Abstract
Turtles, like other amphibious animals, face a trade-off between terrestrial and aquatic hearing. We used laser vibrometry and auditory brainstem responses to measure their sensitivity to vibration stimuli and to airborne versus underwater sound. Turtles are most sensitive to sound underwater, and their sensitivity depends on the large middle ear, which has a compliant tympanic disc attached to the columella. Behind the disc, the middle ear is a large air-filled cavity with a volume of approximately 0.5 ml and a resonance frequency of approximately 500 Hz underwater. Laser vibrometry measurements underwater showed peak vibrations at 500–600 Hz with a maximum of 300 µm s−1 Pa−1, approximately 100 times more than the surrounding water. In air, the auditory brainstem response audiogram showed a best sensitivity to sound of 300–500 Hz. Audiograms before and after removing the skin covering reveal that the cartilaginous tympanic disc shows unchanged sensitivity, indicating that the tympanic disc, and not the overlying skin, is the key sound receiver. If air and water thresholds are compared in terms of sound intensity, thresholds in water are approximately 20–30 dB lower than in air. Therefore, this tympanic ear is specialized for underwater hearing, most probably because sound-induced pulsations of the air in the middle ear cavity drive the tympanic disc.
Keywords: underwater sound, evolution, cochlea, auditory brainstem response
1. Introduction
Recent anurans, lizards, archosaurs (birds, crocodiles and dinosaurs), turtles and mammals have very different middle ears that may be grouped into two types. In anurans, lizards and most archosaurs, the two tympana are coupled through one large, interaural cavity connecting the middle ears that can impart strong directionality to the eardrum, and is assumed to be ancestral in all the tetrapods [1,2]. In some birds, most mammals [3] and turtles, the other, derived configuration has enclosed middle ears with narrow Eustachian tubes connected to the oral cavity. In birds and mammals, the enclosed middle ear may have emerged through brain enlargement or by adaptations to protect the middle ear [4]. Similar enclosed middle ears with narrow Eustachian tubes are also found in turtles [5], but the evolutionary processes leading to these enclosed, unconnected middle ears are unknown.
The general function of the turtle middle ear is puzzling, since the turtle ear is less sensitive to airborne sound than those of other tympanate tetrapods [5]. The origin of the turtle ear is further complicated by three currently viable, but conflicting hypotheses for the origin of turtles. The first hypothesis has turtles as the extant sister group to the archosaurs [6], the second as a sister group to the lizard–tuatara clade [7] and the third as a sister group to the entire diapsid clade [8]. Recent morphological analyses favour the third interpretation, placing turtles outside the Diapsida [8]. Paleontological evidence shows also that the tympanic ear of turtles originated independently from that of the other tetrapod lines [9].
Turtle ears have several interesting anatomical features ([10]; see review in Hetherington [11]). The primary ossicle, the columella, is long and thin and connected to the external surface by the large disc-shaped extracollumella, which is covered by normal skin. The other end of the columella runs through connective tissue to the oval window and is the primary transmitter of sound, as demonstrated by Wever & Vernon [10], who reported that hearing was greatly reduced after the columella was clipped. In most turtle species, the ear is relatively insensitive. The auditory papilla is small but is organized tonotopically, such that higher frequency sounds excite the hair cells at the base, with the tones to which hair cells respond becoming lower towards the apex [12].
Hearing sensitivity is of general interest both for the turtle as a model of hair cell function and to understand the functionality and evolution of vertebrate auditory systems. Very little is known about turtle hearing, except for the inner ear of the turtle Trachemys, which has been extensively studied because of its tolerance to low oxygen tension and thus viability of in vitro preparations of its inner ear and brain [12–15]. In comparison, few studies [16] have addressed the general sensitivity of this turtle ear in vivo, and it has been acknowledged that physiological measurements are needed to understand its function [11]. Here, we compare the sensitivity of the red-eared slider turtle, Trachemys scripta elegans, to airborne and underwater sound. The results of this and a recent anatomical study [17] show that the ear is adapted for underwater hearing, explaining both the poor sensitivity to airborne sound and the turtle middle ear structure.
