal communities in many temperate and sub-polar regions of the northern and southern hemispheres [17]. As in other Mytilus species, the mechanisms underlying fertilization dynamics in M. galloprovincialis are poorly described, although there is evidence that gamete-level interactions limit interspecific fertilization within the genus [18,19]. Nevertheless, sperm chemotaxis has not been documented explicitly in this group, and therefore the initial focus of the current study was to determine whether the phenomenon occurs in M. galloprovincialis. Then, as a first step towards addressing its possible role in sexual selection, we evaluated the potential for sperm chemoattraction to promote male–female interactions at fertilization, thus specifically determining whether sperm from individual males respond differentially towards the chemoattractants of different females. In a two-step experiment, we tested for the main and interacting effects of males and females at fertilization, one in which gametes interacted directly but sperm were not given a choice of eggs (a ‘no-choice’ experiment) and a second in which sperm were offered the ‘choice’ of eggs from two females (an ‘egg-choice’ experiment). Our results underscore the importance of considering chemosensory cues when evaluating gamete ‘compatibility’ in broadcast spawning invertebrates and highlight a potentially novel mechanism of sexual selection in these and other systems.
2. Material and methods
(a). Study species and spawning procedures
Mytilus galloprovincialis are sedentary as adults and release gametes into the water column during the spawning season, which in the Western Australian population used in our study spans ca from June to September. Mussels used in this study were collected from an estuarine population at Claremont Jetty, Western Australia (31°59′20.1″ S, 115°46′53.5″ E) during the peak spawning season in August 2010. Upon collection, mussels were placed in recirculating sea water tanks at the University of Western Australia and maintained at 16°C until required (always within 4 days of collection). To induce spawning, mussels were removed from their holding tanks and placed in 60 × 37 × 27 cm plastic containers filled to approximately 6 cm (approx. 13 l) with sea water that was raised to 30°C [20]. When an individual commenced spawning, it was immediately washed in clean sea water (to prevent contamination by gametes from other individuals) and placed individually in a cup containing 250 ml sea water where it continued to spawn. When sperm and eggs were available in appropriately high concentrations, mussels were removed from the cup and gametes were retrieved, and diluted when required, to obtain the desired gamete concentration for each experiment (see below).
(b). Test for sperm chemotaxis
We tested for sperm chemotaxis using a dichotomous choice assay [1] in which sperm from a single male were placed in the centre of a dichotomous chamber fitted with two distal wells (25 mm depth): one containing 7 × 104 eggs from a single randomly selected female, and one containing no eggs but otherwise treated identically with sea water (sham control; figure 1). The plastic dichotomous chamber was fabricated from plastic plumber's tubing (inside diameter: 18 mm) and consisted of two distal wells containing either eggs or a sea water control (figure 1). Eggs (or sea water) were placed in their respective wells and left for 1 h (in the case of eggs to set up a chemoattractant gradient). We then added 1 ml of the sperm solution (sperm density: 1 × 105 cells µl−1) from a single male to the centre of the dichotomous chamber (n = 41 trials were performed during which we reciprocated the position of eggs in successive trials to control for side biases and used different males and females in each trial). Three hours after adding sperm to the chamber, a 200 µl sample was collected from each distal well of the chamber (19 mm above the base of each well and thus not directly at the site of fertilization; figure 1), and sperm were counted using an improved Neubauer haemocytometer. As sperm counts did not exhibit normal distributions, and could not be normalized using standard transformations, we analysed the resultant paired data using the non-parametric Wilcoxon signed-rank test.
Figure 1.
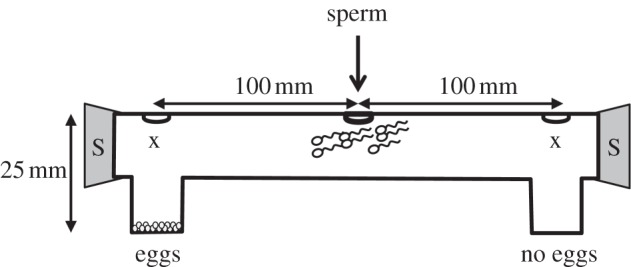
The experimental setup used to test for sperm chemotaxis in the mussel, Mytilus galloprovincialis. The dichotomous chamber pictured here was used to assess whether sperm choose between chemoattractant-containing (eggs) and chemoattractant-free (no eggs) distal wells. Each distal end of the chamber was sealed with a rubber stopper (S). Sperm cells were added to the centre of each chamber and samples were collected 3 h later from point ‘x’ (19 mm above the base of each well).
