Abstract
Aposematic passion-vine butterflies from the genus Heliconius form communal roosts on a nightly basis. This behaviour has been hypothesized to be beneficial in terms of information sharing and/or anti-predator defence. To better understand the adaptive value of communal roosting, we tested these two hypotheses in field studies. The information-sharing hypothesis was addressed by examining following behaviour of butterflies departing from natural roosts. We found no evidence of roost mates following one another to resources, thus providing no support for this hypothesis. The anti-predator defence hypothesis was tested using avian-indiscriminable Heliconius erato models placed singly and in aggregations at field sites. A significantly higher number of predation attempts were observed on solitary models versus aggregations of models. This relationship between aggregation size and attack rate suggests that communally roosting butterflies enjoy the benefits of both overall decreased attack frequency as well as a prey dilution effect. Communal roosts probably deter predators through collective aposematism in which aggregations of conspicuous, unpalatable prey communicate a more effective repel signal to predators. On the basis of our results, we propose that predation by birds is a key selective pressure maintaining Heliconius communal roosting behaviour.
Keywords: communal roosting, collective aposematism, dilution effect, aggregation, predation, Heliconius
1. Introduction
In 1867, the naturalist J. A. Allen first described the spectacular aggregations of migratory monarchs, and was thus the first to report the phenomenon of communal roosting in butterflies [1,2]. Shortly thereafter W. H. Edwards, the entomologist who inspired Bates and Wallace to visit the Amazon, reported communal roosting in the tropical Heliconius passion-vine butterflies [3]. Since the mid-nineteenth century, this unusual behaviour has generated a great deal of scientific and popular interest. After 140 years of work on butterfly roosting, however, it still remains unclear what the benefit of being a social butterfly is. Here, we test the major hypotheses for why Heliconius butterflies roost communally, and present experimental data assessing the adaptive function of this behaviour.
Communal roosting is observed in many types of animals, including birds, bats and primates [4–7], and is especially widespread in insects, having been observed in bees, wasps, beetles, dragonflies, butterflies and moths [8–10]. In butterflies, communal roosting is described as ‘a behavior in which individuals aggregate quiescently in close proximity to each other at a site for more than a few hours’ ([9] p. 90). This behaviour is known primarily from the heliconiines, acraeines, ithomiines and danaiines [11,12]. Many species of Heliconius in particular have been observed to form communal roosts in which adults repeatedly gather in a particular location in their home range to roost for the night (figure 1a). Butterflies arrive at their roost sites as early as 3 h before sunset, and depart from roosts within the first 2 h after sunrise. Roost mates are generally conspecifics, but sometimes different species—often Müllerian co-mimics—roost together [13]. The evolution of communal roosting behaviour in Heliconius is believed to be facilitated by unpalatability, slow reproductive rate [14], limited learned home range [12,15] and long lifespan owing to pollen consumption [16,17].
Figure 1.
(a) Natural and (b) artificial roosts of Heliconius erato. (c) Beak pinch at end of model abdomen, and triangular beak mark imprint on wax wing (indicated by arrow). (d) Dorsal view of abdominal beak pinch.
Heliconius butterflies are well known for their brightly coloured wing patterns and their unpalatability owing to cyanogenic glycosides [18]. Because of these features, Heliconius butterflies serve as a textbook example of warning signalling—also known as aposematism. Aposematism is a major theme in the evolution of animal phenotypes, where its principal function is to provide warning signals associated with unprofitability to predators, such as toxicity, unpalatability or capture costs [19–21]. Aposematism is widespread in invertebrates and is often achieved through visual signalling via conspicuous colour patterns. Collective aposematism is a phenomenon in which aposematic prey form aggregations to enhance the effects of warning signals [22,23]. Despite the substantial amount that is known about Heliconius natural history, little is known about their communal roosting behaviour and its possible relationship to collective aposematism.
There is a broad literature on Heliconius roosting [12,13,24–29]; however, relatively few experimental studies have been performed to address the function of this behaviour. Although the adaptive consequences of roosting remain unclear, it is unlikely that aggregations are involved with thermoregulation [10], kin selection [13] or mating (females usually mate once in their lifetime, within hours or days after eclosion) [30,31]. The favoured explanations have been narrowed to two major hypotheses: information sharing and/or anti-predator defence. The information-sharing hypothesis proposes that new roost mates, presumably related individuals, follow experienced members from the roost to food sources [27,32]. This form of information sharing is a common behaviour in other communal animals such as birds [33,34]. Conversely, the anti-predator hypothesis suggests predators avoid Heliconius aggregations as a result of collective aposematism or predator confusion [12,13,35]. In other aposematic insects, gregarious behaviour contributes to collective enhancement of warning signalling, resulting in more effective predator deterrence [22,36–38]. Another potential anti-predator mechanism is the prey dilution effect, often known as ‘safety in numbers’, which posits that the probability of a single individual being attacked in a group is lower with increasing density [39–42].
In field studies in Panama and Costa Rica, we tested these hypotheses to determine why Heliconius passion-vine butterflies assemble in communal roosts. To test whether Heliconius butterflies rely on roosts as information-sharing centres, we examined following behaviour by butterflies during departures from natural aggregations. The anti-predator defence hypothesis was tested using avian-indiscriminable artificial butterfly models placed singly and in aggregations in the forest. Following the predation study, we investigated whether naturally occurring roost sizes correspond with optimal roost sizes inferred from experimental data.
2. Material and methods
(a). Field sites
All data collection was completed in Costa Rica and Panama. Field sites for this work were chosen based on the abundance and accessibility of Heliconius butterflies and communal roosts. The Panama sites were part of the Smithsonian Tropical Research Institute; we used areas in Gamboa and Soberanía National Park along Pipeline Road. Data were collected in Panama from June through September of 2010 and 2011 during the rainy season. The 2010 visit resulted in natural roost data collected from Heliconius erato, and the 2011 visit resulted in the predation experiment data. In Costa Rica, we worked at the Organization for Tropical Studies' La Selva Tropical Biological Station in Sarapiquí. La Selva was visited in April and May of 2011, during the end of the dry season into the beginning of the rainy season. This site was used for collecting natural roost data from Heliconius sara and model predation experiments.
