Abstract
Tradeoffs occur between a variety of traits in a diversity of organisms, and these tradeoffs can have major effects on ecological and evolutionary processes. Far less is known, however, about tradeoffs between male traits that affect mate attraction than about tradeoffs between other types of traits. Previous results indicate that females of the variable field cricket, Gryllus lineaticeps, prefer male songs with higher chirp rates and longer chirp durations. In the current study, we tested the hypothesis that a tradeoff between these traits affects the evolution of male song. The two traits were negatively correlated among full-sibling families, consistent with a genetically based tradeoff, and the tradeoff was stronger when nutrients were limiting. In addition, for males from 12 populations reared in a common environment, the traits were negatively correlated within populations, the strength of the tradeoff was largely invariant across populations, and the within-population tradeoff predicted how the traits have evolved among populations. A widespread tradeoff thus affects male trait evolution. Finally, for males from four populations assayed in the field, the traits were negatively correlated within and among populations. The tradeoff is thus robust to the presence of environmental factors that might mask its effects. Together, our results indicate there is a fundamental tradeoff between male traits that: (i) limits the ability of males to produce multiple attractive traits; (ii) limits how male traits evolve; and (iii) might favour plasticity in female mating preferences.
Keywords: sexual selection, preferred male traits, female choice, tradeoffs, field crickets
1. Introduction
Resource allocation tradeoffs are fundamental for understanding most ecological and evolutionary process. If resources are limiting, any allele that increases the amount of resources allocated to one trait can have negative pleiotropic consequences, reducing the resources that can be allocated to other traits [1,2]. Detecting tradeoffs can sometimes be difficult because variation in resource acquisition can mask variation in resource allocation; individuals able to acquire more resources may be able to allocate more resources to all of their traits, causing traits to be positively rather than negatively genetically correlated [3]. Nonetheless, there is widespread evidence that allocation tradeoffs occur [4–7]. When they do occur, allocation tradeoffs can affect a variety of processes, including the social and environmental interactions of individuals [8], population dynamics [9], community structure [10] and the evolution of physiological, behavioural, morphological and life-history traits [4–7,11]. While previous studies have examined tradeoffs involving sexually selected traits [12–17], less is known about tradeoffs between the traits that males use to attract females. This information is important, however, for understanding the limitations on how male traits can evolve in response to female mate choice, and potentially for understanding the evolution of female mating preferences.
When males advertise for mates, they often produce multiple signal types, and signals with multiple components, many of which may affect female mate choice [18]. Females often prefer these signals because the males producing them provide high fitness alleles to offspring or material resources to females or offspring [19,20]. Male traits preferred by females can be costly to produce and maintain [21], and if resources are limited, males may often be forced to trade off the resources they allocate to different traits. One reason for the paucity of studies on tradeoffs between male traits preferred by females might be the general focus of sexual selection research on the good gene mechanism [22], combined with an expectation that when two male traits are positively correlated with male fitness, they should be positively correlated with each other [23]. For example, males of higher fitness, because they acquire more resources, might be able to simultaneously allocate more to all of their traits. Whether male traits positively or negatively covary with each other, however, will depend, in part, on the relative variation among males in resource acquisition and resource allocation [3,24–26]. If there is more variation among males in how they allocate resources to two traits than there is in the resources they acquire, there will tend to be a negative correlation between the traits. Research on life-history traits, for example, has shown that allocation tradeoffs can occur even in the presence of substantial genetic variation in resource acquisition [27]. In addition, tradeoffs may result from allocation decisions that are largely unrelated to resource acquisition. For example, time is a fixed resource, and if two signals cannot be produced simultaneously, an increase in the time spent producing one may require a reduction in the time spent producing another. Finally, tradeoffs can result from biomechanical constraints on signal production; morphological structures that allow for the production of one type of signal may preclude the production of others [28].
In some animals, there is a negative phenotypic correlation between male traits preferred by females [29–31], suggesting that tradeoffs may be common. Phenotypic correlations, however, can sometimes be unreliable predictors of genetically based tradeoffs [32]. One of the few studies to examine genetically based tradeoffs between male traits preferred by females found negative genetic correlations [33]. In at least some animals, it thus appears that males may be limited in their ability to produce multiple attractive traits. Whether tradeoffs limit how male traits evolve is not clear. Comparisons among species of birds, for example, have provided mixed support for the hypothesis of evolutionarily important tradeoffs [34–37], although the results of such studies may be difficult to interpret [38].
We report the results of three studies that tested the hypothesis that there are tradeoffs between male traits, and that these tradeoffs limit how male signals evolve. Our study animal was the variable field cricket, Gryllus lineaticeps. In this species, males produce a calling song to attract females, and male songs vary in chirp rate and chirp duration. The chirps consist of strings of pulses, and each pulse is produced using one cycle of opening and closing of the forewings. Higher chirp rates result from the production of more pulse strings per unit time, which requires more wing movement, while longer chirp durations result from the production of longer pulse strings, which also requires more wing movement. Resource or biomechanical limitations might preclude males from increasing the number of wing movements per unit time, and thus from simultaneously producing high rates and long durations. Both song traits tend to be expensive for males to produce [39,40], but both increase a male's attractiveness to females [41–43]. Females appear to express preferences because they receive a fecundity benefit when they mate with males that produce higher chirp rates, and a longevity benefit when they mate with males that produce longer chirp durations [31], although these benefits can be environment-dependent [44]. In addition, there is a negative phenotypic correlation between the song traits in at least one population [31]. First, we examined the relationship between chirp rate and duration within and among 24 full-sibling families in two nutritional environments. If there is a genetically based tradeoff between these traits, then the traits should be negatively correlated among families. In addition, if the tradeoff results from a nutrient allocation tradeoff, then the negative correlation should be stronger in a lower nutrition environment [25,45]. Second, we examined the relationship between chirp rate and duration within and among 12 populations using males reared in a common environment. If a tradeoff affects how these traits evolve, then the traits should be negatively correlated among populations. In addition, because functionally related traits should be largely unaffected by geographical variation in selection on the traits [26], the strength of the tradeoff should vary little among populations. And third, we examined the relationship between chirp rate and duration within and among four populations using field recordings of wild males. This allowed us to assess whether, despite possible genetic and environmental variation in the resources acquired by males, a tradeoff found under controlled environmental conditions affects how male signals covary in nature.