(c). Testing the potential for sperm chemotaxis to facilitate sexual selection
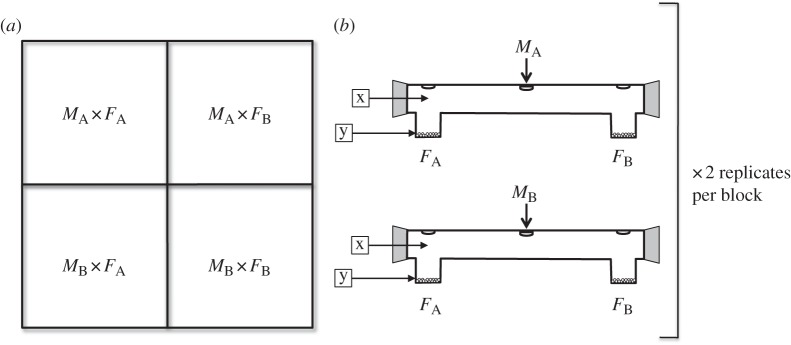
To examine the role that egg chemoattractants play in influencing intraspecific patterns of fertilization, we used a crossing design comprising 15 blocks of factorial crosses. This cross-classified block design [21] enabled us to explicitly test for the interacting and main effects of males and females at fertilization. We conducted two such cross-classified mating experiments (described in detail below), each comprising the same individual males and females crossed in identical male × female combinations but under experimental conditions that offered sperm ‘no-choice’ or a ‘choice’ of a female's eggs to fertilize.
(i). No-choice experiment
In the no-choice experiment, fertilizations were performed in 5.5 cm diameter Petri dishes to analyse sources of variation in fertilization rates when sperm were added directly to eggs. In each block, two males (MA and MB) and two females (FA and FB) were crossed for the four possible combinations (figure 2a), with replicate (n = 2) crosses performed for each pair (i.e. 2 × [MA × FA; MA × FB; MB × FA; MB × FB]). This block design yielded 60 unique crosses (four male–female combinations × 15 replicates) with replication within each cross (120 crosses in total). In this way, we partitioned sources of variation in fertilization rates using a traditional in vitro fertilization protocol [11].
Figure 2.
The experimental design and setup used to determine the role of sperm chemotaxis in sexual selection in Mytilus galloprovincialis. (a) A single block in the cross-classified design used in the no-choice experiment. In these trials, sperm and eggs from two males (denoted MA and MB) and two females (FA and FB) were mixed in all four combinations in Petri dishes, with replicate (×2) crosses performed for each M–F combination. A total of n = 15 blocks were performed (see main text). Fertilization rates were then measured as described in the main text. (b) A single block in the experimental design used to test whether sperm from individual males (MA or MB) consistently ‘choose’ eggs from one of the two females used in each trial (FA and FB). Importantly, each block in (b) comprises the same two males and females used in the no-choice experiment (a). The dichotomous chambers used in the egg-choice experiment (b) were the same as those described in figure 1. In these trials, sperm were sampled 19 mm above the eggs for the sperm counts (corresponding to point x), whereas eggs were sampled from the bottom of each well (point y) to obtain fertilization data.
For the no-choice experiment, sperm concentrations were estimated using an improved Neubauer haemocytometer and adjusted to 4 × 105 sperm ml−1. Eggs were counted using the same methods and adjusted to 2 × 105 eggs ml−1. These gamete concentrations were determined in a pilot study to ensure that ceiling (approx. 100% fertilization rates) or basement (approx. 0% fertilization rates) effects did not hamper the interpretation of our results (see also Fitzpatrick et al. [22]) and are consistent with previous studies that report monospermic fertilizations when using this sperm-to-egg ratio [23]. Gamete concentrations from both sexes were therefore standardized across all crosses both within and among blocks. For each block of the design, we added 10 ml of egg solution to each Petri dish from the two females and left them under aeration for 1 h. We then added 1 ml of sperm solution from the two males to each Petri dish in all 2 × 2 combinations described earlier. After 1 h, the sperm–egg solutions were fixed with a 10 per cent buffered formalin solution; fertilization rates (proportion of eggs undergoing cleavage) were then scored from a haphazardly selected subsample of 100 eggs per sample. These experimental crosses resulted in highly variable fertilization rates across male–female pairs (mean ± s.e. fertilization rates = 0.40 ± 0.02 s.e.; range = 0.10–0.96, n = 120).