(b). Natural roost observations
To assess the information-sharing hypothesis, which predicts that butterflies use roosts to learn the locations of foraging sites from other roost mates, we observed following behaviour of H. erato and H. sara individuals departing natural roosting aggregations. Following was confirmed only if a butterfly was observed departing the roost with another roost mate to subsequently arrive at a flowering plant with that same roost mate. The roosts were within 30 m of the first visited flowering plant, and it was feasible to follow butterflies to these plants. We began all observations approximately 30 min before sunrise, before butterflies left to forage, and stayed until only one individual remained. All H. erato roost members were given unique identification numbers using a Sharpie marker, and were sexed and age determined based on wing wear [43]. Butterflies were captured and marked after departing their roosts to prevent them from associating the roost with danger [44]. Average roost sizes were determined by recording the number of individuals in each roost nightly.
(c). Models
We used artificial butterfly models to test the hypothesis that Heliconius roosts provide an anti-predatory benefit. Wing images were designed in Adobe Illustrator using high-resolution scans of ventral Heliconius erato petiverana wings as a reference. Model butterfly wings were printed on Whatman filter paper, which produces reflectance spectra close in brightness to actual wings, using an Epson Stylus Pro 4880 printer with UltraChrome K3 ink. A 3-hydroxy-dl-kynurenine (3-OHK) pigment solution of 1.0 mg 3-OHK dissolved in 100 µl of methanol was applied to the yellow bands on the hindwing to provide accurate UV reflectance, because printed yellows do not accurately mimic Heliconius yellow in the shape of their reflectance spectra. 3-OHK is the same yellow wing pigment as found in the wings of the butterflies themselves [45,46]. Portions of the artificial wings were dipped in clear wax to allow imprints of beak and bite marks, then Krylon matte finishing spray was applied lightly (before spectra measurements were taken) to coat the 3-OHK with a waterproofing element. Model abdomens were made of Newplast Plasticine.
Birds, in particular jacamars, flycatchers and tanagers, are major Heliconius predators [27,36,47–50]. Therefore, artificial butterfly models were designed and assessed using tetrachromatic bird colour-vision models in order to ensure that avian predators would find colour stimuli presented by the models indiscriminable from actual butterflies. Reflectance spectra of yellow, pink and black from the models and the ventral surface of natural H. erato petiverana wings were measured using an Ocean Optics USB2000 fibre optic spectrometer. A deuterium–halogen tungsten lamp (DH-2000, Ocean Optics) was used as a standardized light source, and measurements were taken using a bifurcating fibre cable (R400-7-UV–vis, Ocean Optics). The axis of the illuminating and detecting fibre was at an elevation of 45° to the plane of the wing and pointed left with respect to the body axis for every measurement. A white spectralon standard (WS-1-SL, Labsphere) was used to calibrate the spectrometer. Spectra measurements from the fibre optic spectrometer were processed using MATLAB software (see Briscoe et al. [46]). The quantum catches for stimuli [51] were calculated, and discriminability between artificial models and natural wing reflectance spectra was determined using tetrachromatic bird-vision models from Vorobyev & Osorio [52]. The comparisons were made using the blue tit (Parus caeruleus) and chicken (Gallus gallus) cone sensitivities, which represent the UV- and violet-type avian visual systems, respectively. Low light intensity and open habitat irradiance spectra were used [53]. All spectral comparisons represented by an average of wing measurements (n = 12) fell below the threshold of one just noticeable difference (figure 2 and table 1); therefore, the reflectance spectra of models and actual butterfly wings were inferred to be indiscriminable to birds.
Figure 2.
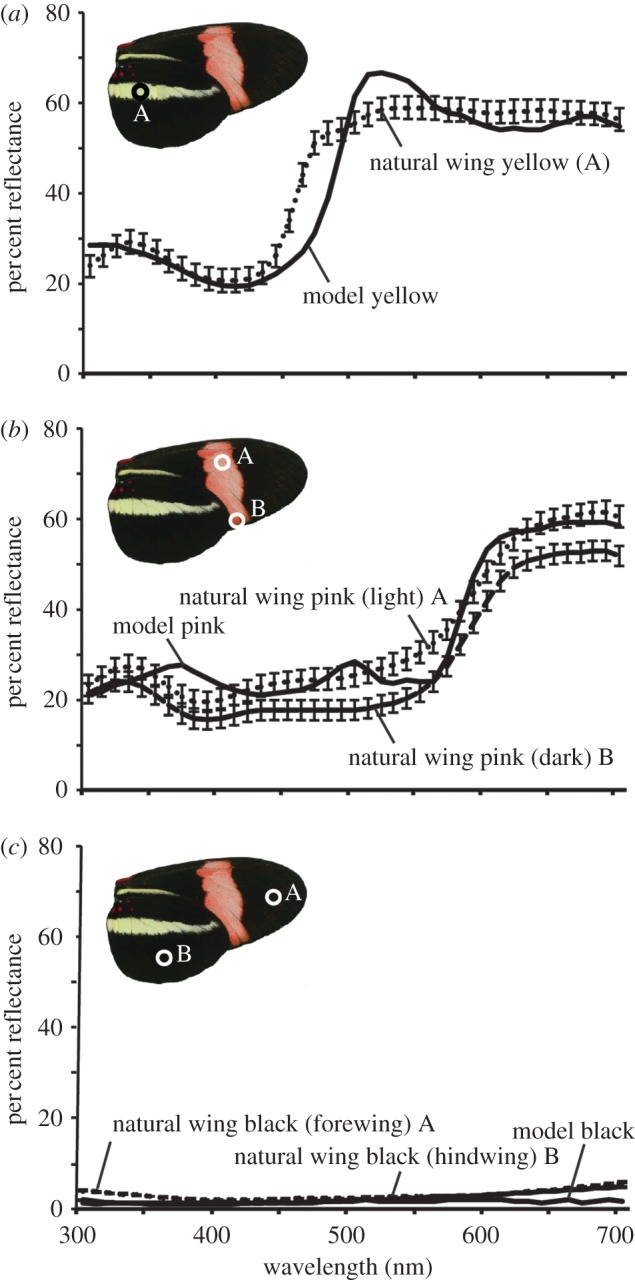
Reflectance spectra of natural and artificial butterfly wings used in discriminability calculations. Shown are the mean values and standard errors (each n = 12). Graphs include a ventral image of Heliconius erato petiverana with circled regions indicating where wing reflectance measurements were taken. (a) Yellow reflectance spectra. (b) Pink reflectance spectra, comparisons made using lightest A and darkest B pink regions on the wing. (c) Black reflectance spectra, comparisons made using both forewing A and hindwing B regions.