2. Methods
(a). Tradeoffs within and among genotypes in two nutritional environments
To examine tradeoffs within and among genotypes, we used the second- and third-generation offspring of females collected from Tucker's Grove County Park, Santa Barbara, CA, USA (34.4527, −119.7842). Random matings between individuals from different families were used to propagate the crickets in the laboratory [44].
The rearing environments and the song recording methods have been previously described [44]. In brief, nymphs from 24 full-sibling families were selected at the third instar and half were individually raised on a high-nutrition diet and half were individually raised on a low-nutrition diet. Upon their final moult, males were randomly assigned a high-nutrition or low-nutrition diet, and were maintained on this diet throughout the experiment. Each family thus had males represented in all juvenile and adult nutrition combinations. A study using the same dataset indicated that the juvenile diet had no effect on adult chirp rate [44], and exploratory analyses for the current study indicated that juvenile diet had no effect on the relationship between chirp duration and chirp rate. It was thus not included as a factor in the analyses we report. Male songs were recorded 8 days after their final moult during the 10 h dark period of the 14 L : 10 D cycle. We recorded approximately 2 min of singing by each male, and for each recording, we calculated the male's chirp rate (chirps/s) and the mean duration of all chirps produced (ms). The temperatures at which males were recorded varied from 22.5°C to 26°C. Because male song characters are affected by temperature [40,42], we adjusted each song character to 25°C prior to analysis, using the statistical relationship between each character and temperature [31,44]. We recorded 185 males from the 24 families (mean = 7.7 males/family).
We used linear mixed models (the xtmixed function of Stata v. 10, StataCorp, with maximum-likelihood estimation) to examine the effects of chirp duration and adult nutritional environment on chirp rate. Because we were interested in separating the within- and among-family effects of chirp duration on chirp rate, two separate predictor variables were derived for each male from the chirp duration measures [46,47]: mean family chirp duration (the mean of the male's family) and within-family deviation in chirp duration (the male's deviation from the family mean). The models included chirp rate as the dependent variable and four fixed factors: mean family chirp duration, within-family deviation in chirp duration, nutritional environment and the interaction between mean family chirp duration and nutritional environment. Male family was included as a random effect. We used a likelihood ratio test to determine whether a model with random within-family slopes (variation among families in the strength of the within-family tradeoff) and random intercepts (variation among families in chirp rate) provided a significantly better fit to the data than did a model with only random intercepts. If not, this would indicate little variation among families in the strength of the within-family tradeoff, in which case, we used the model with only random intercepts [47]. In follow-up analyses that examined tradeoffs within each nutritional environment, critical p-values were adjusted using a Bonferroni correction. A negative effect of mean family chirp duration would be consistent with a genetically based tradeoff between chirp duration and rate (although the strength of the relationship does not provide a reliable estimate of the strength of the genetic correlation [48]). A negative effect of within-family deviation in chirp duration would be consistent with a phenotypic tradeoff within families between the two traits. Finally, an effect of the interaction between mean family chirp duration and nutritional environment would be consistent with an effect of diet on the strength of a genetically based tradeoff.
(b). Tradeoffs within and among populations: males reared in a common environment
To examine the relationship between chirp rate and chirp duration within and among populations, we collected females from 12 populations in California (electronic supplementary material, figure S1). These females had mated in the field and most laid eggs in the laboratory. The average pairwise linear distance between the populations was 221.1 km (n = 66 pairs, minimum = 32 km, maximum = 604.6 km). The rearing and recording methods were identical to those of the first study, except that males were fed ad libitum cat chow throughout their lives and were not separated into individual containers until shortly before their final moult. This diet has effects on male traits that are similar to the low-nutrition diet described above. For example, both the cat chow diet and the low-nutrition diet result in a positive relationship between male chirp rate and female fecundity benefits, whereas the high-nutrition diet results in a negative relationship (cf. [31,44]). All males used were from the second or third laboratory generation. This common garden-rearing design reduced environmental effects on chirp rate and duration. As a result, variation among populations in these traits can largely be attributed to evolved genetic differences.
We recorded 915 males from the 12 populations (mean = 76.3 males/population, range = 71–83) during the 10 h dark period of the 14 L : 10 D cycle. Within each population, we recorded more than one male from most full-sibling families (mean = 2.4 males/family/population, range = 1.7–3.1). The temperatures at which males were recorded varied from 21.8°C to 24°C, and prior to analyses, we adjusted each song character to the average recording temperature (see above).