(ii). Egg-choice experiment
In the egg-choice experiment, crosses were performed in dichotomous chambers where sperm from each male were given the ‘choice’ of eggs from the two females in each block (figure 2). Importantly, for each individual block of the design, we used the same combinations of males and females that were used in the no-choice experiment described above. For each block, we conducted twice-replicated egg-choice trials using the following combinations of gametes from both sexes: sperm from MA could choose to swim towards eggs from either FA or FB and sperm from MB could choose to swim towards eggs from either FA or FB (or 2 × [MA × FA + FB; MB × FA + FB]). This resulted in four potential male–female pairs, each conducted in replicate (figure 2b). Eggs from the two females were adjusted to 2 × 105 eggs ml−1 (the same densities as in the no-choice experiment) and then assigned to either of the two distal wells of the dichotomous chamber (with position reciprocated between replicates to avoid side biases). Eggs were left for 1 h to set up a concentration gradient of chemoattractants before 1 ml of sperm solution was added to the centre of each chamber (figure 2b). In this experiment, sperm concentrations varied among blocks (but not between replicates within a given block). This is because we maximized sperm concentrations in each trial to ensure that sperm were recoverable from the distal well of each dichotomous chamber in sufficient numbers to facilitate sperm counts after 3 h. For this reason, the significant male effects detected in the egg-choice experiment (see §3 and table 1) are not interpreted in this study, as they are almost certainly attributable to variation in initial sperm concentrations in these trials. Three hours after adding sperm to each dichotomous chamber, we sampled 200 µl of water from both distal wells, ensuring that each sample was taken 19 mm above the eggs (thus, sperm were sampled on their way to the eggs, not at the site of fertilization). We then collected 1 ml of eggs from the bottom of each distal well to estimate fertilization rates. All samples (eggs and sperm) were fixed in buffered formalin solution; sperm counts were performed using an improved Neubauer haemocytometer, while fertilization rates were estimated as in the no-choice experiment described earlier.
Table 1.
Sources of variation in fertilization rates when sperm have direct access to eggs (no-choice experiment) and the direction of sperm movement and subsequent fertilization rates in dichotomous choice trials (egg-choice experiment) in the mussel, Mytilus galloprovincialis. For each model, sums of squares (SS) and degrees of freedom (d.f.) were computed individually for each block and summed to estimate the mean squares (MS) for each analysis. Significance levels for male and female effects were assessed by dividing their respective mean squares by the interaction mean squares [21]. Significant effects are presented in bold text. The d.f. associated with the error variance for each block was calculated from the formula NM NF (n − 1), where NM = 2, NF = 2, and n = number of replicate crosses = 2. As with d.f. associated with main effects, error d.f. were summed across blocks for the final analysis [21].
| source of variation | d.f. | SS | MS | F | p |
|---|---|---|---|---|---|
| (a) no-choice experiment: fertilization | |||||
| male | 15 | 0.091 | 0.006 | 1.076 | 0.445 |
| female | 15 | 3.548 | 0.237 | 41.94 | <0.0001 |
| interaction | 15 | 0.085 | 0.006 | 1.945 | 0.036 |
| error | 60 | 0.174 | 0.003 | ||
| (b) egg-choice experiment: sperm numbers | |||||
| male | 15 | 3 544 513 | 236 301 | 2.820 | 0.027 |
| female | 15 | 2 169 863 | 144 658 | 1.727 | 0.151 |
| interaction | 15 | 1 256 763 | 83 784 | 2.049 | 0.026 |
| error | 60 | 2 453 150 | 40 886 | ||
| (c) egg-choice experiment: fertilization | |||||
| male | 13 | 1.560 | 0.120 | 4.065 | 0.008 |
| female | 13 | 1.062 | 0.082 | 2.767 | 0.039 |
| interactiona | 13 | 0.384 | 0.030 | 1.472 | 0.160 |
| error | 52 | 1.043 | 0.020 | ||
aThe interaction term for fertilization rates in the egg-choice experiment was marginally significant (p = 0.05) when data from one block with high residual error variance (on average 5.2 times higher than all other replicates) was removed from the analysis.
