Abstract
Plasticity in behaviour is of fundamental significance when environments are variable. Such plasticity is particularly important in the context of rapid changes in the socio-sexual environment. Males can exhibit adaptive plastic responses to variation in the overall level of reproductive competition. However, the extent of behavioural flexibility within individuals, and the degree to which rapidly changing plastic responses map onto fitness are unknown. We addressed this by determining the behaviour and fitness profiles of individual Drosophila melanogaster males subjected to up to three episodes of exposure to rivals or no rivals, in all combinations. Behaviour (mating duration) was remarkably sensitive to the level of competition and fully reversible, suggesting that substantial costs arise from the incorrect expression of even highly flexible behaviour. However, changes in mating duration matched fitness outcomes (offspring number) only in scenarios in which males experienced zero then high competition. Following the removal of competition, mating duration, but not offspring production, decreased to below control levels. This indicates that the benefit of increasing reproductive investment when encountering rivals may exceed that of decreasing investment when rivals disappear. Such asymmetric fitness benefits and mismatches with behavioural responses are expected to exert strong selection on the evolution of plasticity.
Keywords: Drosophila melanogaster, sperm competition, phenotypic plasticity, male–male competition
1. Introduction
Phenotypic plasticity is a widespread and important component of fitness [1]. It can be manifested in several ways. For example, during development, the environment that is encountered can trigger a switch to one of several possible fixed and irreversible strategies. Alternatively, plasticity may remain highly flexible and reversible throughout life [2]. It is not yet clear whether these alternatives represent distinct phenomena or opposite ends of a continuum [3]. The type of phenotypic plasticity that is favoured is likely to depend on the rapidity of changes in key environmental variables, the reliability of environmental cues and the time lag that occurs between cue detection and the resulting plastic response [4]. Behavioural plasticity is of particular significance in animals as it allows the expression of rapid and reversible responses to the environment. It is assumed that such responses are relatively ‘inexpensive’ [3,5,6] and hence that behaviour is limitlessly flexible. However, in recent years, much research has been focused on why the expression of plasticity can sometimes be limited [7], leading instead to consistent inter-individual variation in behaviour (i.e. behavioural syndromes [8]). We conclude that while consistency in behaviour might sometimes be selected [9], behavioural plasticity is also predicted to be adaptive as it enables animals to respond to short-term environmental fluctuations and therefore maximize fitness in the face of uncertainty [10].
One important and prevalent factor that can change rapidly is the socio-sexual environment. For example, local sex ratios, which signal both mating opportunities and the amount of competition an individual is likely to encounter, can change within a breeding session [11]. Under polygamy, males will encounter competition both before and after mating in each of the matings they obtain. Hence, males are predicted to trade-off resources between present and future matings and to adjust their investment according to the level of competition they encounter [12,13]. Examples of plastic male mating strategies in response to the socio-sexual environment are widespread and include the strategic allocation of ejaculate components [14] and diverse types of behavioural adjustments [15]. However, very few studies have assessed whether plasticity is reversible [15]. The few such studies that address this issue measure responses in treatment cohorts and hence reaction norms for populations rather than individuals [6]. Measurements of the extent to which individuals can express reversible plasticity can be assessed only using paired or repeated measures designs [16]. Such tests are an important and currently missing component of studies of plasticity. They can show whether, rather than merely representing facets of sexual maturation or learning, changes in behaviour that occur upon exposure to a low then high competitive environment are indeed fully plastic. Plasticity observed following reciprocal manipulations of competition levels can therefore provide evidence of responses that are independent of sexual experience. Another important omission is measures of the relationship between observed plasticity and indices of fitness. These are needed in order to understand the adaptive value of plasticity. For example, reversible responses to the presence of rivals have been observed in courtship [17–19], copulation number or duration [16,20] and the amount of sperm ejaculated [21]. However, for none of these studies, do we yet have direct measures of fitness, e.g. number of offspring produced.
In this study, we tested whether individual males could exhibit reversible plastic behaviour, measured as mating duration, over multiple exposures to high and low competition, and determined the number of offspring subsequently produced by those males. We measured the consistent plastic responses of males to rivals in the fruitfly, Drosophila melanogaster. In this species, males respond by mating for significantly longer following single episodes of exposure to rival males prior to mating. This confers significant fitness benefits, i.e. increases in offspring, for males in both competitive and non-competitive situations, including increased egg laying, increased egg-adult survival, decreased female remating and an increased share of paternity [22]. These benefits are associated with increased transfer by males of seminal fluid accessory gland proteins (Acps) in the longer matings that occur following exposure to rivals [23]. Male responses are initiated following a minimum length of exposure to rivals and are accurately calibrated according to exposure duration but not rival density [24]. Males use a set of multiple, but interchangeable cues to detect rivals, suggesting that there are substantial fitness benefits of responding correctly to the current socio-sexual environment [25]. Given that mating duration appears primarily under male control [26], the most parsimonious explanation is that extended mating duration following exposure to rivals is indeed a male-derived effect to increase paternity share. Nevertheless, it is possible that females could detect whether their mate has been exposed to rivals and hence respond differently to them, but this has not yet been tested explicitly.
Here, we investigated the extent to which individual males could respond to the presence or the absence of rivals in up to three episodes of high and low competition, in all combinations. We measured both behavioural responses (mating duration) and fitness (offspring number) for each of the matings, to determine whether the benefits of responding in each scenario were consistent. We used the number of offspring produced in the 24 h after mating under non-competitive conditions as a logistically manageable proxy for fitness. Our previous work showed that this measure is highly correlated—specifically in this experimental context—with other fitness measures, including offspring gained under competitive conditions [22]: it was therefore an appropriate index of reproductive success and fitness for this study (see also §4). We could therefore establish how accurately reproductive success tracked changes in the plastic behavioural response of mating duration. If plastic behaviour is associated with a physiological response (e.g. increased sperm production), then behaviour and fitness benefits could become uncoupled if behaviour is more rapidly flexible than physiological responses. We also tested whether the ultimate costs or benefits of responding to rival males differed according to the direction of change in the level of competition (i.e. high to low versus low to high). For example, it may be more important to increase investment in traits that secure fitness when competition is highly likely, than to decrease investment when competition is less likely.