Table 1.
Results from discriminability calculations using low light intensity and open habitat irradiance. Comparisons were made using the blue tit (Parus caeruleus) and chicken (Gallus gallus) cone sensitivities, and are based on mean values (n = 12). Spectra comparisons fell below the threshold of one just noticeable difference (JND). FV, forewing ventral; HV, hindwing ventral; L, light; D, dark.
| JND comparisons | FV-L pink versus model pink | FV-D pink versus model pink | HV yellow versus model yellow | FV black versus model black | HV black versus model black |
|---|---|---|---|---|---|
| P. caeruleus | 0.597 | 0.374 | 0.736 | 0.336 | 0.440 |
| G. gallus | 0.556 | 0.424 | 0.858 | 0.315 | 0.533 |
(d). Predation experiments
Butterfly models were tied to branches with thread in appropriate roosting habitats and in natural roosting postures [10,13] (figure 1b). At our Costa Rica field site, a total of 320 aggregations containing five butterflies each and 320 single butterflies were used for the first predation experiment. The models were placed in 80 different forest sites, each containing four roosts and four single individuals. All 80 sites were at least 250 m apart to control for the home range territories of primary Heliconius avian predators. Predator home range sizes vary between 100 and 250 m, and have been determined by other researchers through radio tracking (flycatchers [54]), harmonic distance method and core area use (tanagers [55]), minimum convex polygon modelling (flycatchers [54]) and observation (tanagers [56]; jacamars [57]; C. E. G. Pinheiro 2011, personal communication; L. E. Gilbert 2011, personal communication). Tree Tanglefoot was applied to the base of plant stems containing artificial butterflies to avoid removal or attack of the models by ants and other small arthropods, and it was also effective in preventing small vertebrates such as lizards from reaching the butterfly models [58].
The models were left at their sites for a total of 96 h (4 days), and each model was examined daily for predation evidence and replaced if attacked. None of the 80 sites were used twice in the study because predator forgetting time varies between bird species, and is affected by prey conspicuousness and distastefulness [59–61], both difficult to measure for this project. A butterfly was considered attacked if damage to the abdomen and wings appeared in the form of beak marks and/or large indentations in the abdomen (figure 1c,d; see also [62,63]). Small chew-like marks, probably from mandibular insects such as grasshoppers, were not considered in the data analyses. If a model was attacked twice on two separate days, then it was counted only as a single attack. If multiple butterflies in a roost were attacked, this was also counted as a single attack (i.e. each roost was treated as a unit), because in nature when a roost is disturbed most or all individuals depart from the roosting site [13,29] (S. D. Finkbeiner 2010, personal observation), thereby reducing the probability of further predation attempts on individual roost mates. Predation differences were analysed using a Wilcoxon signed-rank test with continuity correction, using each site as a sample.
To assess the per capita attack risk of individual butterflies, single (focal) individuals were randomly selected from each roost to compare attack rates under the conservative assumption that one attack leads to the dispersal of the other roosting butterflies. For roosts of one, this single individual was the focal individual. The binomial response of attack (yes, no) was modelled as dependent upon roost size using generalized linear models. In these analyses, site was included as a random effect to account for potential non-independence among replicate roosts within sites.
As a control for our models, we compared the attack rate on models with real wings (and Plasticine abdomens) to the attack rate on models with artificial wings, using five forest sites separated 250 m apart, each with four models with real wings and four models with artificial wings. They were left for 96 h and checked daily for attacks. We found no difference in attacks between real-wing models and artificial-wing models (Wilcoxon signed-rank test: W = 249.5, p = 0.836, n = 20).
The second predation study, conducted in Panama, investigated the association between roost size and predation frequency. This was tested using the same artificial butterfly models described earlier. One hundred forest sites were chosen, approximately 250 m apart, and each of these sites contained two roosts of 2, two roosts of 5 and two roosts of 10 butterflies. This totalled 600 artificial roosts: 200 roosts for each treatment. Roosts were removed after 4 days, each of the 100 sites was used only once and attacked models were replaced when necessary. If a roost was attacked more than once or if more than one butterfly in the roost was attacked, then it was counted only as one attack; thus each roost was considered as a single unit in the analysis. These predation data were analysed using a Kruskal–Wallis multiple comparison test with a post hoc Bonferroni correction, using each site as a sample. Individual per capita attack risk was determined by comparing attack rates between randomly selected single (focal) individuals in each roost treatment, and the binomial response of attack was modelled as dependent upon roost size using generalized linear models, with site included as a random effect. The post hoc tests for the significance of pairwise comparisons were made using a Tukey test.
We determined at what time of day roosts are most susceptible to attack to gain further information about the roost predators. This study was conducted with 100 artificial roosts in Panama: 52 roosts of two placed randomly along two trails in Soberanía National Park, with the remaining 48 roosts already being observed for predation data (16 more roosts of 2, 5 and 10 each). The artificial roosts were checked for attacks every 3 h from 06.00 (15 min prior to sunrise) to 18.00 (just before sunset) for 9 days.