(d). Statistical analysis
We used sequential two-way ANOVA models to estimate the main and interactive effects of males and females on fertilization rates in the no-choice experiment, and the directionality of sperm movement and subsequent fertilization rates in the egg-choice experiment. As we used a series (n = 15) of factorial crosses, analyses were performed individually for each block and combined in a final model, as prescribed for the block ‘North Carolina II’ design [21] and implemented previously in externally fertilizing species [11]. In the egg-choice experiment, it was not possible to estimate fertilization rates in two blocks owing to problems with the fixative (formalin). Thus, in the egg choice experiment, analyses of fertilization rates are confined to 13 blocks.
Next, we determined whether the fertilization patterns generated in the no-choice experiment predicted the directionality of sperm movement and fertilization rates in the egg-choice experiment. Thus, we asked to what extent might patterns of male-by-female interaction in assays where sperm are not given a choice of eggs reflect the chemotactic responses of sperm when given the simultaneous choice of eggs. To address this question, we calculated the mean fertilization rates in the no-choice experiment and egg-choice experiment, and the mean sperm counts from the egg-choice experiment. Mean values were calculated from the two replicated M × F crosses performed for each block. We then calculated the difference in mean fertilization rates for a given male and the two females to which he was assigned in the no-choice experiment (i.e. fertilization rates of [MA × FA] – [MA × FB]) as a measure of sperm–egg ‘compatibility’ and correlated these compatibility values with the corresponding differences for the sperm counts (a measure of sperm preference) and fertilization rates (a measure of sperm–egg compatibility following sperm choice) between distal wells in the egg-choice experiment. As each male was represented twice within each block (i.e. each male was crossed with both females in a block), we used a resampling procedure to extract data for just one male from each block at random to preserve statistical independence in our analysis of correlation coefficients. A total of 10 000 iterations of the resampling protocol were performed for the calculation of each distribution of correlation coefficients. We then calculated the mean and 95% confidence limits (CLs) for each distribution to assess the strength and significance of each correlation. Resampling procedures were carried out using PopTools v. 3.1.1 [24].
3. Results
(a). Test for sperm chemotaxis
Our chemotaxis experiment revealed that sperm densities were almost five times higher in the distal wells of the dichotomous chamber containing eggs compared with the sham control (median [interquartile range]: egg containing distal wells = 5.8 × 104 sperm cells per sample [3.6 × 104 – 10.2 × 104]; sham control distal wells = 1.2 × 104 [0.2 × 106 – 3.6 × 106]). This difference in sperm number between the two distal wells was highly significant (Wilcoxon signed-rank test: W = 331, p < 0.001), indicating that sperm preferentially swam towards the side of the dichotomous chamber containing eggs. These findings are consistent with a large body of literature from other taxa revealing the chemotactic responses of sperm to eggs [1].
(b). No-choice and egg-choice experiments
In the no-choice experiment, fertilization rates were influenced both by female identity and the interactive effect of males and females (table 1a). These results indicate that in no-choice experimental conditions fertilization success depends on the specific combination of sperm and eggs present.
In the egg-choice experiment, we also detected a significant male-by-female interaction underlying the number of sperm recovered from the distal well of the dichotomous chamber (table 1b). We also detected a weak interaction effect on fertilization rates in the egg-choice experiment after a single outlier was removed from the analysis (see note accompanying table 1). Importantly, in the egg-choice experiment, sperm were sampled on their way to the eggs, not directly at the site of fertilization (see §2). Thus, even when gametes from both sexes do not have the potential to interact physically, as in the no-choice experiment, sperm are capable of differentiating between the eggs of different females using remote chemical cues and consistently alter their swimming path to move towards eggs from specific females.
The finding that patterns of sperm migration in the egg-choice experiment can be attributed to the interacting effects of males and females raises the question of whether these patterns could potentially explain fertilization rates when sperm and eggs interacted directly within a Petri dish (i.e. in the no-choice experiment). Using a resampling analysis (see §2), we confirmed this prediction by showing an overall significant positive correlation between fertilization rates in the no-choice experiment and (i) the directionality of sperm movement (mean correlation coefficient = 0.41; 95% CLs = 0.12–0.69; figure 3a) and (ii) fertilization rates in the egg-choice experiment (mean correlation coefficient = 0.72; 95% CL = 0.52–0.88; figure 3b).
Figure 3.