We used linear mixed models to examine the effect of chirp duration on chirp rate. We used the approach described above to separate among- and within-population effects of chirp duration. The models included chirp rate as the dependent variable and two fixed factors: mean population chirp duration and within-population deviation in chirp duration. Population was included as a random effect. In addition, because more than one male was recorded from the full-sibling families within each population, male family was included as a random effect that was nested within population. As described above, we used a likelihood ratio test to determine whether a model with random within-population slopes and intercepts provided a significantly better fit to the data than did a model with only random intercepts. If not, this would indicate that there is little variation among populations in the strength of the within-population tradeoff, in which case we used the model with only random intercepts. A negative effect of mean population chirp duration would be consistent with an evolutionary tradeoff between chirp duration and rate. A negative effect of within-population deviation in chirp duration would be consistent with a phenotypic tradeoff within populations between the two traits. After estimating the model, we tested whether the slope of the among-population tradeoff differed from the slope of the within-population tradeoff (i.e. whether the among-population effect minus the within-population effect significantly differed from zero). Similar slopes would suggest that the phenotypic tradeoff within populations predicts the evolutionary tradeoff.
(c). Tradeoffs within and among populations: males under natural conditions
To examine the relationship between chirp rate and chirp duration in wild populations, we recorded the songs of males from four of the populations used in the above studies: Santa Barbara (n = 14), Sedgwick Reserve (n = 35), Hastings Natural History Reservation (n = 23), and Academy (n = 10). Recordings were made and analysed as previously described [31]. Temperatures were recorded from a singing male's position immediately following each recording, and prior to analysis, chirp rate and duration were adjusted to the average recording temperature (see above). Our analytical approach was identical to that used for the common environment population study, except that the relatedness of males within a population was not known, so male family was not included in the statistical models.
3. Results
(a). Tradeoffs within and among genotypes in two nutritional environments
A model with random within-family slopes and random intercepts did not provide a significantly better fit to the data than did a model with only random intercepts (likelihood ratio test: 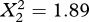 , p = 0.389). There was thus no evidence that the families differed in the strength of the tradeoff between the two traits (figure 1a). Subsequent tests used only the model with random intercepts (table 1).
, p = 0.389). There was thus no evidence that the families differed in the strength of the tradeoff between the two traits (figure 1a). Subsequent tests used only the model with random intercepts (table 1).
Figure 1.
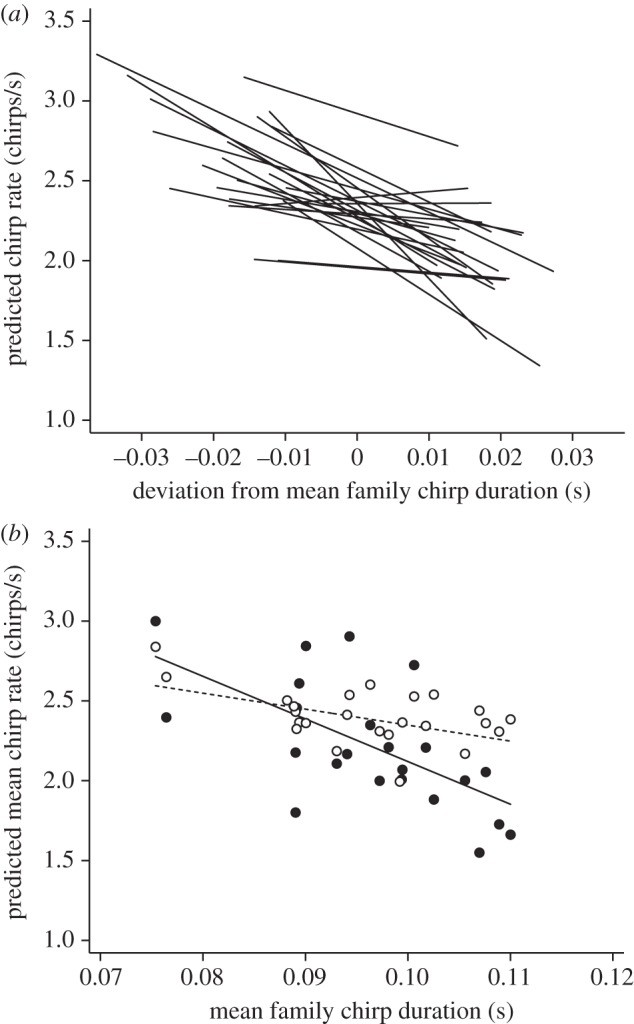
Relationship within and among families between chirp duration and chirp rate in G. lineaticeps. (a) Relationship between deviation from the mean family chirp duration and predicted chirp rate within the 24 full sibling families (based on a model with random intercepts and random within-family slopes). (b) Relationship between mean family chirp duration and mean predicted chirp rate for males raised in a low-nutrition environment (filled circles, solid line) and a high-nutrition environment (open circles, dashed line; based on a model with random intercepts). For details, see the electronic supplementary material, statistical methods.
Table 1.
Family and diet effects on male chirp rate in G. lineaticeps. Data were analysed using a linear mixed model. Chirp duration (mean) is the among-family effect of chirp duration. Chirp duration (deviation) is the within-family effect of chirp duration. The fixed effects were tested using Wald tests. The random effect was tested using a likelihood ratio test that compared models with and without the random effect. Regression coefficients are presented for the fixed effects, while variance component estimates are presented for the random effect and error terms.
| fixed effects | coefficient | s.e. | 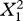 |
p |
|---|---|---|---|---|
| chirp duration (mean) | −44.349 | 14.874 | 8.89 | 0.003 |
| chirp duration (deviation) | −16.345 | 3.300 | 25.62 | <0.001 |
| adult diet | −1.577 | 0.823 | 3.67 | 0.055 |
| duration (mean) × adult diet | 17.478 | 1.428 | 4.14 | 0.042 |
| random effect and error | estimate | s.e. | 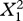 |
p |
| male family | 0.068 | 0.030 | 17.21 | <0.001 |
| error | 0.249 | 0.028 | ||
There was a significant within-family (phenotypic) effect of chirp duration on chirp rate: males that produced longer chirp durations, relative to their siblings, produced lower chirp rates. There was also a significant effect of the interaction between mean family chirp duration and nutritional environment, which suggests that there is a genetically based tradeoff that might be contingent upon environmental conditions (figure 1b). Follow-up analyses within each nutrition environment showed that there was a significant negative effect of mean family chirp duration on chirp rate in the low-nutrition environment (coefficient = −26.04, s.e. = 10.12, X12=6.62, p = 0.010, critical p = 0.025), but not in the high-nutrition environment (coefficient = −10.94, s.e. = 7.24, 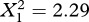 , p = 0.130, critical p = 0.025).