2. Methods
(a). Head reconstruction and middle ear
Two forms of biomedical imaging, submillimeter, ultrahigh resolution computerized tomography (CT; UHRCT) and micro-magnetic resonance imaging (microMRI), were employed to document middle ear tissues and morphometry of the turtle ear. CT scans of two live turtles were obtained with a Siemens Volume Zoom CT scanner at the Woods Hole Oceanographic Institution Imaging Center (http://csi.whoi.edu). Three-dimensional reconstructions from DICOM files were obtained using Siemens software for shaded-surface display (SSD) and volume-rendered technique (VRT) multi-tissue segmentations and with Amira software (Visage Imaging Inc., CA, USA). Volumes of the air spaces in live turtles were calculated and compared from the CT scan data by two methods: Siemens Volume ROI software and the morphometry volume tool in Amira (see electronic supplementary material).
Images of ear regions were also obtained using magnetic resonance imaging on post-mortem fixed tissues. MR images were acquired using a 7.2 T Micro MRI at the Armed Forces Institute of Pathology, Rockville, MD, USA. Coronal and sagittal plane two-dimensional images were provided in DICOM format and were processed and analysed using Neurolucida (Microbrightfield, Williston, VT, USA). Although histological preparations of the whole head sections yielded better detail, both UHRCT and microMRI imaging provided faster and more accurate results because of the lack of distortion. Additionally, volumes were obtained from two heads by filling the cavity with epoxy and weighing the casts, and from a fixed head that was decalcified in EDTA, embedded in celloidin, sectioned at 200 µm and reconstructed with Neurolucida (Microbrightfield, VT, USA).
(b). Laser vibrometry
The turtles were lightly anaesthetized with propofol (10 mg kg−1, Warner-Lambert/Parke-Davis, Denmark) injected in the dorsal sinus just beneath the carapace. Eardrum vibrations from underwater sound were measured in a water tank (depth 54 cm, length 100 cm, width 90 cm). To enhance laser light reflections, a small reflector (3 M) was glued on the exposed tympanic disc using a drop of tissue glue (Histoacryl Blau, Braun-Melsungen). The animal was placed on an acrylic platform suspended 10 cm below the water surface in the centre of the tank. The laser (OFV-505 sensor and OFV-5000 vibrometer, Polytec, Waldbronn, Germany) was placed outside the tank, approximately 1 m from the animal, and focused on the tympanic disc through a transparent window. Sound at the tympanic disc was measured by a small hydrophone (Brüel and Kjaer 8103, Nærum, Denmark) placed approximately 1 cm from the disc. The particle velocities in the direction of disc vibrations were measured with the animal in place using two B&K 8103 hydrophones (see below). Finally, the speed of sound in the tank was determined to be 1370 m s−1 (s.d. ± 5, n = 3) based on transient travel time to two B&K 8103 hydrophones 0.74 m apart. Thus, impedance of this tank was 9 per cent lower than in a large body of fresh water [18], probably owing to slight pressure release at the tank walls and water surface. Laser and hydrophone signals were recorded using a TDT RM2 digital signal processor and customized software.
The measured responses were compared with a model of sound-induced vibrations in an underwater air cavity. This model was developed for fish swimbladders and was used to assess the contribution of the air cavity to the frequency response of the ear. Values for damping coefficient and stiffness were taken from fish tissue [19]. In SI units, the model states that
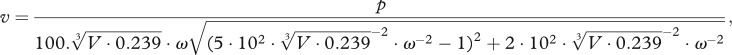 |
where v is vibration velocity, p pressure, V cavity volume and ω angular frequency.