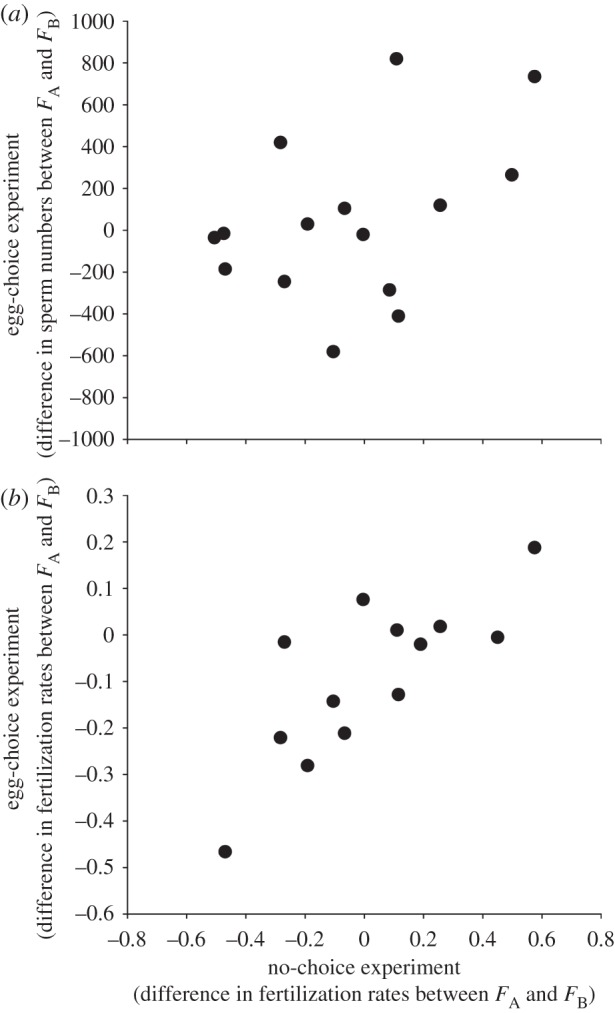
(a) The relationship between fertilization rates in the no-choice experiment (i.e. the difference in mean fertilization scores for a given male and the two females to which he was assigned) and sperm migration patterns in the egg-choice experiment (the corresponding differences in the number of sperm recovered from the distal wells of the dichotomous choice chambers; see text for details). (b) The relationship between the difference in mean fertilization scores in the no-choice and egg-choice experiments. For each relationship, we illustrate these patterns using a scatterplot of a single set of resampled data that corresponds to a correlation coefficient equal to the mean correlation coefficient obtained after 10 000 iterations of the resampling protocol.
4. Discussion
Our results provide evidence that the male–female interaction at fertilization can be attributable to chemosensory interactions between eggs and sperm, therefore revealing a previously unanticipated function of sperm chemoattractants in sessile marine broadcast spawning invertebrates. These findings imply that egg chemoattractants function as intraspecific cues that enable sperm to consistently differentiate between the eggs from different females, thus exposing the potential for chemoattractants to function within the context of sexual selection.
Until now, the known evolutionary and ecological roles of sperm chemoattractants were limited to (i) providing remote chemical signals that increase the effective target size of eggs and thereby promote sperm–egg encounters [4,5] and (ii) providing an efficient mechanism of avoiding costly interspecific hybridization [8,9]. The latter of these two functions emphasizes the variation that must exist among sperm chemoattractants among divergent taxa. The findings from the present study suggest that egg chemoattractants not only vary in substance among taxa, but also among individuals of the same species. This conclusion, which is consistent with the high level of variation that exists in many other forms of animal chemical communication [25], especially in the context of sexual selection [26], is likely to have important evolutionary implications in sessile broadcast spawning invertebrates, where the opportunities for direct mate assessment are limited. We anticipate that M. galloprovincialis, along with other marine invertebrates, are likely to provide exciting model systems for increasing our understanding of chemotaxis and exploring its role in sexual selection. However, our findings may also have wider implications for other species in which sperm chemotaxis has been documented, including humans and other mammals [2], where the mechanisms stimulating sperm activation and chemotaxis are now being revealed [27,28].
Our design in the dichotomous egg-choice experiment involved sampling sperm on their way to eggs rather than at the site of fertilization. This indicates that the male–female interactions uncovered in these trials were attributable to differential chemosensory responses and not direct sperm–egg interactions. In accordance with this conclusion, we detected no significant effect of females on the number of sperm recovered from each well of the choice chamber in the egg-choice trials (i.e. where there was no possibility for gametes from either sex to interact directly), despite the finding that female effects at fertilization are generally important in broadcast spawning marine invertebrates ([29]; note that in table 1 we also report significant female effects in experiment 1, but in the presence of significant male-by-female interaction, we do not interpret these in our study).