, p = 0.130, critical p = 0.025).
(b). Tradeoffs within and among populations: males reared in a common environment
A model with random within-population slopes and random intercepts did not provide a significantly better fit to the data than did a model with only random intercepts (likelihood ratio test: 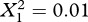 , p = 0.942). There was thus no evidence that the populations differed in the strength of the tradeoff between the two traits (figure 2a). Subsequent tests used only the model with random intercepts (table 2).
, p = 0.942). There was thus no evidence that the populations differed in the strength of the tradeoff between the two traits (figure 2a). Subsequent tests used only the model with random intercepts (table 2).
Figure 2.
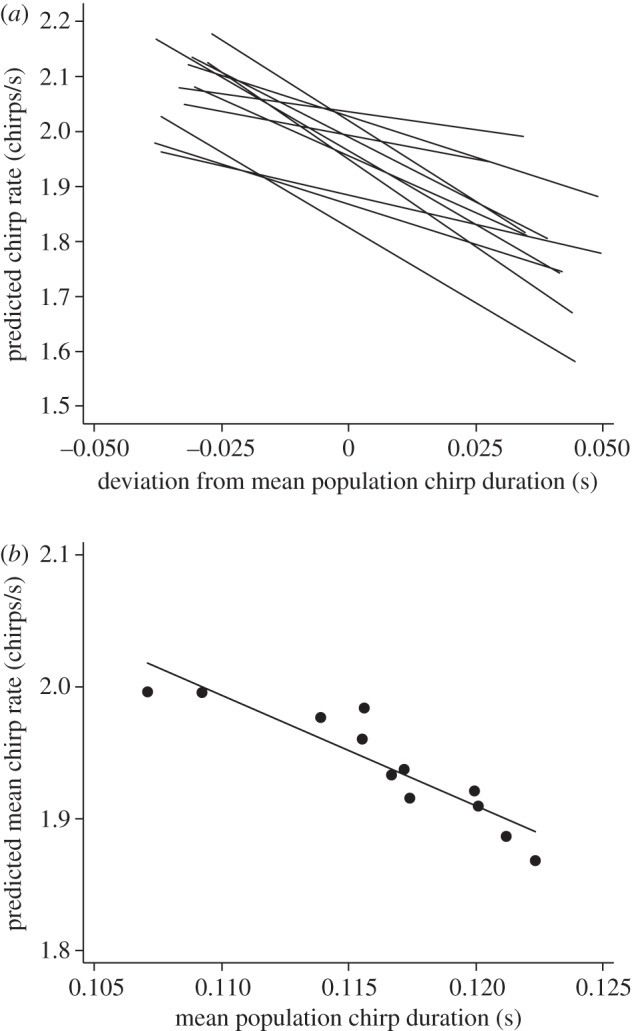
Relationship within and among populations between chirp duration and chirp rate in G. lineaticeps. (a) Relationship between deviation from the mean population chirp duration and predicted chirp rate within the 12 populations (based on a model with random intercepts and random within-population slopes). (b) Relationship between mean population chirp duration and mean predicted chirp rate (based on a model with random intercepts). For details, see the electronic supplementary material, statistical methods.
Table 2.
Population effects on male chirp rate in G. lineaticeps. Data were analysed using a linear mixed model. Chirp duration (mean) is the among-population effect of chirp duration. Chirp duration (deviation) is the within-population effect of chirp duration. The fixed and random effects were tested and are presented as in table 1.
| fixed effects | coefficient | s.e. | 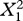 |
p |
|---|---|---|---|---|
| chirp duration (mean) | −8.299 | 3.404 | 5.93 | 0.015 |
| chirp duration (deviation) | −4.308 | 0.897 | 23.96 | <0.001 |
| random effects and error | estimate | s.e. | 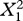 |
p |
| population | 0 | 0.001 | 0.14 | 0.708 |
| male family | 0.019 | 0.006 | 11.20 | <0.001 |
| error | 0.127 | 0.008 | ||
There was a significant within-population (phenotypic) effect of chirp duration on chirp rate: males that produced longer chirp durations, relative to other males from the same population, produced lower chirp rates. There was also a significant among-population (evolutionary) effect of chirp duration on chirp rate: males from populations that produced longer mean chirp durations produced lower chirp rates (figure 2b). Finally, there was not a significant difference in the slope of the within-population tradeoff and the slope of the among-population tradeoff (z1 = −1.13, p = 0.257). The phenotypic tradeoff within populations is thus similar to the evolutionary tradeoff.
(c). Tradeoffs within and among populations: males under natural conditions
A model with random within-population slopes and random intercepts did not provide a significantly better fit to the data than did a model with only random intercepts (likelihood ratio test: 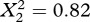 , p = 0.665). There was thus no evidence that the populations differed in the strength of the tradeoff between the two traits (electronic supplementary material, figure S2a). Subsequent tests used only the model with random intercepts (electronic supplementary material, table S1).
, p = 0.665). There was thus no evidence that the populations differed in the strength of the tradeoff between the two traits (electronic supplementary material, figure S2a). Subsequent tests used only the model with random intercepts (electronic supplementary material, table S1).