3. Results
(a). Observations of roost departure following
To assess whether Heliconius butterflies rely on communal roosts as information-sharing centres, we observed following behaviour during morning roost departures. Of 256 H. erato departures by at least 66 unique individuals from nine different roosts, only one instance of following from the roost to a flowering plant was observed (Wilcoxon signed-rank test: W = 256, p < 0.0001, n = 256, based on the null hypothesis that there is no difference between the number of butterflies that do and do not follow). Additionally, out of 74 H. sara butterfly departures from at least 25 unique individuals and three different roosts, we observed no incidence of following. When butterflies departed roosts in the morning occasionally more than one individual would leave at the same time, but they were never observed to follow one another. Most of the time the butterflies left individually, even when there was a disturbance event.
Lantana and Psychotria plants, considered to be key Heliconius resources, were common at our study sites, and it is important to note that spatial distribution and density of nectar and pollen sources may influence whether following happens. We observed that many roosting butterflies shared the same flower resources and followed each other between flowering plants, as previously described by Waller & Gilbert [27]. As well, on multiple occasions, we observed a new recruit following an established roost member to the aggregation, suggesting following behaviour may play a role in roost recruitment.
(b). Observations on roost size and proximity
The average H. erato roost size was observed to be 4.3 individuals (s.d. = 1.6, n = 233 observations across nine natural roosts) from roosts in Gamboa, Panama. It was common to find multiple roosts within 15 m of each other, some as close as 3 m apart, and in line-of-sight from one another in a given part of the home range. We observed this in both H. erato and H. sara. When butterflies of H. erato were exercising pre-roosting behaviour, the butterflies often interacted with one another before convening at their preferred roosts.
(c). Effect of roost size on predation frequency
To determine whether communal roosts provide an anti-predatory benefit, we first tested whether there is a difference in predation between single butterfly models or models placed in aggregations of five. We observed a very strong difference in attack frequencies between roosting and solitary butterflies. Of 320 artificial H. erato roosts, only 25 were attacked compared with 68 attacks on 320 single individuals (Wilcoxon signed-rank test: W = 4141.5, p = 0.000262, n = 80 sites; table 2). Individual attack risk also differed significantly between roosts of 1 (single butterflies) and roosts of 5 (F1,559 = 36.85, p < 0.0001, n = 640 roosts total), with 21.3 per cent of focal individuals being attacked in roosts of 1 but only 3.4 per cent of focal individuals being attacked in roosts of 5 (table 2).
Table 2.
Data representing attacks on butterfly models at field sites in Costa Rica and Panama, categorized by aggregation size. Roost attack risk was calculated by dividing attacked roosts by the overall number of roosts used in that treatment. Individual attack risk was determined by comparing attack rates between randomly selected single (focal) individuals in each roost treatment, based on the assumption that one attack leads to the dispersal of the other roosting butterflies. Probability values between pairwise comparisons are indicated by asterisks.
| aggregation size | total observations | roosts attacked | attack risk (per roost, %) | attack risk (per individual, %) |
|---|---|---|---|---|
| Costa Rica | ||||
| single individuals | 320 | 68*** | 21.3 | 21.3*** |
| roost of five | 320 | 25 | 7.8 | 3.4 |
| Panama | ||||
| roost of two | 200 | 21* | 10.5 | 9.5** |
| roost of five | 200 | 8 | 4.0 | 1.5 |
| roost of 10 | 200 | *24 | 12.0 | 4.5 |
*p < 0.05.
**p < 0.001.
***p < 0.0005.
Considering the difference we found in predation between single butterflies and roosts, we decided to investigate whether the predator deterrence effect was similar across roosts of different sizes (table 2). We found significantly higher predation on roosts of 2 and 10 than on roosts of 5 (Kruskal–Wallis test: χ2 = 8.7356, p = 0.0127, n = 100 sites). The post hoc Bonferroni correction showed that roosts of 10 were attacked more than roosts of 5 (p = 0.016), and roosts of 2 were attacked more than roosts of 5 (p = 0.028). There was no significant difference in predation frequency between roosts of 2 and 10 (p = 1.000). Individual attack rate also differed significantly between the three roost sizes (F2,498 = 5.51, p = 0.0043, n = 600 roosts total), with attack rates on focal individuals in roosts of 2, 5 and 10 being 9.5 per cent, 1.5 per cent and 4.5 per cent, respectively (table 2). Adjusting significance levels to account for multiple comparisons, Tukey tests showed that a single butterfly in a roost of 2 has a higher attack risk than a single butterfly in a roost of 5 (p = 0.00064), but there is no difference in individual attack risk between roosts of 5 and 10 butterflies (p = 0.22), or between roosts of 2 and 10 butterflies (p = 0.13).
(d). Timing and nature of predation
We sought to determine at which time of day roosts are most susceptible to attack. We found that all attacks occurred during the morning hours, as previously observed by Mallet [13]. Ten out of 12 attacks, from 100 different artificial roosts, occurred between the hours of 06.00 and 09.00, and two attacks occurred between the hours of 09.00 and 12.00. Triangular beak marks observed in the model abdomens supports previous findings [48–50] that birds are the primary predators of Heliconius. This is further supported because all attacks on models occurred between the hours of 06.00 and 12.00 when birds are most active [56,64].
4. Discussion
In this study, we assessed the two major hypotheses for explaining why Heliconius butterflies participate in communal roosting behaviour. We found no support for the information-sharing hypothesis, because there was little evidence of roost mates following each other to resources upon departure from roosts. These findings are in agreement with Mallet [13], who observed a similar lack of following and even a predictable tendency for roost mates to visit different flowers.