The male-by-female interactions that often characterize fertilization rates in marine broadcast spawning invertebrates [11,12,16] have traditionally been attributed to the presence of highly differentiated reproductive proteins expressed on the surfaces of gametes [30], which in turn are activated upon direct contact between sperm and eggs [31]. These proteins are typically highly polymorphic [13,14] and are known to mediate gamete-level differentiation among individuals of the same species. For example, in two separate studies of the sea urchins Echinometra mathaei and Strongylocentrotus franciscanus, variation in the gamete-recognition protein bindin mediates intraspecific patterns of fertilization [15,16]. Similarly, intraspecific variation in the gamete-recognition protein M7 lysin has been reported in three Mytlilus species, including M. galloprovincialis [18] and there is recent evidence for intraspecific (temporally based) variation in the expression of these acrosomal proteins [32]. These gamete surface proteins are usually invoked to account for the male-by-female interactions at fertilization observed in sea urchins and other marine invertebrates, thus supporting their role in mediating the selection of genetically compatible mates [11,12,16]. Our study reveals an additional, or possibly alternative, process by which such interactions can arise. We show that sperm are differentially attracted towards unfertilized eggs, presumably on the basis of variation in the chemical signals they emit. Importantly, we used the same combinations of males and females in two contexts (direct sperm–egg encounters in the no-choice experiment; chemical attraction in the egg-choice experiment) and showed that fertilization rates in the no-choice experiment strongly predicted the direction in which sperm travelled in the egg-choice experiment. We can think of two possible explanations for this finding. First, egg-derived chemoattractants may be functionally related to the cell-surface receptors that mediate the binding and passage of sperm through the egg envelope and ultimately the fusion of gametes. According to this scenario, gamete surface proteins may act to refine patterns of sperm–egg communication initiated prior to gamete contact during sperm chemotaxis. Second, egg chemoattractants may be the primary mediators of sperm–egg interactions at the intraspecific level, and there may be no direct relationship between egg chemoattractants and egg surface receptors. According to this latter scenario, the patterns of male-by-female interaction that we observed in the no-choice experiment may be attributable predominantly to the action of egg chemoattractants, and not gamete surface proteins as often assumed. Clearly, as in most other cases where chemotaxis has been reported, there is a need to identify the molecular and biochemical properties of the chemoattractants (and their receptors in sperm) to test between these different scenarios [4]. We anticipate that our results will stimulate such an investigation.
In summary, we provide evidence that sperm differentially swim towards eggs from different females on the basis of chemical cues emitted from eggs, thus exposing a novel role for sperm chemotaxis in mediating intraspecific patterns of fertilization. Given the widespread occurrence of sperm chemotaxis across a diverse range of taxa [3], we anticipate that our findings will have implications that extend beyond marine invertebrates. One important direction for future research is to explore the fitness implications associated with these chemically mediated sperm–egg interactions. For example, such work could determine the extent to which sperm chemotaxis functions to avoid reproductive incompatibilities that may impair offspring survival or fitness. It would also be interesting to determine the mechanistic basis for the interactions uncovered in our study, and in particular to uncover functional relationships (if any) between these mechanisms and those already known to generate assortative patterns of fertilization in broadcast spawning marine invertebrates (e.g. cell-surface gamete-recognition proteins). Finally, it would be interesting to determine whether egg chemoattractants have the potential to mediate competitive fertilization success when ejaculates from two (or more) males compete directly for fertilization with a single female's eggs. Such a scenario would represent a novel mechanism of gamete choice, where physiological effects attributable to females (in this case chemoattractants) influence the relative success of competing ejaculates [33]. Our study highlights the potential of chemical communication between gametes to serve such a purpose and sets the stage for a robust investigation of the importance of sexual selection at the gametic level in sessile organisms.
Acknowledgements
We thank Cameron Duggin for technical assistance, Janne Kotiaho, Bob Montgomerie, Joe Tomkins and Leigh Simmons for comments on previous drafts of the manuscript; and Gary Carvalho, Jacek Radwan and two anonymous referees for comments during the review process. We also acknowledge the Australian Research Council for financial support.