There was a significant within-population (phenotypic) effect of chirp duration on chirp rate: males that produced longer chirp durations, relative to other males from the same population, produced lower chirp rates. There was also a significant among-population (evolutionary and/or environmental) effect of chirp duration on chirp rate: males from populations that produced longer mean chirp durations produced lower chirp rates (electronic supplementary material, figure S2b). Finally, there was not a significant difference in the slope of the within-population tradeoff and the slope of the among-population tradeoff (z1 = 0.10, p = 0.922). The phenotypic tradeoff within populations is thus similar to the evolutionary and/or environmental tradeoff.
4. Discussion
The combined results of our study provide strong evidence for a fundamental, widespread and evolutionarily important tradeoff between two male traits used by females in mate choice. This tradeoff appears to limit the ability of males to simultaneously produce multiple attractive traits, and limit how male signals evolve. First, we found a negative correlation between chirp rate and duration among full-sibling families. This result is consistent with a genetically based tradeoff that limits the ability of males to produce attractive values of both traits, although traits can covary among full-sibling families because of shared dominance, epistatic and maternal effects. Second, using males reared in a common environment, we found a negative correlation within and among populations between chirp rate and duration, with little variation among populations in the strength of the tradeoff. These results suggest that the tradeoff is widespread and largely unaffected by geographical variation in selection. They also suggest that the tradeoff has affected how male traits have evolved: an evolutionary increase in one trait has been accompanied by an evolutionary decrease in the other. And third, using field-recorded males, we found a negative correlation within and among populations between chirp rate and duration. The tradeoff thus appears to be expressed across a range of natural environmental conditions. Tradeoffs between male traits preferred by females may be common, and may help to explain broad patterns of correlated trait evolution. For example, there is a negative correlation between call rate and duration across a large taxonomic range of acoustic animals [49], despite the fact that sexual selection commonly favours males with both faster and longer calls [50].
It is not known why there is a tradeoff between chirp rate and duration within and among populations of G. lineaticeps. One major reason that traits can be negatively correlated within populations is correlational selection. Correlational selection occurs when selection favours some trait combinations and disfavours others, which can cause linkage disequilibrium between alleles that affect the expression of each trait [51]. Given that the relationship between chirp rate and duration was largely identical within each population, the pattern of correlational selection would also need to be largely identical to explain our results. While it is possible that correlational selection could produce very similar tradeoffs within each population, selection probably cannot explain the negative correlation among populations. Female choice appears to favour higher values of both traits [41–43], whereas predation by phonotactic parasitoid flies, when it occurs, appears to favour lower values of both traits [41,52,53]. These two major sources of selection on male song predict that the traits will be positively correlated among populations rather than negatively correlated; males should produce higher chirp rates and longer chirp durations in populations in which sexual selection is stronger and/or natural selection is weaker.
A second major reason that traits can be negatively correlated is antagonistic pleiotropy: alleles that mediate resource allocation decisions can have pleiotropic consequences for the expression of multiple traits [4,54]. Higher chirp rates and longer chirp durations both require more wing movement, and if males have limited energy resources to power singing, alleles that cause males to produce higher chirp rates may require them to produce shorter chirp durations. Our result showing that the tradeoff might be stronger in a low-nutrition environment than in a high-nutrition environment is consistent with an energy allocation tradeoff. A strong test of this hypothesis, however, requires an understanding of the mechanistic basis of the tradeoff [6,55].
There is one type of resource allocation tradeoff that we can unambiguously reject. For acoustic animals, time is a limiting resource because there is an absolute ceiling on the proportion of time that an individual can produce sound. An individual can increase the rate at which it produces a signal, the duration of its signal, or both, but once it approaches continuous sound production, it cannot increase one signal character without reducing the other. In our laboratories studies, the mean proportion of time that males produced song (chirp rate × chirp duration) was 0.22 (maximum = 0.41). And in our field study, the mean proportion of time that males produced song was 0.32 (maximum = 0.44). Males could thus produce substantially greater values of both signal types and still not be forced by temporal limitations to trade off one trait for the other. Furthermore, the time allocation hypothesis predicts that the strength of the tradeoff will remain constant across nutritional environments, which is inconsistent with our results.
A third major reason that traits can be negatively correlated is biomechanical constraints: the expression of some traits can preclude the expression of others [56–58]. In birds, for example, the vocal tract features that allow the production of songs with a high trill rate appear to preclude the production of songs with a broad frequency range [28,59,60]. The biomechanical constraint hypothesis predicts that the strength of the tradeoff between chirp rate and duration in G. lineaticeps will remain constant across adult nutritional environments, which is inconsistent with our results.
Tradeoffs between traits may not only have consequences for male signal evolution, but also for female preference evolution. In G. lineaticeps, for example, males with high chirp rates provide seminal fluid products to females during mating that increase female fecundity, whereas males with longer chirp durations provide seminal fluid products that increase female longevity [31]. These male-provided direct benefits have the greatest effect on female fecundity and life span in a low-nutrition environment [31,44], which is the environment in which the tradeoff between chirp rate and duration is strongest. Females might thus be forced to trade off one benefit for the other in some environments. Such tradeoffs might favour plasticity in female-mating preferences. For example, environmental conditions might affect the extent to which the two direct benefits increase female fitness, and if so, females should adjust their preferences for each trait based on environmental conditions. Relatively, little is known about tradeoffs in female-mating benefits in other systems, although there is some evidence that females of some species may trade off male parental care and male genetic quality [61,62]. An important area of future research will be to determine whether tradeoffs between male traits cause tradeoffs in female-mating benefits, and if so, to determine the factors that affect how females balance these mating benefits when selecting a mate.