(c). Auditory brainstem response
Hearing sensitivity was measured using the auditory brainstem response (ABR) in 21 turtles (Trachemys scripta elegans, formerly Chrysemys scripta elegans) (150–500 g, both sexes). The turtles were sedated with ketamine (40–80 mg kg−1) plus 20 mg kg−1 xylazine injected into the muscles of the forelimb. We measured the ABR in both air and water using four different modes of stimulation: (i) airborne sound, closed-field (coupler sealed over the tympanum), (ii) underwater sound, (iii) dorsoventral vibration, and (iv) direct motion of the tympanic disc. In all cases, audiograms were measured by stimulating alternately with a brief pulse and with the pulse with an added tonal masker. Sensitivity to the masker was assayed by subtracting masked from unmasked responses [20,21]. The masked ABR enabled us to measure auditory responses at very low frequencies, where it is difficult to get a good response using tone burst ABR measurements. All experiments were performed at room temperature (ca 22°C).
(d). Auditory brainstem response recording
ABRs were measured by two differential stainless steel electrodes of approximately 1 kΩ impedance, inserted subdermally, one above the ear and the other above the brainstem with reference to a ground electrode placed dorsally post-cranial. The electrode signal was passed through a headstage and preamplifier (TDT, PA4 & RA4). The stimulus presentation, ABR acquisition, equipment control and data management are similar to those in prior studies [2,22]. Stimulation, recording and data analysis were performed by custom software (QuickABR) running on a PC and a digital signal processor (Tucker-Davis Technologies (TDT; Gainesville, FL, USA) RM2).
(e). Airborne sound stimulation
Turtles were stimulated by airborne sound in a closed coupler that was sealed over the tympanum using an earmould compound (Gold Velvet II; All American Mold Laboratories, Oklahoma City, OK, USA). The coupler consisted of a brass housing containing a headphone (Beyer 48.0A) and a Brüel & Kjaer (B&K) half inch microphone for calibration.
(f). Underwater sound stimulation
Underwater hearing sensitivity was measured in four turtles in a 1 × 1 m PVC tank with 70 cm deep water. The turtles were suspended in a sling at a depth of 20 cm, 50 cm above an UW30 (Lubell Labs, Inc., Columbus, OH) underwater loudspeaker. We measured particle velocities along all three orthogonal axes (and +5 cm in all directions) in the tank using two B&K 8103 hydrophones spaced 2 cm apart connected to two B&K 2635 charge amplifiers recorded on a TDT RM2 digital signal processor. The pressure gradient was estimated by integrating and scaling the pressure difference between the two hydrophones (see [23] for further details) using custom software. The hydrophones were calibrated using a B&K 4228 piston phone.
(g). Disc vibration
The tympanic disc was exposed by careful removal of the skin covering in four turtles. The pointed tip of a vibration probe was connected to a B&K 4810 vibration exciter and glued to the disc using a drop of tissue glue (Vetbond, WPI). The vibration of the probe was calibrated using a small accelerometer (B&K 4500-A) connected to a B&K 2635 charge amplifier (see further details of the stimulation in Elliott et al. [24]).
(h). Whole-body vibration
Vibration sensitivity was measured in five turtles by placing the turtles on a platform on B&K Vibration exciter 4809, so that both carapace and head rested on the platform. The shaker was calibrated using a B&K accelerometer and calibration exciter. The shaker was placed on alternating layers of mineral wool and flagstone to minimize vibrational noise coupling from the floor.