References
- 1.Eisenbach M. 1999. Sperm chemotaxis. Rev. Reprod. 4, 56–66 10.1530/ror.0.0040056 (doi:10.1530/ror.0.0040056) [DOI] [PubMed] [Google Scholar]
- 2.Eisenbach M., Giojalas L. C. 2006. Sperm guidance in mammals: an unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 7, 276–285 10.1038/nrm1893 (doi:10.1038/nrm1893) [DOI] [PubMed] [Google Scholar]
- 3.Miller R. L. 1985. Sperm chemo-orientation in the metazoa. In Biology of fertilization (eds Metz C. B., Monroy A.), pp. 275–337 New York, NY: Academic Press [Google Scholar]
- 4.Riffell J. A., Krug P. J., Zimmer R. K. 2004. The ecological and evolutionary consequences of sperm chemoattraction. Proc. Natl Acad. Sci. USA 101, 4501–4506 10.1073/pnas.0304594101 (doi:10.1073/pnas.0304594101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmer R. K., Riffell J. A. 2011. Sperm chemotaxis, fluid shear, and the evolution of sexual reproduction. Proc. Natl Acad. Sci. USA 108, 13 200–13 205 10.1073/pnas.1017675108 (doi:10.1073/pnas.1017675108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jantzen T. M., de Nys R., Havenhand J. N. 2001. Fertilization success and the effects of sperm chemoattractants on effective egg size in marine invertebrates. Mar. Biol. 138, 1153–1161 10.1007/s002270100537 (doi:10.1007/s002270100537) [DOI] [Google Scholar]
- 7.Krug P. J., Riffell J. A., Zimmer R. K. 2009. Endogenous signaling pathways and chemical communication between sperm and egg. J. Exp. Biol. 212, 1092–1100 10.1242/jeb.027029 (doi:10.1242/jeb.027029) [DOI] [PubMed] [Google Scholar]
- 8.Miller R. L. 1997. Specificity of sperm chemotaxis among great barrier reef shallow-water holothurians and ophiuroids. J. Exp. Zool. 279, 189–200 (doi:10.1002/(SICI)1097-010X(19971001)279:2<189::AID-JEZ10>3.0.CO;2-B) [DOI] [Google Scholar]
- 9.Howard D. J., Palumbi S. R., Birge L. M., Manier M. K. 2009. Sperm and speciation. In Sperm biology: an evolutionary perspective (eds Birkhead T. R., Hosken D. J., Pitnick S.), pp. 367–403 Burlington, MA: Academic Press [Google Scholar]
- 10.Swanson W. J., Vacquier V. D. 2002. The rapid evolution of reproductive proteins. Nature Rev. Gen. 3, 137–144 10.1038/nrg733 (doi:10.1038/nrg733) [DOI] [PubMed] [Google Scholar]
- 11.Evans J. P., Marshall D. J. 2005. Male-by-female interactions influence fertilization success and mediate the benefits of polyandry in the sea urchin Heliocidaris erythrogramma. Evolution 59, 106–112 10.1111/j.0014-3820.2005.tb00898.x (doi:10.1111/j.0014-3820.2005.tb00898.x) [DOI] [PubMed] [Google Scholar]
- 12.Marshall D. J., Evans J. P. 2005. The benefits of polyandry in the free-spawning polychaete Galeolaria caespitosa. J. Evol. Biol. 18, 735–741 10.1111/j.1420-9101.2004.00873.x (doi:10.1111/j.1420-9101.2004.00873.x) [DOI] [PubMed] [Google Scholar]
- 13.Moy G. W., Springer S. A., Adams S. L., Swanson W. J., Vacquier V. D. 2008. Extraordinary intraspecific diversity in oyster sperm bindin. Proc. Natl Acad. Sci. USA 105, 1993–1998 10.1073/pnas.0711862105 (doi:10.1073/pnas.0711862105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metz E. C., Palumbi S. R. 1996. Positive selection and sequence rearrangements generate extensive polymorphism in the gamete recognition protein bindin. Mol. Biol. Evol. 13, 397–406 [DOI] [PubMed] [Google Scholar]
- 15.Levitan D. R., Ferrell D. L. 2006. Selection on gamete recognition proteins depends on sex, density, and genotype frequency. Science 312, 267–269 10.1126/science.1122183 (doi:10.1126/science.1122183) [DOI] [PubMed] [Google Scholar]