Acknowledgements
We thank S. Beran, S. Fine, S. Fountain, A. Huebner, J. McNair, A. Smith, E. Steckler, W. Steinbach and M. Sullivan for assistance with the research. We also thank E. Hebets, C. Mitra and A. Zera for feedback on the manuscript. The research was supported by the School of Biological Sciences and Initiative for Ecological and Evolutionary Analysis at the University of Nebraska-Lincoln (AET), a GAANN award from the US Department of Education (AET), and NSF grants IOB 0521743 and IOS 0818116 (WEW).
References
- 1.Lack D. 1947. The significance of clutch-size. Ibis 89, 302–352 10.1111/j.1474-919X.1947.tb04155.x (doi:10.1111/j.1474-919X.1947.tb04155.x) [DOI] [Google Scholar]
- 2.Williams G. C. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 10.2307/2406060 (doi:10.2307/2406060) [DOI] [Google Scholar]
- 3.van Noordwijk A. J., de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142 10.1086/284547 (doi:10.1086/284547) [DOI] [Google Scholar]
- 4.Stearns S. C. 1992. The evolution of life histories. New York, NY: Oxford University Press [Google Scholar]
- 5.Roff D. A. 2002. Life history evolution. Sunderland, MA: Sinauer Associates [Google Scholar]
- 6.Zera A. J., Harshman L. G. 2001. The physiology of life history trade-offs in animals. Ann. Rev. Ecol. Syst. 32, 95–126 10.1146/annurev.ecolsys.32.081501.114006 (doi:10.1146/annurev.ecolsys.32.081501.114006) [DOI] [Google Scholar]
- 7.Zera A. J., Harshman L. G. 2009. Laboratory selection studies of life history physiology in insects. In Experimental evolution: concepts, methods, and applications (eds Garland T., Jr., Rose M. R.), pp. 217–262 Berkeley, CA: University of California Press [Google Scholar]
- 8.Biro P. A., Stamps J. A. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368 10.1016/j.tree.2008.04.003 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 9.Beckerman A., Benton T. G., Ranta E., Kaitala V., Lundberg P. 2002. Population dynamic consequences of delayed life-history effects. Trends Ecol. Evol. 17, 263–269 10.1016/S0169-5347(02)02469-2 (doi:10.1016/S0169-5347(02)02469-2) [DOI] [Google Scholar]
- 10.Kneitel J. M., Chase J. M. 2003. Trade-offs in community ecology: linking spatial scales and species coexistence. Ecol. Lett. 7, 69–80 10.1046/j.1461-0248.2003.00551.x (doi:10.1046/j.1461-0248.2003.00551.x) [DOI] [Google Scholar]
- 11.Sih A., Bell A. M., Johnson J. C., Ziemba R. E. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 10.1086/422893 (doi:10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 12.Gustaffson L., Qvarnström A., Sheldon B. C. 1995. Trade-offs between life-history traits and a secondary sexual character in male collared flycatchers. Nature 375, 311–313 10.1038/375311a0 (doi:10.1038/375311a0) [DOI] [Google Scholar]
- 13.Andersson S., Pryke S. R., Örnborg J., Lawes M. J., Andersson M. 2002. Multiple receivers, multiple ornaments, and a trade-off between agonistic and epigamic signaling in a widowbird. Am. Nat. 160, 683–691 10.1086/342817 (doi:10.1086/342817) [DOI] [PubMed] [Google Scholar]
- 14.Roff D. A., Crnokrak P., Fairbairn D. J. 2003. The evolution of trade-offs: geographic variation in call duration and flight ability in the sand cricket, Gryllus firmus. J. Evol. Biol. 16, 744–753 10.1046/j.1420-9101.2003.00570.x (doi:10.1046/j.1420-9101.2003.00570.x) [DOI] [PubMed] [Google Scholar]
- 15.Simmons L. W., Emlen D. J. 2006. Evolutionary trade-off between weapons and testes. Proc. Natl Acad. Sci. USA 10, 16 346–16 351 10.1073/pnas.0603474103 (doi:10.1073/pnas.0603474103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Immler S., Pitnick S., Parker G. A., Durrant K. L., Lüpold S., Calhim S., Birkhead T. 2011. Resolving variation in the reproductive tradeoff between sperm size and number. Proc. Natl Acad. Sci. USA 108, 5325–5330 10.1073/pnas.1009059108 (doi:10.1073/pnas.1009059108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engqvist L. 2011. Male attractiveness is negatively genetically associated with investment in copulations. Behav. Ecol. 22, 345–349 10.1093/beheco/arq211 (doi:10.1093/beheco/arq211) [DOI] [Google Scholar]
- 18.Hebets E. A., Papaj D. R. 2005. Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214 10.1007/s00265-004-0865-7 (doi:10.1007/s00265-004-0865-7) [DOI] [Google Scholar]
- 19.Jones A. G., Ratterman N. L. 2009. Mate choice and sexual selection: what have we learned since Darwin? Proc. Natl. Acad. Sci. USA 106, 10 001–10 008 10.1073/pnas.0901129106 (doi:10.1073/pnas.0901129106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner W. E., Jr 2011. Direct benefits and the evolution of female mating preferences: conceptual problems, potential solutions, and a field cricket. Adv. Stud. Behav. 43, 273–319 10.1016/B978-0-12-380896-7.00006-X (doi:10.1016/B978-0-12-380896-7.00006-X) [DOI] [Google Scholar]