3. Results
(a). Anatomy
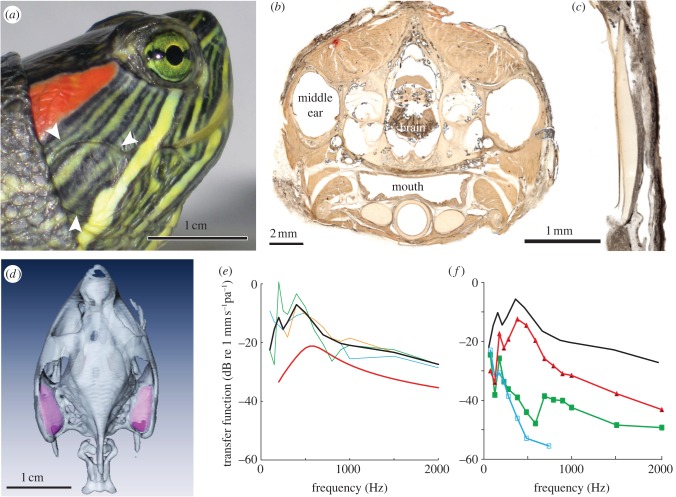
The red-ear slider tympanum (figure 1a, arrows) consists of relatively thin skin covering a soft, pliable, ovoid subdermal layer that is continuous with a cartilaginous tympanal disc forming the main body of the extracolumella (figure 1c). The extracolumella is attached at its inner edges by a heavy posterior ligament, which acts as a hinge, and by a thin anterior ligament [5]. It articulates with a long, thin columella that extends to the pericapsular recess and ends at the oval window [5]. The two large, bilaterally symmetrical middle ear cavities are ovoid, with the long axis directed anteroposteriorly (figure 1b,d). The volume of the pneumatized space of the cavity as measured from CT scans (figure 1d) varied according to animal mass [17]. In the smaller animal, the volumes were 0.22 (right ear) and 0.22 (left) ml; in the larger animal, the volume was 0.50 (right) and 0.44 (left) ml. The larger animal also had distended mucosa and fluid in the left ear, which may have reduced residual volume.
Figure 1.
Ear structure and laser vibrometry. (a) The turtle ear is characterized by a covering of relatively undifferentiated skin (arrows). Scale bar, 1 cm. (b) Transverse thick (200 µm) section through a decalcified head embedded in celloidin at the level of the middle ear (ear). Scale bar, 2 mm. (c) The extracolumella forms a cartilaginous tympanic disc, shown here with overlying epidermis. Scale bar, 1 mm. (d) CT Scan of the turtle skull, with middle ear cavities shown in purple. The volume of the cavities measured from the scans is approximately 0.5 ml. Scale bar, 1 cm. (e) Laser measurement of disc vibration stimulated by underwater sound for three animals. Y-axis, eardrum vibration transfer functions; eardrum vibration velocities scaled by the sound pressure measured at the tympanic disc. Maximal disc vibrations are seen at 500–800 Hz, where disc vibrations are approximately 0.3 mm s−1 Pa−1. The red line shows model data of fish swimbladder vibrations in a sound field [19] for 0.5 ml volume. (f) Eardrum vibration transfer function before (black, median) and after filling the middle ear cavity with water (red). Also shown are vibration transfer functions of an adjacent region of the head (green) and particle velocity (blue).
(b). Laser vibrometry
We used laser vibrometry to measure tympanic disc vibration with underwater sound stimulation (figure 1e,f). The transfer functions in three animals showed a peak at 400–500 Hz, with maximal amplitudes around 300 µm s−1 pa−1. The disc vibrations were 30–40 dB larger than vibrations of adjacent parts of the head or the water particle velocities. After partial filling of the middle ear cavity (figure 1f) in three turtles, the disc vibrations decreased by up to 30 dB. We compared these responses with a model of fish swimbladder vibration using the measured cavity volumes (see §2 and smooth red curve, figure 1e) to estimate disc vibration. The frequency maxima and shape of the model curve were very similar to the measured eardrum data, although the eardrum vibrations were consistently larger than predicted by the model.