In contrast, we found very strong support for the anti-predator defence hypothesis. Our field experiments in Costa Rica using avian-indiscriminable butterfly models showed predation attempts on singly placed models were nearly three times higher than predation attempts on roosts of five models, and the predation risk for a single butterfly is over six times the per capita predation risk for an individual butterfly in a roost of 5 (table 2). A second field experiment in Panama showed the same trend, with attack rates more than twice as high on roosts of 2 versus 5, and individual risk over six times higher in a roost of 2 than a roost of 5 (table 2). Surprisingly, however, the Panama experiment also showed that attacks on roosts of 10 were three times as high as on roosts of 5 (table 2), thus suggesting the predator deterrence effect may be weak or non-existent in large aggregations. In the Panama experiment, the greatest individual fitness benefit was seen in roosts of 5, with an individual predation risk of 1.5 per cent; however, individuals in roosts of 10 benefited only slightly less (not significant) than those in roosts of 5 (individual risk of 4.5% in roosts of 10). Therefore, even though roosts of 10 did not enjoy a significantly decreased predation rate compared with roosts of 2 or 5, a simple prey dilution effect [39] would still favour large roost sizes.
Our field studies suggest that the most beneficial minimum roost size, with respect to group advantage, may be around 5 individuals. This is because our experimental aggregations of five models experienced the lowest overall attack rates and also offered the lowest per capita attack risk for individuals. Interestingly, this experimentally determined minimum roost size corresponds closely to naturally observed H. erato roost sizes (4.3). This correspondence implies that predator deterrence, coupled with a prey dilution effect, could help explain roost sizes observed in natural populations. An optimal or minimum roost size may be important for predator deterrence when butterfly densities are too low for assembling in larger aggregations; however, roost sizes are probably influenced by foraging and resource availability as well.
Because medium-sized roosts provide an anti-predatory benefit through collective aposematism, it is unclear why larger aggregations appear to lose their ability to deter predators. It is possible that solitary individuals or very small roosts are too small to communicate an effective warning signal, whereas very large roosts may be conspicuous enough to attract naïve predators. In support of this idea, Salcedo [29] noted that the most frequent predator disturbances on H. sara roosts occurred on the largest aggregation studied (10–16 individuals), suggesting that oversized aggregations of Heliconius may increase the frequency of predator attacks. Although there is much evidence that predator wariness and aggregation-induced phobias increase with the size of aposematic prey aggregations [61], greater aggregation distinctiveness increases detectability costs and, in some cases, larger aggregation sizes of defended animals result in higher predation [23,65]. There may be other costs to forming larger groups such that small movements made by other butterflies could produce incidental disturbances or ‘false alarms’, causing unexpected or premature roost departures, but this would not explain higher predation on larger roosts from our experimental data.
We propose that it is no coincidence that multiple roosts are found in the same location in a home range and often in line-of-sight from one another. We have observed this in H. erato, H. sara, H. melpomene and H. charithonia, with some roosts neighbouring the roosts of heterospecifics (in Costa Rica). Others have observed this Heliconius behaviour of preferentially forming smaller aggregations as well [13,66] (C. Boggs 2011, personal communication). Salcedo [29] proposes these may be early-stage aggregations made up of individuals who have not yet located a larger roost. In contrast, however, our field observations of pre-roosting interactions between butterflies from different roosts suggest that the butterflies are aware of other roosts in their home range, yet still choose to join smaller aggregations, despite plenty of substrate (i.e. dry branches and twigs) to sustain much larger aggregations. On the basis of the assumption that individual fitness should increase with larger aggregations owing to a reduced per capita predation risk, again it is unclear why local butterflies do not simply choose to form very large roosts. There is the possibility that an interaction among roosts is introduced, so that if individuals in one group are attacked the predator is inhibited from attacking individuals in other groups [42]. This repeated warning display could therefore facilitate rapid learning in naïve birds whose feeding areas may include multiple Heliconius roosting sites [67,68]. It is also possible that very large roosts could attract high enough levels of predation to cancel out the prey dilution benefit, although experimental work is required to test this idea.
Here, we show that Heliconius butterflies do not rely on communal roosts as information-sharing centres. Instead, our field studies indicate that this behaviour confers an anti-predatory benefit at both the individual level (prey dilution) and group level (collective aposematism), and attack risks vary between individuals in different aggregation sizes. This correspondence suggests that predation may be the key selective pressure maintaining communal roosting in Heliconius, thus providing insight into the types of ecological pressures that contribute to the evolution of social behaviour in historically solitary animals.
Acknowledgments
We thank Kailen Mooney for aid in project design and statistical analyses; Jim Mallet for advice and comments; Christian Salcedo and Daniel Osorio for feedback on the manuscript; Nancy Burley, Owen McMillan and Larry Gilbert for advice; Jasmine Velez, Talia Gustafson, Jimena Golcher and Maranatha Kellinger for aid in field observations and assisting in the preparation of butterfly models; Johannes Spaethe, Chris Jiggins and Patricio Salazar for tips on butterfly models; Dave Krueger and UCI ImageWorks for aid in designing and printing models; the Smithsonian Tropical Research Institute (STRI) and Organization for Tropical Studies (OTS) for use of field sites; La Autoridad Nacional del Ambiente (ANAM, Panama) and El Ministerio del Ambiente, Energía, y Telecomunicaciones (MINAET, Costa Rica) for research permit approval; and our funding sources: the Smithsonian Tropical Research Institue, the Organization for Tropical Studies, the U.S. Department of Education GAANN, the National Geographic Society; this material is based upon work supported by the National Science Foundation (NSF) Graduate Research Fellowship under award no. DGE-0808392 to S.D.F. and NSF grant no. IOS-1025106 to A.D.B. and R.D.R.