We predicted that males maintained in stable environments, either consistently with or without rivals, would not show significant changes in mating duration or offspring production across matings. By contrast, males that were switched males between environments were expected to change mating duration and offspring production, with those exposed to a rival then held alone expected to decrease mating duration and offspring production, and those held alone then exposed to a rival to increase mating duration and offspring production.
2. Material and methods
(a). General fly rearing and experimental procedure
Fly rearing and all experiments were conducted in a 25°C humidified room, with a 12 L : 12 D cycle. Flies were maintained in glass vials (75 × 25 mm) containing 7 ml standard sugar–yeast-agar medium (100 g Brewer's yeast powder, 100 g sugar, 20 g agar, 30 ml Nipagin (10% w/v solution) and 3 ml propionic acid per litre of medium) [27]. Wild-type flies were from a large laboratory population originally collected in the 1970s in Dahomey (Benin) and were from the strain used previously in our related studies [22,24,25]. Larvae for all experiments were raised at a standard density of 100 per vial, supplemented with live yeast liquid. At eclosion, flies were collected and the sexes separated using ice anaesthesia. Males were assigned randomly to treatments in each experiment. Initial sample sizes were 50 per treatment; however, any vials containing dead males (focal or rival) were discarded. Virgin females were stored 10 per vial on medium supplemented with live yeast granules, until 1 day prior to mating when they were transferred to individual mating vials using ice anaesthesia. Rival males were identified by the use of a small wing clip (wing tips were clipped using a scalpel under CO2 anaesthesia). At mating, single focal males were introduced to single females using aspiration. The latency to mate and mating duration was then recorded. Pairs were given 2 h to mate and those that did not mate within this time were discarded. There were 3–7/50 non-maters in the Two Exposures experiment (see §2b) and 7–16/50 in the Three Exposures experiment (see §2c). There was no significant difference in the number of non-maters within the 2 h assay period between treatments (χ23 = 2.04, p = 0.57; χ27 = 9.10, p = 0.24). After mating, males were immediately aspirated into a new exposure vial, which was either empty (for no-exposure treatments) or contained a wing clipped rival (exposure treatments).
(b). Behavioural responses of males (mating duration) to exposure to rivals or no rivals in series: two exposures
We have previously shown that males respond to the potential level of competition they are likely to encounter from rival males by mating for significantly longer following a single episode of exposure to a rival [22,24,25]. To test the prediction that this male behavioural response to rivals is reversible and can accurately be tailored to current levels of competition, we measured the mating duration of individual males after two bouts of exposure to a rival and/or no rival in all combinations. Hence, males were either maintained in a stable exposure regime (‘no change’, i.e. either with or without a rival across both episodes), or switched between regimes. Males were not exposed (0) or exposed to a rival (R) for 3 days, then mated to a virgin female on the fourth day post-eclosion. Immediately after mating, males were transferred to new vials and exposed or not exposed to a rival for a second 3 day period. Following this, males were again mated to a second virgin female on the seventh day post-eclosion. Hence, there were four treatments (00, 0R, R0, RR), which either remained in a ‘no change’ exposure regime (without a rival, 00; always with a rival, RR), or a ‘switched exposure’ regime (0R or R0). Therefore, we would predict that males experiencing ‘no change’ in exposure regime should not show a change in mating duration, whereas those that are ‘switched’ should either (0R) or decrease mating duration (R0).
(c). Behavioural and fitness responses of males (mating duration and offspring number) to exposure to rivals or no rivals in series: three exposures
We then replicated the experiment above and extended it by challenging males to switch behaviour following an additional exposure to rivals or no rivals. In this experiment, we also counted the offspring produced in the 24 h following each mating as a measure of a male's fitness. As noted earlier, our previous work shows that this non-competitive measure is correlated with a male's competitive reproductive success in this experimental context [22]. The method was as described earlier, except that there was a third mating on the 10th day post-eclosion. Hence, there were eight treatments (000, 00R, 0R0, R00, R0R, 0RR, RR0, RRR). The prediction was that males in ‘no change’ regimes would not show a difference between matings (i.e. we expect no change in the duration of first and second matings for 000, 00R, RR0 and RRR treatments and no change in second and third matings for 000, R00, 0RR and RRR), whereas those males that are switched should either increase (0 → R) or decrease mating duration (R → 0). Females were allowed to lay eggs for 24 h after mating. Offspring emerging from those eggs were frozen at 12 days post-laying, for subsequent counting. We predicted that changes in offspring production should follow changes in mating duration, that is where duration is increased (0 → R), offspring production should also increase, and where duration is decreased (R → 0), offspring production should also decrease.