(c). Hearing sensitivity in air
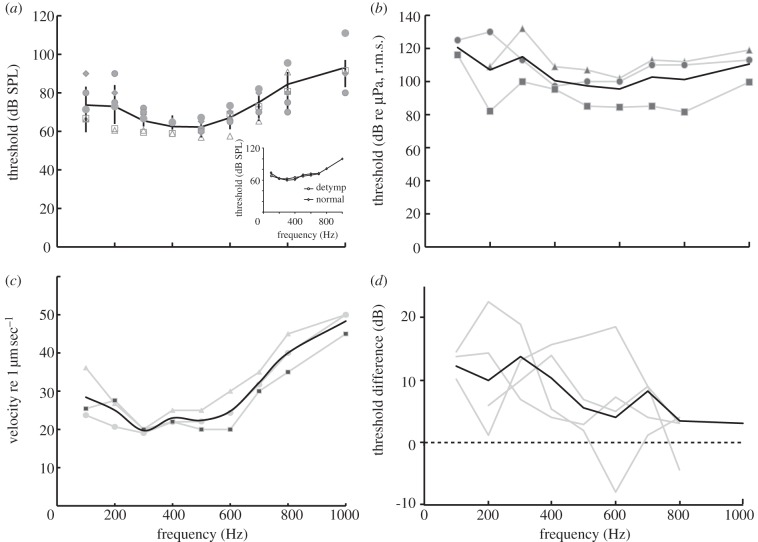
The ABR click response showed two to three prominent peaks occurring within 4–6 ms of stimulus onset (figure 2). Turtles showed a peak sequence similar to other vertebrates, with click thresholds of 69 ± 5 dB, peak–peak, sound pressure level (SPL). Evoked waveforms showed clearly defined peaks in response to a click stimulus and to the click stimulus with an added tonal masker. Audiograms for masked click coupler stimulation were shallow and U-shaped, with best frequencies at 400–500 Hz and lowest thresholds around 60 dB SPL (figure 3a). Removing the ‘tympanum’, i.e. the slightly recessed skin covering the tympanic disc, did not change sensitivity (figure 3a, insert).
Figure 2.
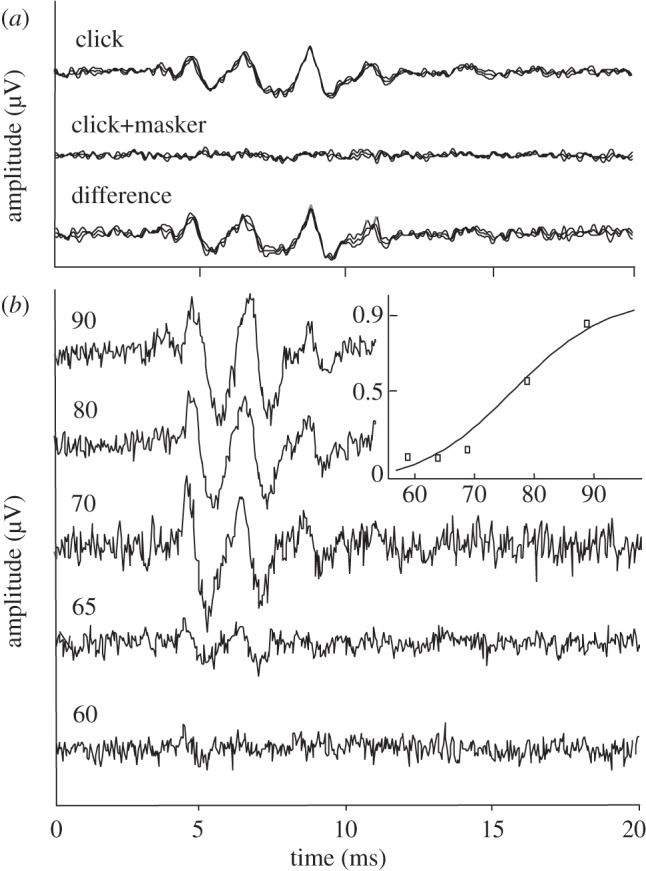
Generation and measurement of the masked ABR. (a) Response to click stimulation (upper trace), masked click stimulation (middle trace) and difference (bottom trace). (b) The ABR difference signal (as measured in A) at five different masker amplitudes (increasing from bottom to top). Insert: average amplitude of the difference signal (normalized by click response) as a function of masker level (symbols). A sigmoid function is fitted to the data (curve). Response thresholds are calculated from such curves as the levels where the response exceeds the average noise level in the recording.
Figure 3.