References
- 1.Scudder S. H., Allen J. A. 1869. A preliminary list of the butterflies of Iowa. Trans. Chicago Acad. Sci. 1, 326–337 [Google Scholar]
- 2.Brower L. P. 1995. Understanding and misunderstanding the migration of the monarch butterfly (Nymphalidae) in North America: 1857–1995. J. Lepid. Soc. 49, 304–385 [Google Scholar]
- 3.Edwards W. H. 1881. On certain habits of Heliconia charitonia Linn., a species of butterfly found in Florida. Papilio 1, 209–215 [Google Scholar]
- 4.Ward P. 1965. Feeding ecology of the black-faced dioch Quellea quellea in Nigeria. Ibis 107, 173–214 10.1111/j.1474-919X.1965.tb07296.x (doi:10.1111/j.1474-919X.1965.tb07296.x) [DOI] [Google Scholar]
- 5.Soini P. 1987. Ecology of the saddle-back tamarin Saguinus fuscicollis-illigeri on the Rio Pacaya, northeastern Peru. Folia Primatol. 49, 11–32 10.1159/000156305 (doi:10.1159/000156305) [DOI] [Google Scholar]
- 6.Lewis S. E. 1995. Roost fidelity of bats: a review. J. Mammal. 76, 481–496 10.2307/1382357 (doi:10.2307/1382357) [DOI] [Google Scholar]
- 7.Merkel F. R., Mosbech A. 2008. Diurnal and nocturnal feeding strategies in common eiders. Waterbirds 31, 580–586 10.1675/1524-4695-31.4.580 (doi:10.1675/1524-4695-31.4.580) [DOI] [Google Scholar]
- 8.Pearson D. L., Anderson J. J. 1985. Perching heights and nocturnal communal roosts of some tiger beetles (Coleoptera: Cicindelidae) in southeastern Peru. Biotropica 17, 126–129 10.2307/2388504 (doi:10.2307/2388504) [DOI] [Google Scholar]
- 9.DeVries P. J., Schull J., Greig N. 1987. Synchronous nocturnal activity and gregarious roosting in the neotropical skipper butterfly Celaenorrhinus fritzgaertneri (Lepidoptera: Hesperiidae). Biol. J. Linn. Soc. 89, 89–103 10.1111/j.1096-3642.1987.tb01345.x (doi:10.1111/j.1096-3642.1987.tb01345.x) [DOI] [Google Scholar]
- 10.Salcedo C. 2010. Environmental elements involved in communal roosting in Heliconius butterflies (Lepidoptera: Nymphalidae). Environ. Entom. 39, 907–911 10.1603/EN09340 (doi:10.1603/EN09340) [DOI] [PubMed] [Google Scholar]
- 11.Benson W. W., Emmel T. C. 1973. Demography of gregariously roosting populations of the nymphaline butterfly Marpesia berania in Costa Rica. Ecology 54, 326–335 10.2307/1934340 (doi:10.2307/1934340) [DOI] [Google Scholar]
- 12.Turner J. R. G. 1975. Communal roosting in relation to warning colour in two heliconiine butterflies (Nymphalidae). J. Lepid. Soc. 29, 221–226 [Google Scholar]
- 13.Mallet J. 1986. Gregarious roosting and home range in Heliconius butterflies. Natl. Geogr. Res. 2, 198–215 [Google Scholar]
- 14.Erlich P. R., Gilbert L. E. 1973. Population structure and dynamics of the tropical butterfly Heliconius ethilla. Biotropica 5, 69–82 10.2307/2989656 (doi:10.2307/2989656) [DOI] [Google Scholar]
- 15.Mallet J. 1986. Dispersal and gene flow in a butterfly with home range behavior: Heliconius erato (Lepidoptera: Nymphalidae). Oecologia 68, 210–217 10.1007/BF00384789 (doi:10.1007/BF00384789) [DOI] [PubMed] [Google Scholar]
- 16.Gilbert L. E. 1972. Pollen feeding and reproductive biology of Heliconius butterflies. Proc. Natl Acad. Sci. USA 69, 1403–1407 10.1073/pnas.69.6.1403 (doi:10.1073/pnas.69.6.1403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boggs C. L., Smiley J. T., Gilbert L. E. 1981. Patterns of pollen exploitation by Heliconius butterflies. Oecologia 48, 284–289 10.1007/BF00347978 (doi:10.1007/BF00347978) [DOI] [PubMed] [Google Scholar]
- 18.Engler-Chaouat H. S., Gilbert L. E. 2007. De novo synthesis versus sequestration: negatively correlated metabolic traits and the evolution of host plant specialization in cyanogenic butterflies. J. Chem. Ecol. 33, 25–42 10.1007/s10886-006-9207-8 (doi:10.1007/s10886-006-9207-8) [DOI] [PubMed] [Google Scholar]
- 19.Cott H. B. 1957. Adaptive coloration in animals. London, UK: Methuen [Google Scholar]
- 20.Guilford T. 1990. The evolution of aposematism. In Insect defenses: adaptive mechanisms and strategies of prey and predators (eds Evans D. L., Schmidt J. O.), pp. 23–61 Albany, NY: State University of New York Press [Google Scholar]
- 21.Santos J. C., Coloma L. A., Cannatella D. C. 2003. Multiple, recurring origins of aposematism and diet specialization in poison frogs. Proc. Natl Acad. Sci. USA 100, 12 792–12 797 10.1073/pnas.2133521100 (doi:10.1073/pnas.2133521100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamberale G., Tullberg B. S. 1998. Aposematism and gregariousness: the combined effect of group size and coloration on signal repellence. Proc. R. Soc. Lond. B 265, 889–894 10.1098/rspb.1998.0374 (doi:10.1098/rspb.1998.0374) [DOI] [Google Scholar]
- 23.Riipi M., Alatalo R. V., Lindström L., Mappes J. 2001. Multiple benefits of gregariousnes cover detectability costs in aposematic aggregations. Nature 413, 512–514 10.1038/35097061 (doi:10.1038/35097061) [DOI] [PubMed] [Google Scholar]
- 24.Jones F. M. 1930. The sleeping heliconias of Florida. Nat. Hist. 30, 635–644 [Google Scholar]