(d). Statistical analysis
Statistical analysis was performed using SPSS v. 16.0 (SPSS Inc., Chicago, IL, USA) and R v. 2.10.1 [28]. Data were not normally distributed (Kolmogorov–Smirnov tests, p > 0.05) and could not be transformed to achieve normality; hence, we used non-parametric tests or applied appropriate error distributions. We used generalized linear mixed models (GLMMs). Mating duration or offspring number were the response variables, and the presence of a rival and mating (first, second or third mating by the male) were fixed effects, with male identity (ID) as a random factor. We used analysis of deviance with removal of factors from the maximal model. Significant effects of presence of a rival indicate the existence of plasticity, whereas interactions between fixed effects and ID show evidence for individual male consistency in mating duration despite alterations to the level of competition. We also investigated the extent of reversibility in responses to rivals by using one-sample Wilcoxon signed-rank tests, to examine whether the difference between mating duration and number of offspring produced showed significant deviations from 0 (i.e. ‘no change’ between the mating duration between the first and second or second and third mating). We then assessed, using Mann–Whitney U-tests, whether treatments that switched regimes (i.e. 0 → R or R → 0) were different from the ‘no change’ regimes (0 → 0 or R → R). To minimize multiple testing, we first grouped treatments by exposure regimes. Hence, between the first and second mating, the ‘no change’ regimes comprised treatments 000, 00R, RR0 and RRR, ‘minus rival’ regimes comprised treatments R00 and R0R, and ‘plus rival’ regimes comprised treatments 0R0 and 0RR. For comparisons between the second and third mating, the ‘no change’ regimes comprised treatments 000, R00, 0RR and RRR, ‘minus rival’ regimes comprised treatments 0R0 and RR0, and ‘plus rival’ regimes comprised treatments 00R and R0R. The effect of mating history was examined by testing the treatments individually for comparisons between the second and third matings. We applied sequential Bonferroni corrections for multiple tests. In order to assess the relative importance of plasticity and consistency, we followed the method of Briffa et al. [29] by comparing effect sizes. We derived effect sizes for plasticity from the difference in duration using paired Wilcoxon signed-rank tests (where effect size = z/√n) and for consistency using rho from Spearman's rank correlations between mating durations. We analysed the effects of mating number and exposure to a rival on latency to mate using GLMMs as described earlier for mating duration. Post hoc Mann–Whitney U-tests were then used to clarify where any significant differences lay.
3. Results
(a). Males modulate mating duration in response to the competitive environment
As predicted, males that were exposed to rivals subsequently mated for significantly longer than males not exposed, for all matings in the Two and Three Exposure experiments (GLMM; Two Exposures: χ21 = 43.81, p < 0.0001, figure 1a; Three Exposures: χ22 = 68.67, p < 0.0001). Mating number (i.e. first, second or third mating) had no significant effect in the Two Exposures experiment (χ22 = 0.64, p = 0.424) but was marginally significant in the Three Exposures experiment (χ22 = 6.31, p = 0.043). In both experiments, removal of the interaction with male ID significantly reduced the fit of the model (p < 0.0001), suggesting that individual males showed consistency as well as plasticity in responses to rivals. There was no consistent pattern of effects of exposure to rivals on mating latency in any of the experiments (see the electronic supplementary material).
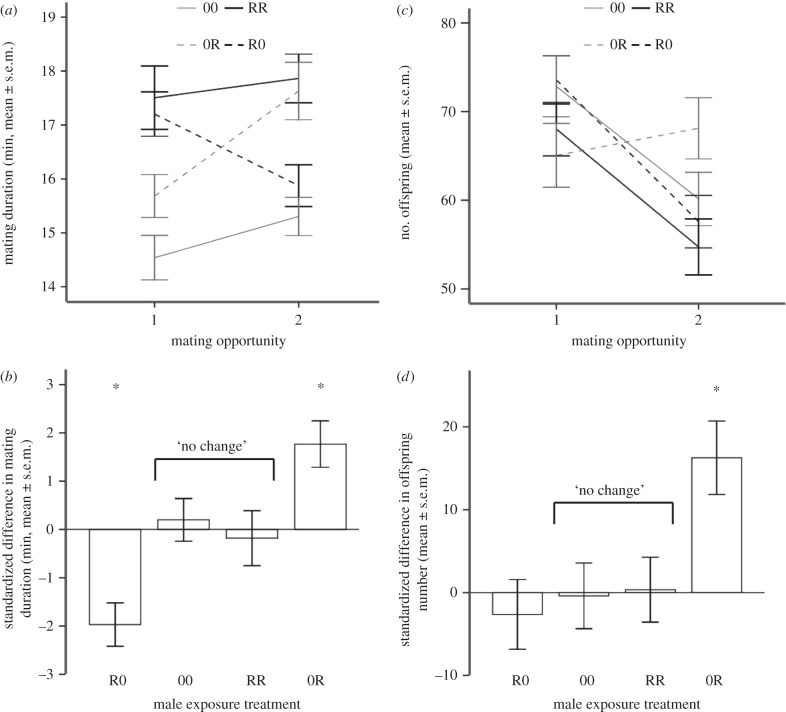
Figure 1.

Mating duration following the first and second matings in the Two Exposures experiment. R = held with a rival, 0 = without a rival prior to each mating (e.g. R0 = first exposure was with a rival, second exposure without a rival). (a) Mating duration at each mating. (b) The difference in mating duration between first and second matings standardized to the mean of the ‘no change’ treatments. Asterisk indicates a significant difference from the ‘no change’ regime, after applying sequential Bonferroni correction.
(b). Males significantly alter mating duration behaviour following a change in the competitive environment
Data from both experiments supported the predictions, namely that stable environments should result in no change to mating duration, and that increases and decreases in exposure to rivals should cause increases and decreases in mating duration, respectively. In the Two Exposures experiment, males in ‘no change’ exposure regimes did not significantly change in mating duration between their first and second mating (Wilcoxon signed-rank test, 00 treatment: V = 358.5, n = 47, p = 0.490; RR treatment: V = 478.0, n = 47, p = 0.744; figure 1a). R → 0 males significantly decreased their mating duration in their second mating (z = 3.06, n = 136, p = 0.002; figure 1a,b), and 0 → R males significantly increased mating duration (z = 3.34, n = 137, p = 0.001; figure 1a,b).