- 21.Kotiaho J. S. 1991. Costs of sexual traits: a mismatch between theoretical consideration and experimental evidence. Biol. Rev. 76, 365–376 10.1017/S1464793101005711 (doi:10.1017/S1464793101005711) [DOI] [PubMed] [Google Scholar]
- 22.Kotiaho J. S., Puurtinen M. 2007. Mate choice for indirect genetic benefits: scrutiny of the current paradigm. Funct. Ecol. 21, 638–644 10.1111/j.1365-2435.2007.01286.x (doi:10.1111/j.1365-2435.2007.01286.x) [DOI] [Google Scholar]
- 23.Kodric-Brown A., Brown J. H. 1984. Truth in advertising: the kinds of traits favored by sexual selection. Am. Nat. 124, 309–323 10.1086/284275 (doi:10.1086/284275) [DOI] [Google Scholar]
- 24.Houle D. 1991. Genetic covariance of fitness correlates: what genetic correlations are made of and why it matters. Evolution 45, 630–648 10.2307/2409916 (doi:10.2307/2409916) [DOI] [PubMed] [Google Scholar]
- 25.Zera A. J., Potts J., Kobus K. 1998. The physiology of life history trade-offs: experimental analysis of a hormonally-induced life-history trade-off in Gryllus assimilis. Am. Nat. 152, 7–23 10.1086/286146 (doi:10.1086/286146) [DOI] [PubMed] [Google Scholar]
- 26.Roff D. A., Fairbairn D. J. 2007. The evolution of trade-offs: where are we? J. Evol. Biol. 20, 433–447 10.1111/j.1420-9101.2006.01255.x (doi:10.1111/j.1420-9101.2006.01255.x) [DOI] [PubMed] [Google Scholar]
- 27.King E. G., Roff D. A., Fairbairn D. J. 2011. The evolutionary genetics of acquisition and allocation in the wing dimorphic cricket, Gryllus firmus. Evolution 65, 2273–2285 10.1111/j.1558-5646.2011.01296.x (doi:10.1111/j.1558-5646.2011.01296.x) [DOI] [PubMed] [Google Scholar]
- 28.Podos J. 1996. Motor constraints on vocal development in a songbird. Anim. Behav. 51, 1061–1070 10.1006/anbe.1996.0107 (doi:10.1006/anbe.1996.0107) [DOI] [Google Scholar]
- 29.Wells K. D., Taigen T. L. 1986. The effect of social interactions on calling energetics in the gray treefrog (Hyla versicolor). Behav. Ecol. Sociobiol. 19, 9–18 10.1007/BF00303837 (doi:10.1007/BF00303837) [DOI] [Google Scholar]
- 30.Drăgănoiu T. I., Nagle L., Kreutzer M. 2002. Directional female preference for an exaggerated male trait in canary (Serinusanaria) song. Proc. R. Soc. Lond. B 269, 2525–2531 10.1098/rspb.2002.2192 (doi:10.1098/rspb.2002.2192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner W. E., Jr, Harper C. J. 2003. Female life span and fertility are increased by the ejaculates of preferred males. Evolution 57, 2054–2066 10.1554/02-548 (doi:10.1554/02-548) [DOI] [PubMed] [Google Scholar]
- 32.Reznick D. N. 1985. Costs of reproduction: an evaluation of the empirical evidence. Oikos 44, 257–267 10.2307/3544698 (doi:10.2307/3544698) [DOI] [Google Scholar]
- 33.Brooks R., Endler J. A. 2001. Direct and indirect sexual selection and quantitative genetics of male traits in guppies (Poecilia reticulata). Evolution 55, 1002–1015 10.1554/0014-3820(2001)055[1002:DAISSA]2.0.CO;2 (doi:10.1554/0014-3820(2001)055[1002:DAISSA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 34.Catchpole C. K., McGregor P. K. 1985. Sexual selection, song complexity and plumage dimorphism in European buntings of the genus Emberiza. Anim. Behav. 33, 1378–1380 10.1016/S0003-3472(85)80209-8 (doi:10.1016/S0003-3472(85)80209-8) [DOI] [Google Scholar]
- 35.Shutler D., Weatherhead P. J. 1990. Targets of sexual selection: song and plumage of wood warblers. Evolution 44, 1967–1977 10.2307/2409607 (doi:10.2307/2409607) [DOI] [PubMed] [Google Scholar]
- 36.Badyaev A. V., Hill G. E., Weckworth B. V. 2002. Species divergence in sexually selected traits: increase in song elaboration is related to decrease in plumage ornamentation in finches. Evolution 56, 412–419 10.1554/0014-3820(2002)056[0412:SDISST]2.0.CO;2 (doi:10.1554/0014-3820(2002)056[0412:SDISST]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 37.Ornelas J. F., González C., Espinosa de los Monteros A. 2009. Uncorrelated evolution between vocal and plumage coloration traits in the trogons: a comparative study. J. Evol. Biol. 22, 471–484 10.1111/j.1420-9101.2008.01679.x (doi:10.1111/j.1420-9101.2008.01679.x) [DOI] [PubMed] [Google Scholar]
- 38.Shutler D. 2011. Sexual selection: when to expect trade-offs. Biol. Lett. 7, 101–104 10.1098/rsbl.2010.0531 (doi:10.1098/rsbl.2010.0531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prestwich K. N. 1994. The energetics of acoustic signaling in anurans and insects. Am. Zool. 34, 625–643 10.1093/icb/34.6.625 (doi:10.1093/icb/34.6.625) [DOI] [Google Scholar]