- 16.Palumbi S. R. 1999. All males are not created equal: fertility differences depend on gamete recognition polymorphisms in sea urchins. Proc. Natl Acad. Sci. USA 96, 12 632–12 637 10.1073/pnas.96.22.12632 (doi:10.1073/pnas.96.22.12632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daguin C., Borsa P. 2000. Genetic relationships of Mytilus galloprovincialis Lamarck populations worldwide: evidence from nuclear-DNA markers. In Evolutionary biology of the Bivalvia, geological society special publication, vol. 177 (eds Harper E. M., Taylor J. D., Crame J. A.), pp. 389–397 Bath, UK: Geological Soc Publishing House [Google Scholar]
- 18.Riginos C., McDonald J. H. 2003. Positive selection on an acrosomal sperm protein, M7 lysin, in three species of the mussel genus Mytilus. Mol. Biol. Evol. 20, 200–207 10.1093/molbev/msg021 (doi:10.1093/molbev/msg021) [DOI] [PubMed] [Google Scholar]
- 19.Bierne N., David P., Boudry P., Bonhomme F. 2002. Assortative fertilization and selection at larval stage in the mussels Mytilus edulis and M. galloprovincialis. Evolution 56, 292–298 [DOI] [PubMed] [Google Scholar]
- 20.Rawson P. D., Slaughter C., Yund P. O. 2003. Patterns of gamete incompatibility between the blue mussels Mytilus edulis and M. trossulus. Mar. Biol. 143, 317–325 10.1007/s00227-003-1084-x (doi:10.1007/s00227-003-1084-x) [DOI] [Google Scholar]
- 21.Lynch M., Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 22.Fitzpatrick J. L., Simmons L. W., Evans J. P. In press. Complex patterns of multivariate selection on the ejaculate of a broadcast spawning marine invertebrate. Evolution. [DOI] [PubMed] [Google Scholar]
- 23.Dufresne-Dubé L., Dubé F., Guerrier P., Couillard P. 1983. Absence of a complete block to polyspermy after fertilization of Mytilus galloprovincialis (Mollusca, Pelecypoda) oocytes. Dev. Biol. 97, 27–33 10.1016/0012-1606(83)90060-X (doi:10.1016/0012-1606(83)90060-X) [DOI] [PubMed] [Google Scholar]
- 24.Hood G. M. 2009. PopTools version 3.1.1. Available on the internet. See http://www.cse.csiro.au/poptools
- 25.Bradbury J. W., Vehrencamp S. L. 1998. Principles of animal communication. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 26.Setchell J. M., Vaglio S., Abbott K. M., Moggi-Cecchi J., Boscaro F., Pieraccini G., Knapp L. A. 2011. Odour signals major histocompatibility complex genotype in an Old World monkey. Proc. R. Soc. B 278, 274–280 10.1098/rspb.2010.0571 (doi:10.1098/rspb.2010.0571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lishko P. V., Botchkina I. L., Kirichok Y. 2011. Progesterone activates the principal Ca2+ channel of human sperm. Nature 471, 387–391 10.1038/nature09767 (doi:10.1038/nature09767) [DOI] [PubMed] [Google Scholar]
- 28.Strünker T., Goodwin N., Brenker C., Kashikar N. D., Weyand I., Seifert R., Kaupp B. 2011. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471, 382–386 10.1038/nature09769 (doi:10.1038/nature09769) [DOI] [PubMed] [Google Scholar]
- 29.Levitan D. R. 2006. The relationship between egg size and fertilization success in broadcast-spawning marine invertebrates. Integr. Comp. Biol. 46, 298–311 10.1093/icb/icj025 (doi:10.1093/icb/icj025) [DOI] [PubMed] [Google Scholar]
- 30.Zigler K. S., McCartney M. A., Levitan D. R., Lessios H. A. 2005. Sea urchin bindin divergence predicts gamete compatibility. Evolution 59, 2399–2404 10.1111/j.0014-3820.2005.tb00949.x (doi:10.1111/j.0014-3820.2005.tb00949.x) [DOI] [PubMed] [Google Scholar]
- 31.Vacquier V. D. 1998. Evolution of gamete recognition proteins. Science 281, 1995–1998 10.1126/science.281.5385.1995 (doi:10.1126/science.281.5385.1995) [DOI] [PubMed] [Google Scholar]
- 32.Banni M., Negri A., Mignone F., Boussetta H., Viarengo A., Dondero F. 2011. Gene expression rhythms in the mussel Mytilus galloprovincialis (Lam.) across an annual cycle. PLoS ONE 6, e18904. 10.1371/journal.pone.0018904 (doi:10.1371/journal.pone.0018904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eberhard W. G. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press [Google Scholar]