In the Three Exposures experiment, there was a marginally significant increase in the second mating duration compared with the first, for ‘no change’ males (V = 6135.5, n = 155, p = 0.046), which is consistent with the significant effect of mating number in the GLMM analysis. Males that were switched between exposure regimes either decreased or increased mating duration in the predicted direction compared with the ‘no change’ treatments (R → 0 ‘minus rival’ significantly decreased mating duration: z = 4.03, n = 239, p < 0.0001; 0 → R ‘plus rival’ showed a significant increase: z = 3.15, n = 234, p = 0.002; figure 2a and electronic supplementary material, figure S1). Comparing the second and third mating durations showed that there was no significant difference in the ‘no change’ regimes compared with 0 (V = 4521.5, n = 155, p = 0.990). Again, males that were switched between exposure regimes changed their mating duration in the predicted direction (R → 0 ‘minus rival’ showed a significant decrease in mating duration: z = 2.83, n = 236, p = 0.005; 0 → R ‘plus rival’ showed a significant increase: z = 4.87, n = 238, p < 0.0001; figure 2b and electronic supplementary material, figure S1).
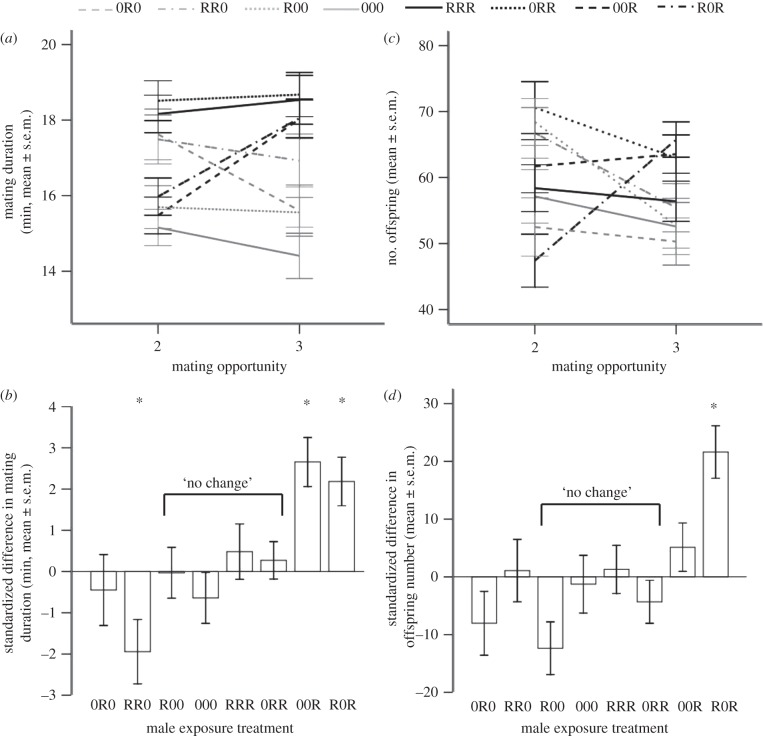
Figure 2.
Mating duration and offspring produced following the first and second matings in the Three Exposures experiment, R = held with a rival, 0 = without a rival prior to each mating (e.g. R0 = first exposure was with a rival, second exposure without a rival). (a) Mating duration at each mating. (b) Difference in mating duration between first and second matings standardized to the mean of the ‘no change’ treatments. (c) The number of offspring produced at each mating and (d) the difference in offspring produced between first and second matings standardized to the mean of the ‘no change’ treatments. Asterisk indicates that the difference is significantly different from the ‘no change’ regime, after sequential Bonferroni correction.
(c). Male fitness (offspring number) tracks behavioural responses under increasing but not decreasing competition
In contrast to the results for mating duration, the fitness data (offspring production) did not fully match the predictions. We saw the expected increase in offspring production under increasing exposure to rivals but not the decrease in offspring when rivals were removed. There was a significant interaction between male exposure to rivals and mating number on the number of offspring produced (GLMM χ22 = 11.65, p = 0.0006), and also a significant interaction with male ID, indicating that males showed individual, consistent responses (p < 0.0001). There was no significant difference in offspring produced between R → 0 and ‘no change’ regimes (first and second mating: z = 0.64, n = 239, p = 0.552, figure 2b and electronic supplementary material, S1; second and third mating: z = 0.10, n = 238, p = 0.919). However, 0 → R males increased offspring output significantly compared with the ‘no change’ regimes (first and second mating: z = 3.38, n = 234, p = 0.001, figure 2b and electronic supplementary material, S1; second and third mating: z = 4.25, n = 238, p < 0.0001). Therefore, 0 → R males significantly increased mating duration and offspring output as predicted when competition increased. By contrast, R → 0 males significantly decreased mating duration but did not fully decrease investment in offspring production when competition levels dropped.
Across the three matings, there was a decline in the number of offspring produced by males in the ‘no change’ exposure regimes (first and second mating: V = 2311.0, n = 155, p < 0.0001, figure 2c and electronic supplementary material, figure S1; second and third mating: V = 3807.0, n = 155, p = 0.0004, figure 3c and electronic supplementary material, figure S1). Overall, males exposed to rivals produced significantly more offspring (z = 3.024, n = 963, p = 0.002). This effect was not significant in the first two matings (first mating: z = 1.235, n = 321, p = 0.217; second mating: z = 1.651, n = 321, p = 0.099), but highly significant in the third (z = 4.057, n = 321, p < 0.0001).
Figure 3.
Mating duration and offspring produced following the second and third matings in the Three Exposures experiment. R = held with a rival, 0 = without a rival prior to each mating (e.g. R0R = first exposure was with a rival, second exposure without a rival, third exposure with a rival). (a) Mating duration at each mating and (b) the difference in mating duration between second and third matings standardized to the mean of the ‘no change’ treatments. (c) Offspring produced at each mating and (d) the difference in offspring produced between second and third matings standardized to the mean of the ‘no change’ treatments. Asterisk indicates that the difference is significantly different from the ‘no change’ regime, after sequential Bonferroni correction.