- 40.Hoback W. W., Wagner W. E., Jr 1997. The energetic cost of calling in the variable field cricket, Gryllus lineaticeps. Physiol. Entomol. 22, 286–290 10.1111/j.1365-3032.1997.tb01170.x (doi:10.1111/j.1365-3032.1997.tb01170.x) [DOI] [Google Scholar]
- 41.Wagner W. E., Jr 1996. Convergent song preferences between female field crickets and acoustically orienting parasitoid flies. Behav. Ecol. 7, 279–285 10.1093/beheco/7.3.279 (doi:10.1093/beheco/7.3.279) [DOI] [Google Scholar]
- 42.Wagner W. E., Jr, Reiser M. G. 2000. The relative importance of calling song and courtship song in female mate choice in the variable field cricket. Anim. Behav. 59, 1219–1226 10.1006/anbe.1999.1428 (doi:10.1006/anbe.1999.1428) [DOI] [PubMed] [Google Scholar]
- 43.Wagner W. E., Jr., Basolo A. L. 2007. The relative importance of different direct benefits in the mate choices of a field cricket. Evolution 61, 617–622 10.1111/j.1558-5646.2007.00062.x (doi:10.1111/j.1558-5646.2007.00062.x) [DOI] [PubMed] [Google Scholar]
- 44.Tolle A. E., Wagner W. E., Jr 2011. Costly signals in a field cricket can indicate high or low quality direct benefits depending upon the environment. Evolution 65, 283–294 10.1111/j.1558-5646.2010.01123.x (doi:10.1111/j.1558-5646.2010.01123.x) [DOI] [PubMed] [Google Scholar]
- 45.Sgrò C. M., Hoffmann A. A. 2004. Genetic correlations, tradeoffs and environmental variation. Heredity 93, 241–248 10.1038/sj.hdy.6800532 (doi:10.1038/sj.hdy.6800532) [DOI] [PubMed] [Google Scholar]
- 46.van de Pol M., Wright J. 2009. A simple method for distinguishing within-versus between-subjects effects using mixed models. Anim. Behav. 77, 753–758 10.1016/j.anbehav.2008.11.006 (doi:10.1016/j.anbehav.2008.11.006) [DOI] [Google Scholar]
- 47.Rabe-Hesketh S., Skrondal A. 2008. Multilevel and longitudinal modeling using Stata, 2nd edn College Station, TX: Stata Press [Google Scholar]
- 48.Lynch M., Walsh J. B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates [Google Scholar]
- 49.Gillooly J. F., Ophir A. G. 2010. The energetic basis of acoustic communication. Proc. R. Soc. B 277, 1325–1331 10.1098/rspb.2009.2134 (doi:10.1098/rspb.2009.2134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryan M. J., Keddy-Hector A. 1992. Directional patterns of female mate choice and the role of sensory biases. Am. Nat. 139, S4–S35 10.1086/285303 (doi:10.1086/285303) [DOI] [Google Scholar]
- 51.Brodie E. D., III. 1992. Correlational selection for color pattern and antipredator behavior in the garter snake, Thamnophis ordinoides. Evolution 46, 1284–1298 10.2307/2409937 (doi:10.2307/2409937) [DOI] [PubMed] [Google Scholar]
- 52.Wagner W. E., Jr, Basolo A. L. 2007. Host preferences in a phonotactic parasitoid of field crickets: the relative importance of host song characters. Ecol. Entomol. 32, 478–484 10.1111/j.1365-2311.2007.00898.x (doi:10.1111/j.1365-2311.2007.00898.x) [DOI] [Google Scholar]
- 53.Martin C. M., Wagner W. E., Jr 2010. Female field incur increased parasitism risk when near preferred song. PLoS ONE 5, e9592. 10.1371/journal.pone.0009592 (doi:10.1371/journal.pone.0009592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rose M. R. 1985. Life history evolution with antagonistic pleiotropy and overlapping generations. Theor. Popul. Biol. 28, 342–358 10.1016/0040-5809(85)90034-6 (doi:10.1016/0040-5809(85)90034-6) [DOI] [Google Scholar]
- 55.Harshman L. G., Zera A. J. 1997. The cost of reproduction: the devil in the details. Trends Ecol. Evol. 22, 80–86 10.1016/j.tree.2006.10.008 (doi:10.1016/j.tree.2006.10.008) [DOI] [PubMed] [Google Scholar]
- 56.Raup D. M. 1966. Geometric analysis of shell coiling: general problems. J. Paleontol. 40, 1178–1190 [Google Scholar]
- 57.Alberch P., Gould S. J., Oster G., Wake D. 1979. Size and shape in ontogeny and phylogeny. Paleobiology 5, 296–317 [Google Scholar]
- 58.Wake D., Larson A. 1987. Multidimensional analysis of an evolving lineage. Science 238, 42–48 10.1126/science.238.4823.42 (doi:10.1126/science.238.4823.42) [DOI] [PubMed] [Google Scholar]
- 59.Podos J. 1997. A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes: Emberizidae). Evolution 51, 537–551 10.2307/2411126 (doi:10.2307/2411126) [DOI] [PubMed] [Google Scholar]
- 60.Podos J. 2001. Correlated evolution of morphology and vocal signal structure in Darwin's finches. Nature 409, 185–188 10.1038/35051570 (doi:10.1038/35051570) [DOI] [PubMed] [Google Scholar]
- 61.Westneat D. F., Sherman P. W., Morton M. L. 1990. The ecology and evolution of extra-pair copulations in birds. Curr. Ornithol. 7, 331–369 [Google Scholar]
- 62.Smith H. G. 1995. Experimental demonstration of a trade-off between mate attraction and paternal care. Proc. R. Soc. Lond. B 260, 45–51 10.1098/rspb.1995.0057 (doi:10.1098/rspb.1995.0057) [DOI] [Google Scholar]