- 25.Young A. M., Thomason J. H. 1975. Notes on communal roosting of Heliconius charitonius (Nymphalidae) in Costa Rica. J. Lepid. Soc. 29, 243–255 [Google Scholar]
- 26.Mallet J. L. B. 1980. A laboratory study of gregarious roosting in the butterfly Heliconius melpomene. MSc thesis, University of Newcastle-upon-Tyne, Newcastle-upon-Tyne, UK [Google Scholar]
- 27.Waller D. A., Gilbert L. E. 1982. Roost recruitment and resource utilization: observations on a Heliconius charitonia L. roost in Mexico . J. Lepid. Soc. 36, 178–184 [Google Scholar]
- 28.Mallet J., Gilbert L. E. 1995. Why are there so many mimicry rings? Correlations between habitat, behaviour and mimicry in Heliconius butterflies. Biol. J. Linn. Soc. 55, 159–180 10.1111/j.1095-8312.1995.tb01057.x (doi:10.1111/j.1095-8312.1995.tb01057.x) [DOI] [Google Scholar]
- 29.Salcedo C. 2011. Evidence of predation and disturbance events at Heliconius (Insecta: Lepidoptera: Nymphalidae) nocturnal aggregations in Panama and Costa Rica. J. Nat. Hist. 45, 1715–1721 10.1080/00222933.2011.559692 (doi:10.1080/00222933.2011.559692) [DOI] [Google Scholar]
- 30.Gilbert L. E. 1976. Postmating female odor in Heliconius butterflies: male-contributed anti-aphrodisiac. Science 193, 419–420 10.1126/science.935877 (doi:10.1126/science.935877) [DOI] [PubMed] [Google Scholar]
- 31.Schulz S., Estrada C., Yildizhan S., Boppré M., Gilbert L. E. 2008. An antiaphrodisiac in Heliconius melpomene butterflies. J. Chem. Ecol. 34, 82–93 10.1007/s10886-007-9393-z (doi:10.1007/s10886-007-9393-z) [DOI] [PubMed] [Google Scholar]
- 32.Gilbert L. E. 1975. Ecological consequences of a coevolved mutualism between butterflies and plants. In Coevolution of animals and plants (eds Gilbert L. E., Raven P. R.), pp. 210–240 Austin, TX: University of Texas Press [Google Scholar]
- 33.Ward P., Zahavi A. 1973. The importance of certain assemblages of birds as ‘information centers’ for food finding. Ibis 115, 517–534 10.1111/j.1474-919X.1973.tb01990.x (doi:10.1111/j.1474-919X.1973.tb01990.x) [DOI] [Google Scholar]
- 34.Dall S. R. X. 2002. Can information sharing explain recruitment to food from communal roosts? Behav. Ecol. 13, 42–51 10.1093/beheco/13.1.42 (doi:10.1093/beheco/13.1.42) [DOI] [Google Scholar]
- 35.Gillett S. D., Hogarth P. J., Noble F. E. 1979. The response of predators to varying densities of Gregaria locust nymphs. Anim. Behav. 27, 592–596 10.1016/0003-3472(79)90195-7 (doi:10.1016/0003-3472(79)90195-7) [DOI] [Google Scholar]
- 36.Benson W. W. 1971. Evidence for the evolution of unpalatability through kin selection in the Heliconiinae (Lepidoptera). Am. Nat. 105, 213–226 10.1086/282719 (doi:10.1086/282719) [DOI] [Google Scholar]
- 37.Edmunds M. 1974. Defence in animals: a survey of anti-predator defences. Harlow, UK: Longman [Google Scholar]
- 38.Vulinek K. 1990. Collective security: aggregation by insects as a defense. In Insect defenses: adaptive mechanisms and strategies of prey and predators (eds Evans D. L., Schmidt J. O.), pp. 251–288 Albany, NY: State University of New York Press [Google Scholar]
- 39.Bertram B. C. R. 1978. Living in groups: predators and prey. In Behavioural ecology: an evolutionary approach (eds Krebs J. R., Davies N. B.), pp. 64–96 Sunderland, MA: Sinauer Associates [Google Scholar]
- 40.Hamilton W. D. 1971. Geometry for the selfish herd. J. Theor. Biol. 31, 295–311 10.1016/0022-5193(71)90189-5 (doi:10.1016/0022-5193(71)90189-5) [DOI] [PubMed] [Google Scholar]
- 41.Turner G. F., Pitcher T. J. 1986. Attack abatement: a model for group protection by combined avoidance and dilution. Am. Nat. 128, 228–240 10.1086/284556 (doi:10.1086/284556) [DOI] [Google Scholar]
- 42.Sillén-Tullberg B., Leimar O. 1988. The evolution of gregariousness in distasteful insects as a defense against predators. Am. Nat. 132, 723–734 10.1086/284884 (doi:10.1086/284884) [DOI] [Google Scholar]
- 43.Karlsson B. 1987. Variation in egg weight, oviposition rate and reproductive reserves with female age in a natural population of the speckled wood butterfly, Pararge aegeria. Ecol. Entomol. 12, 473–476 10.1111/j.1365-2311.1987.tb01029.x (doi:10.1111/j.1365-2311.1987.tb01029.x) [DOI] [Google Scholar]
- 44.Mallet J., Longino J. T., Murawski D., Murawski A., Simpson de Gamboa A. 1987. Handling effects in Heliconius: where do all the butterflies go? J. Anim. Ecol. 56, 377–386 10.2307/5054 (doi:10.2307/5054) [DOI] [Google Scholar]
- 45.Reed R. D., McMillan W. O., Nagy L. M. 2008. Gene expression underlying adaptive variation in Heliconius wing patterns: non-modular regulation of overlapping cinnabar and vermilion prepatterns. Proc. R. Soc. B 275, 37–45 10.1098/rspb.2007.1115 (doi:10.1098/rspb.2007.1115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Briscoe A. D., Bybee S. M., Bernard G. D., Yuan F., Sison-Mangus M. P., Reed R. D., Warren A. D., Llorente-Bosquets J., Chiao C.-C. 2010. Positive selection of a duplicated UV-sensitive visual pigment coincides with wing pigment evolution in Heliconius butterflies. Proc. Natl Acad Sci. USA 107, 3628–3633 10.1073/pnas.0910085107 (doi:10.1073/pnas.0910085107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chai P. 1986. Field observations and feeding experiments on the responses of rufous-tailed jacamars (Galbula ruficauda) to free-flying butterflies in a tropical rainforest. Biol. J. Linn. Soc. 29, 161–189 10.1111/j.1095-8312.1986.tb01772.x (doi:10.1111/j.1095-8312.1986.tb01772.x) [DOI] [Google Scholar]