(d). Exposure history has a significant effect on behavioural (mating duration) and fitness (offspring number) responses to rivals
Although males changed their behaviour according to current levels of competition, the first exposure regime had a significant effect on subsequent behaviour and fitness in second and third matings. In the latter comparison of the minus rival regimes (0R0 and RR0), only RR0 significantly decreased mating duration (RR0: z = 2.89, n = 196, p = 0.004; 0R0: z = 1.56, n = 199, p = 0.120; figure 3a and electronic supplementary material, S1). Neither 0R0 nor RR0 differed significantly in offspring number from the ‘no change’ treatments (RR0: z = 0.72, n = 193, p = 0.475; 0R0: z = 0.53, n = 198, p = 0.596; figure 3b and electronic supplementary material, S1). Therefore, treatments initially exposed to a rival (RR0) subsequently decreased mating duration, but did not suffer a decrease in offspring output. The plus rival regimes (00R and R0R) both increased mating duration compared with ‘no change’ treatments (00R: z = 3.99, n = 199, p < 0.0001; R0R: z = 3.58, n = 196, p < 0.0001; figure 3a and electronic supplementary material, figure S1), and both significantly increased offspring production (00R: z = 1.89, n = 197, p = 0.048; R0R: z = 4.73, n = 196, p < 0.0001; figure 3b and electronic supplementary material, figure S1). However, R0R produced significantly more offspring than 00R (z = 2.52, n = 83, p = 0.012). Taken together, these analyses suggested that prior exposure to rivals increased a male's competitiveness in subsequent reproductive episodes.
(e). Males show consistent individual responses as well as plasticity
In the analyses above, significant effects of exposure to males and of interactions with male ID indicated evidence for both individual male plasticity and consistency in mating duration, respectively. Male consistency was also evidenced by significant positive correlations between mating duration across the different matings in both experiments (Two Exposures experiment Spearman's rank correlation: rho = 0.46, n = 182, p < 0.0001; Three Exposures experiment Kendall's coefficient of concordance: W = 0.01, n = 321, p = 0.044). Comparing effects sizes for the difference between exposure regimes from Wilcoxon signed-rank tests (plasticity) and correlations between mating durations from Spearman's rho (consistency) showed that both plasticity and consistency had moderate to large effect sizes, though in six of eight comparisons the effect size associated with plasticity was greater (electronic supplementary material, table S1).
4. Discussion
Males were highly flexible in their responses to the likelihood of competition, and altered their mating duration behaviour accordingly with great accuracy. Males increased mating duration when they were exposed to rivals prior to mating and reduced mating duration when they were not. However, the fitness benefit of responding to rivals, measured as number of offspring produced in a non-competitive context, only partially matched onto the apparently fully flexible behavioural responses. This suggests that there were asymmetric fitness benefits. Behaviour and offspring production were aligned when increased investment occurred following exposure to rivals after exposure to no rivals. However, there was a mismatch when decreased investment was expected (i.e. exposure to rivals then no rivals), as males significantly decreased mating duration but not offspring production, in comparison with the stable ‘no change’ treatments.
Behaviour is generally predicted to be highly flexible and ‘inexpensive’ [3,5,6]. Our study reveals that males can match their behaviour to the current competitive environment very precisely. This suggests that there are significant costs associated with expressing the wrong mating duration behaviour for the current social context. Such costs could be separate to those resulting from the actual expression of the behaviour per se, and this would be interesting to test in the future. Importantly, the ability to reverse plastic responses in either direction, or maintain mating duration under a stable social environment shows that the changes in mating duration are not simply a sexual maturation or age effect. Hence, males do not require prior experience of other individuals (either male or female) to tailor mating duration to the conditions, unlike for example, male courtship, whereby males learn to avoid directing courtship towards other males [30] or towards immature females [31].
Our interpretation of the results assumes that mating duration is largely under male control. As noted in §1, evidence supporting this view comes from the finding that mating duration in crosses between lines artificially selected for long or short matings follows the male line of origin [26]. We therefore interpret extended mating duration following exposure to rivals as a male-derived effect to increase paternity share in an environment in which the likelihood of sperm competition is increased. Nevertheless, it is important to consider other possible alternatives. For instance, extended mating duration following exposure of males to rivals could also be influenced by differential responses of females to males. This would require that females can detect males who have previously been exposed to rivals and respond in a way that causes an increase in mating duration. Possible mechanisms include the detection by females of differences in a male's odour profile [32], stress responses or behaviour. The benefits to females of extending mating duration have not yet been determined but could include increased productivity and decreased overall mating frequency. However, if the benefit of longer mating derives from increased seminal fluid protein synthesis (see below), then female-mediated effects on mating duration per se would not necessarily alter reproductive success. As yet, we have no evidence that females control mating duration and hence influence a male's response to rivals in this respect. However, explicit tests of these ideas would be very useful.
While male mating duration behaviour generally tracked the competitive environment, there were mismatches in terms of fitness measured as the number of offspring produced. As discussed in §1, we used the number of offspring produced under non-competitive conditions as an index of fitness. This assay was employed for logistical reasons, and on the basis that it is highly correlated with offspring produced under competitive scenarios in this specific experimental context [22]. We suggest that our short-term measure of a male's non-competitive reproductive output is a good index for fitness in our experimental design [25], but is not necessarily a suitable general fitness measure. We present mating duration data as indicative of behaviour, mapping onto a non-competitive fitness estimate. In reality, the distinction between behavioural and fitness adjustments is unlikely to be as clear-cut. For instance, mating duration behaviour may also represent an opportunity for males to alter their reproductive investment via physiological mechanisms, such as increased seminal fluid synthesis and transfer. The fitness measure we used does indicate investment in mechanisms leading directly to the production of increased offspring numbers, but could omit other types of investment and overlook trade-offs with other life-history traits. Nevertheless, despite the simplifications we have employed, our results support the idea that responses associated with behaviour can show more flexibility than those more closely linked to investment in progeny production.
We found here and in previous work [22,25] that extended mating duration following exposure to rivals increased reproductive success. However, in general, there is no simple relationship between offspring production and the duration of mating in D. melanogaster. Sperm delivery during mating, for example, is quantal and not linear with time [33]. Hence, the association between mating duration and reproductive success in this species is often loose or absent [34]. Mating duration does, however, strongly affect offspring production when males have previously been exposed to rivals as in this study. Under these conditions, longer matings lead to elevated seminal fluid transfer to females [23] and increased progeny production under both competitive and non-competitive conditions [22]. Our previous work indicates that this is because males need more than 24 h to detect and respond adaptively to the presence of rivals [24]. We propose that this period is needed in order for males to increase their production of seminal fluid proteins [23]. Therefore, it is not mating duration in general that is positively associated with increased progeny production, but male-mediated extension of mating duration following exposure males to rivals. Increased mating duration associated with increased offspring production is therefore probably a specific response to the level of sperm competition (as simulated by exposure to rivals).
Overall, as expected, males exposed to rivals prior to mating produced significantly more offspring than those not so exposed. However, surprisingly this was significant for only the third mating, in contrast to the expectation from our previous work [22,25]. Nevertheless, the number of offspring produced increased significantly when males moved from no- to high-exposure treatments. However, when moving from high to no competition, the number of offspring produced was equivalent to the ‘no change’ treatments, rather than the predicted decrease below the no change level, as was observed for mating duration. This suggests two possible scenarios: that the cost of not responding to decreased levels of competition is negligible, or that once males are physiologically primed for an increasing competitive environment they cannot easily or rapidly decrease their investment in offspring.
Selection on males to reduce investment when levels of competition decrease will depend on the likelihood of future matings and hence the encounter rate with females [35]. If a future mating is unlikely, preserving reproductive effort will not be strongly selected. Costs associated with these responses have not been measured, and while the presence of plasticity implies that they exist, they are not necessarily equal across all competitive environments. For example, our data suggest that failing to respond under increasing threat levels from rivals is more costly than over-investing in the absence of competition. The quality of the information available to males may also play a role. For example, the presence of a rival may be a better indicator of the level of competition than the absence of a rival is of the lack of competition.
The only previous study of which we are aware, to measure fitness indices and reversibility in behavioural responses to rivals, was in burying beetles (Nicrophorus vespilloides) [16]. In this, subordinate males increased mating duration in the presence of dominant males; however, this was not linked to changes in paternity success [16]. More studies are therefore needed to establish a general picture of costs and benefits associated with plastic responses to mating rivals. Asymmetric costs of responding incorrectly are clearly predicted in some contexts. For example, not responding to a predator when there is one present is more costly than responding when one is absent. Nevertheless, such asymmetries are not necessarily obvious in regard to responses to mating competition and have not yet been incorporated into theory [12,13,36].
The inability to decrease investment in offspring production when competition decreases may be an unavoidable physiological consequence of increased previous investment. There may be a physiological carry-over effect, whereby behaviour is matched to the current conditions, but investment in sperm or Acps is matched to previous levels of competition. Carry-over effects are almost an inevitable consequence of sequential actions, and can be observed in long-lived animals from one season to the next [37]. It has been suggested that such carry-over effects can lock an individual into a behavioural pattern [38]. Our findings show that while behaviour remains flexible, investment patterns do not remain flexible to the same degree. We have previously found that male responses to rivals are not instantaneous and require more than 24 h exposure. As noted earlier, this implies that responses involve physiological changes in addition to reallocation of existing resources [24]. Increases in mating duration following exposure to rivals are associated with increased seminal protein transfer. However, contrary to predictions, the expression of three seminal protein and four spermatogenesis genes was either unaltered or was decreased following exposure to rivals [39]. This could indicate that increases in reproductive investment arise through increased transfer of seminal proteins and sperm rather than through their increased production.
Our data also showed that exposure history influenced subsequent behavioural adjustments. We found that exposure to rivals prior to first matings affected the difference between the second and third mating durations. Males that were initially exposed to rivals remained more competitive than those not so exposed. The initial exposure to a rival may raise the male's perception of the average level of competition at the population level. For instance, males encountering a rival in two out of three exposures experienced higher levels of competition on average, and may hence have invested more than those encountering a rival in one out of three exposures. In addition, it has been proposed that early experiences set behavioural tendencies, reducing the flexibility of a trait over time [4,8]. Our data suggest that behaviour remains flexible, but more strongly so in response to increasing levels of competition.
While we showed that mating duration was flexible, our data also provided evidence for individual consistency. If, for example, males mated for relatively longer on average, then they continued to do so even after adjustments in response to the competitive environment. It is possible that males in relatively better condition can invest more in each mating regardless of the level of competition they face. Coexistence of plasticity and consistency has been found in other contexts. For example, startle responses in hermit crabs, Pagurus bernhardus [29], show significant plasticity, but the effect is greatly outweighed by consistency. Likewise, the degree of boldness in fishing spiders, Dolomedes triton, is plastic but also correlated across different contexts of foraging and courtship [40]. This is consistent with the behavioural syndrome framework [8], in which behaviours observed in different contexts are correlated. However, unlike boldness, copulation duration can be observed only in one context (i.e. when mating), but could be correlated with other behaviours, such as courtship or aggression.