- 48.Pinheiro C. E. G. 2003. Does Müllerian mimicry work in nature? Experiments with butterflies and birds (Tyrannidae). Biotropica 35, 356–364 10.1111/j.1744-7429.2003.tb00589.x (doi:10.1111/j.1744-7429.2003.tb00589.x) [DOI] [Google Scholar]
- 49.Langham G. M. 2004. Specialized avian predators repeatedly attack novel color morphs of Heliconius butterflies. Evolution 58, 2783–2787. [DOI] [PubMed] [Google Scholar]
- 50.Pinheiro C. E. G. 2011. On the evolution of warning coloration, Batesian and Müllerian mimicry in Neotropical butterflies: the role of jacamars (Galbulidae) and tyrant-flycatchers (Tyrannidae). J. Avian Biol. 42, 277–281 10.1111/j.1600-048X.2011.05435.x (doi:10.1111/j.1600-048X.2011.05435.x) [DOI] [Google Scholar]
- 51.Kelber A., Vorobyev M., Osorio D. 2003. Animal colour vision: behavioural tests and physiological concepts. Biol. Rev. 78, 81–118 10.1017/S1464793102005985 (doi:10.1017/S1464793102005985) [DOI] [PubMed] [Google Scholar]
- 52.Vorobyev M., Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358 10.1098/rspb.1998.0302 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bybee S. M., Yuan F., Ramstetter M. D., Llorente-Bousquets J., Reed R. D., Osorio D., Briscoe A. D. 2011. UV Photoreceptors and UV-yellow wing pigments in Heliconius butterflies allow a color signal to serve both mimicry and intraspecific communication. Am. Nat. 179, 38–51 10.1086/663192 (doi:10.1086/663192) [DOI] [PubMed] [Google Scholar]
- 54.Ning W., Zhang Y., Zheng G. 2007. Home ranges and habitat vegetation characters in breeding season of Narcissus Flycatcher and Yellow-rumped Flycatcher. Front. Biol. China 2, 345–350 10.1007/s11515-007-0051-1 (doi:10.1007/s11515-007-0051-1) [DOI] [Google Scholar]
- 55.Samuel M. D., Pierce D. J., Garton E. O. 1985. Identifying areas of concentrated use within home range. J. Anim. Ecol. 54, 711–719 10.2307/4373 (doi:10.2307/4373) [DOI] [Google Scholar]
- 56.Buskirk W. H., Powell G. V. N., Wittenberger J. F., Buskirk R. E., Powell T. U. 1972. Interspecific bird flocks in tropical highland Panama. The Auk 89, 612–624 [Google Scholar]
- 57.Pinheiro C. E. G., Bagno M. A., Brandão R. A. 2003. Diet and foraging behavior of the rufous-tailed jacamar (Galbula ruficauda, Galbulidae) in central Brazil. Ararajuba 11, 241–243 [Google Scholar]
- 58.Dial R., Roughgarden J. 1995. Experimental removal of insectivores from rain forest canopy: direct and indirect effects. Ecology 76, 1821–1834 10.2307/1940714 (doi:10.2307/1940714) [DOI] [Google Scholar]
- 59.Servedio M. R. 2000. The effects of predator learning, forgetting, and recognition errors on the evolution of warning coloration. Evolution 54, 751–763 10.1111/j.0014-3820.2000.tb00077.x (doi:10.1111/j.0014-3820.2000.tb00077.x) [DOI] [PubMed] [Google Scholar]
- 60.Speed M. P. 2000. Warning signals, receiver psychology and predator memory. Anim. Behav. 60, 269–278 10.1006/anbe.2000.1430 (doi:10.1006/anbe.2000.1430) [DOI] [PubMed] [Google Scholar]
- 61.Ruxton G. D., Sherratt T. N., Speed M. P. 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. Oxford, UK: Oxford University Press [Google Scholar]
- 62.Brodie E. D. 1993. Differential avoidance of coral snake banded patterns by free-ranging avian predators in Costa Rica. Evolution 47, 227–235 10.2307/2410131 (doi:10.2307/2410131) [DOI] [PubMed] [Google Scholar]
- 63.Stobbe N., Schaefer H. M. 2008. Enhancement of chromatic contrast increases predation risk for striped butterflies. Proc. R. Soc. B 275, 1535–1541 10.1098/rspb.2008.0209 (doi:10.1098/rspb.2008.0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DesLauriers J. V., Francis C. M. 1991. The effect of time of day on mist-net captures of passerines on spring migration. J. Field Ornithol. 62, 107–116 [Google Scholar]
- 65.Lindstedt C., Huttunen H., Kakko M., Mappes J. 2011. Disentangling the evolution of weak warning signals: high detection risk and low production costs of chemical defences in gregarious pine sawfly larvae. Evol. Ecol. 25, 1029–1046 10.1007/s10682-010-9456-4 (doi:10.1007/s10682-010-9456-4) [DOI] [Google Scholar]
- 66.Salcedo C. 2006. Spatial dynamics of night roosting in Heliconius erato petiverana (Lepidoptera: Nymphalidae). MSc thesis, University of Florida, Gainesville, FL [Google Scholar]
- 67.Poulton E. B. 1931. The gregarious sleeping habits of Heliconius charitonius L. Proc. R. Entomol. Soc. Lond. 6, 4–10 [Google Scholar]
- 68.Tuskes P. M., Brower L. P. 1978. Overwintering ecology of the monarch butterfly, Danaus plexippus L., in California. Ecol. Entomol. 3, 141–153 10.1111/j.1365-2311.1978.tb00912.x (doi:10.1111/j.1365-2311.1978.tb00912.x) [DOI] [Google Scholar]