5. Conclusion
We showed that behavioural changes (mating duration) in response to mating rivals were reversible in either direction. The findings are generally important because they provide evidence for the rarely tested assumption that individual behaviour is highly flexible. Our results also showed that behavioural responses correlated with fitness benefits (offspring production) only in an arena of increasing competition. Reproductive investment patterns under high competition were therefore strongly linked to reproductive output, and our results exemplify how fitness gains can be context-dependent.
Acknowledgements
We thank Claudia Fricke, Dominic Edward and Darrell Green for help with experimental work. We thank Tom Price for discussion of the manuscript before submission and two anonymous reviewers and Associate Editor Mike Ritchie for comments that substantially improved the manuscript. This work was funded by a BBSRC grant (no. BB/H002499/1) to T.C., M.J.G.G. and A B.
References
- 1.West-Eberhard M. J. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Piersma T., Drent J. 2003. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 18, 228–233 10.1016/S0169-5347(03)00036-3 (doi:10.1016/S0169-5347(03)00036-3) [DOI] [Google Scholar]
- 3.Ghalambor C. K., Angeloni L. M., Carroll S. P. 2010. Behavior as phenotypic plasticity. In Evolutionary behavioral ecology (eds Westneat D. F., Fox C. W.), pp. 90–107 Oxford, UK: Oxford University Press [Google Scholar]
- 4.Gabriel W., Luttbeg B., Sih A., Tollrian R. 2005. Environmental tolerance, heterogeneity, and the evolution of reversible plastic responses. Am. Nat. 166, 339–353 10.1086/432558 (doi:10.1086/432558) [DOI] [PubMed] [Google Scholar]
- 5.Parker G. A. 1982. Phenotype-limited evolutionary stable strategies. In Current problems in sociobiology (ed. King's College Sociobiology Group C), pp. 173–201 Cambridge, UK: Cambridge University Press [Google Scholar]
- 6.Sih A. 2004. A behavioural ecological view of phenotypic plasticity. In Phenotypic plasticity: functional and conceptual approaches (eds DeWitt T. J., Scheiner S. M.), pp. 112–125 Oxford, UK: Oxford University Press [Google Scholar]
- 7.Auld J. R., Agrawal A. A., Relyea R. A. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511 10.1098/rspb.2009.1355 (doi:10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sih A., Bell A. M., Johnson J. C. 2004. Behavioural syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 9.Schuett W., Tregenza T., Dall S. R. X. 2010. Sexual selection and animal personality. Biol. Rev. 85, 217–249 10.1111/j.1469-185X.2009.00101.x (doi:10.1111/j.1469-185X.2009.00101.x) [DOI] [PubMed] [Google Scholar]
- 10.Komers P. E. 1997. Behavioural plasticity in variable environments. Can. J. Zool. 75, 161–169 10.1139/z97-023 (doi:10.1139/z97-023) [DOI] [Google Scholar]
- 11.Kasumovic M. M., Bruce M. J., Andrade M. C. B., Herberstein M. E. 2008. Spatial and temporal demographic variation drives within-season fluctuations in sexual selection. Evolution 62, 2316–2325 10.1111/j.1558-5646.2008.00446.x (doi:10.1111/j.1558-5646.2008.00446.x) [DOI] [PubMed] [Google Scholar]
- 12.Parker G. A., Ball M. A., Stockley P., Gage M. J. G. 1996. Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc. R. Soc. Lond. B 263, 1291–1297 10.1098/rspb.1996.0189 (doi:10.1098/rspb.1996.0189) [DOI] [Google Scholar]
- 13.Parker G. A., Ball M. A., Stockley P., Gage M. J. G. 1997. Sperm competition games: a prospective analysis of risk assessment. Proc. R. Soc. Lond. B 264, 1793–1802 10.1098/rspb.1997.0249 (doi:10.1098/rspb.1997.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wedell N., Gage M. J. G., Parker G. A. 2002. Sperm competition, male prudence and sperm limited females. Trends Ecol. Evol. 17, 313–320 10.1016/S0169-5347(02)02533-8 (doi:10.1016/S0169-5347(02)02533-8) [DOI] [Google Scholar]
- 15.Bretman A., Gage M. J. G., Chapman T. 2011. Quick-change artists: adult behavioural plasticity at mating. Trends Ecol. Evol. 26, 467–473 10.1016/j.tree.2011.05.002 (doi:10.1016/j.tree.2011.05.002) [DOI] [PubMed] [Google Scholar]
- 16.Sakaluk S. K., Muller J. K. 2008. Risk of sperm competition mediates copulation duration, but not paternity, of male burying beetles. J. Insect Behav. 21, 153–163 10.1007/s10905-007-9115-y (doi:10.1007/s10905-007-9115-y) [DOI] [Google Scholar]
- 17.Verrell P. A., Krenz J. D. 1998. Competition for mates in the mole salamander, Ambystoma talpoideum: tactics that may maximize male mating success. Behaviour 135, 121–138 [Google Scholar]
- 18.Dzieweczynski T. L., Lyman S., Poor E. A. 2009. Male Siamese fighting fish, Betta splendens, increase rather than conceal courtship behavior when a rival is present. Ethology 115, 186–195 10.1111/j.1439-0310.2008.01602.x (doi:10.1111/j.1439-0310.2008.01602.x) [DOI] [Google Scholar]
- 19.Lyons C., Barnard C. J. 2006. A learned response to sperm competition in the field cricket, Gryllus bimaculatus (de Geer). Anim. Behav. 72, 673–680 10.1016/j.anbehav.2005.12.006 (doi:10.1016/j.anbehav.2005.12.006) [DOI] [Google Scholar]
- 20.Alonso-Pimentel H., Papaj D. R. 1996. Operational sex ratio versus gender density as determinants of copulation duration in the walnut fly, Rhagoletis juglandis (Diptera: Tephritidae). Behav. Ecol. Sociobiol. 39, 171–180 10.1007/s002650050278 (doi:10.1007/s002650050278) [DOI] [Google Scholar]
- 21.Pound N., Gage M. J. G. 2004. Prudent sperm allocation in Norway rats, Rattus norvegicus: a mammalian model of adaptive ejaculate adjustment. Anim. Behav. 68, 819–823 10.1016/j.anbehav.2004.02.004 (doi:10.1016/j.anbehav.2004.02.004) [DOI] [Google Scholar]
- 22.Bretman A., Fricke C., Chapman T. 2009. Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. Proc. R. Soc. B 276, 1705–1711 10.1098/rspb.2008.1878 (doi:10.1098/rspb.2008.1878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wigby S., Sirot L. K., Linklater J. R., Buehner N., Calboli F. C. F., Bretman A., Wolfner M. F., Chapman T. 2009. Drosophila melanogaster males modify seminal fluid protein transfer in response to social cues and artificial selection on accessory gland size. Curr. Biol. 19, 751–757 10.1016/j.cub.2009.03.036 (doi:10.1016/j.cub.2009.03.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bretman A., Fricke C., Hetherington P., Stone R., Chapman T. 2010. Exposure to rivals and plastic responses to sperm competition in Drosophila melanogaster. Behav. Ecol. 21, 317–321 10.1093/beheco/arp189 (doi:10.1093/beheco/arp189) [DOI] [Google Scholar]
- 25.Bretman A., Westmancoat J., Gage M. J. G., Chapman T. 2011. Males use multiple, redundant cues to detect mating rivals. Curr. Biol. 21, 1–6 10.1016/j.cub.2010.11.056 (doi:10.1016/j.cub.2010.11.056) [DOI] [PubMed] [Google Scholar]
- 26.MacBean I. T., Parsons P. A. 1967. Directional selection for duration of copulation in Drosophila melanogaster. Genetics 56, 233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bass T. M., Grandison R. C., Wong R., Martinez P., Partridge L., Piper M. D. W. 2007. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. A 62, 1071–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ihaka R., Gentleman R. 1996. R: a language for data analysis and graphics. J. Comp. Graph. Stat. 5, 299–314 10.2307/1390807 (doi:10.2307/1390807) [DOI] [Google Scholar]
- 29.Briffa M., Rundle S. D., Fryer A. 2008. Comparing the strength of behavioural plasticity and consistency across situations: animal personalities in the hermit crab Pagurus bernhardus. Proc. R. Soc. B 275, 1305–1311 10.1098/rspb.2008.0025 (doi:10.1098/rspb.2008.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dukas R. 2010. Causes and consequences of male–male courtship in fruit flies. Anim. Behav. 80, 913–919 10.1016/j.anbehav.2010.08.017 (doi:10.1016/j.anbehav.2010.08.017) [DOI] [Google Scholar]
- 31.Ejima A., Smith B. P. C., Lucas C., van der Goes van Naters W., Miller C. J., Carlson J. R., Levine J. D., Griffith L. C. 2007. Generalization of courtship learning in Drosophila melanogaster is mediated by cis-vaccenyl acetate. Curr. Biol. 17, 599–605 10.1016/j.cub.2007.01.053 (doi:10.1016/j.cub.2007.01.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grillet M., Dartevelle L., Ferveur J.-F. 2006. A Drosophila male pheromone affects female sexual receptivity. Proc. R. Soc. B 273, 315–323 10.1098/rspb.2005.3332 (doi:10.1098/rspb.2005.3332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilchrist A. S., Partridge L. 2000. Why it is difficult to model sperm displacement in Drosophila melanogaster: the relation between sperm transfer and copulation duration. Evolution 54, 534–542 [DOI] [PubMed] [Google Scholar]
- 34.Fricke C., Wigby S., Hobbs R., Chapman T. 2009. The benefits of male ejaculate sex peptide transfer in Drosophila melanogaster . J. Evol. Biol. 22, 275–286 10.1111/j.1420-9101.2008.01638.x (doi:10.1111/j.1420-9101.2008.01638.x) [DOI] [PubMed] [Google Scholar]
- 35.Parker G. A. 1982. Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J. Theor. Biol. 96, 281–294 10.1016/0022-5193(82)90225-9 (doi:10.1016/0022-5193(82)90225-9) [DOI] [PubMed] [Google Scholar]
- 36.Parker G. A., Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934 10.1111/j.1469-185X.2010.00140.x (doi:10.1111/j.1469-185X.2010.00140.x) [DOI] [PubMed] [Google Scholar]
- 37.Harrison X. A., Blount J. D., Inger R., Norris D. R., Bearhop S. 2011. Carry-over effects as drivers of fitness differences in animals. J. Anim. Ecol. 80, 4–18 10.1111/j.1365-2656.2010.01740.x (doi:10.1111/j.1365-2656.2010.01740.x) [DOI] [PubMed] [Google Scholar]
- 38.Dall S. R. X., Houston A. I., McNamara J. M. 2004. The behavioural ecology of personality: consistent individual differences from an adpative perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- 39.Fedorka K. M., Winterhalter W. E., Ware B. 2011. Perceived sperm competition intensity influences seminal fluid protein production prior to courtship and mating. Evolution 65, 584–590 10.1111/j.1558-5646.2010.01141.x (doi:10.1111/j.1558-5646.2010.01141.x) [DOI] [PubMed] [Google Scholar]
- 40.Johnson J. C., Sih A. 2007. Fear, food, sex and parental care: a syndrome of boldness in the fishing spider, Dolmedes triton. Anim. Behav. 74, 1131–1138 10.1016/j.anbehav.2007.02.006 (doi:10.1016/j.anbehav.2007.02.006) [DOI] [Google Scholar]