Audiograms for airborne and underwater sound and for direct vibration of the tympanic disc. (a) The masked ABR audiogram of six turtles in response to closed coupler stimulation, with best frequencies at 400–500 Hz and lowest thresholds around 60 dB SPL (bars ± 1s.d.). Insert: audiogram in one animal before and after removing the skin covering the tympanic disc. (b) The masked ABR audiogram in water (note reference level of 1 µPa). Data from four turtles. (c) The masked ABR response to direct vibration of the tympanic disc by vibration probe, showing thresholds as vibration velocities in dB re 1 µm s−1. (d) Comparison of sensitivity to sound pressure in air and water. Thresholds to underwater and airborne sound (recalculated in dB re 1 µPa) were measured in the same animal and the difference is shown for each animal. (a) Grey symbols in air; black line, mean. (b) Grey line, in water; black line, mean. (c) Grey line, data; black line, mean. (d) Grey line, data; black line, median.
(d). Underwater audiograms
Audiograms in four animals were measured in both air and water (figure 3b). The underwater audiograms had the same shape as audiograms in air, with best frequency of 400–500 Hz and lowest thresholds at 80 dB re 1 µPa (r.m.s.). Sound pressure thresholds in water were elevated by 10–20 dB compared with thresholds for the same animals in air (threshold differences in figure 3d). The median differences between air and water sound pressure thresholds in each animal ranged from 5 to 12 dB. Owing to the very different impedances, sound in water contains 30 dB less energy than in air at equal sound pressures, and the ear is, therefore, stimulated more efficiently by underwater sound.
(e). Direct vibration of the tympanic disc
Audiograms constructed from stimulation of the tympanic disc by vibration probe had a similar shape to the sound audiograms, with lowest vibration velocity thresholds around 2–5 µm s−1, corresponding to displacement thresholds near 1 nm at 500 Hz (n = 4, figure 3c). Assuming that the disc vibration was comparable to vibration with sound stimuli, the lowest sound thresholds at 60 dB SPL were equivalent to a disc vibration of 2–5 µm s−1 at 500 Hz, corresponding to a disc transfer function of 0.1–0.25 mm s−1 pa−1.
(f). Whole-body vibration
Sensitivity to dorso-ventral vibration of the whole body was investigated in five animals. The resulting sensitivity function was not similar to the audiograms, with a peak at 150–200 Hz, and thresholds of 20–50 µm s−1 (median 28 µm s−1; figure 4). Thus, dorso-ventral vibration was not as effective as disc vibration in stimulating the auditory system.
Figure 4.
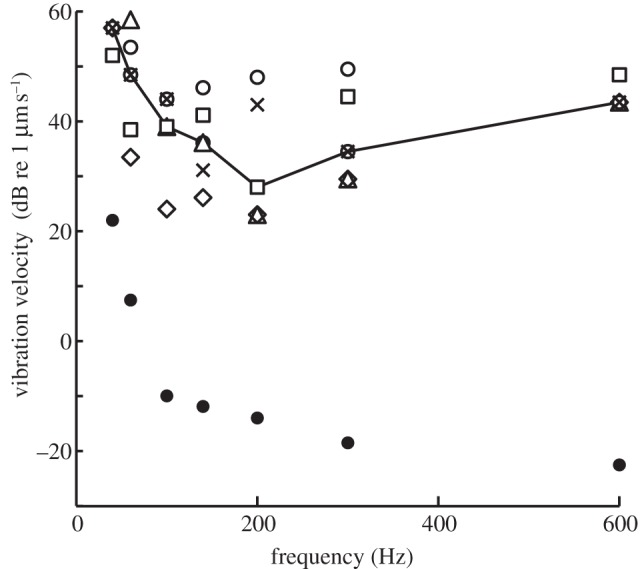
Sensitivity to whole-body dorso-ventral vibration. The masked ABR vibrogram in response to whole-body vibration (i.e. particle motion stimulation). Thresholds in substrate vibration velocities in dB re 1 µm s−1. Open symbols, data from individual animals; line, median threshold; closed circles, noise level.
4. Discussion
One of the problems faced by amphibious animals such as turtles, frogs, crocodiles or seals is to adapt their sensory systems to the very different properties of air and water. For hearing, the much denser medium of water is characterized by higher sound speeds, smaller particle motion components and higher sound pressures than air (at equal sound energy). Also, the impedance of water is close to the impedance of animal tissues, so sound can enter the head and body with little reflection. Thus, whereas the main task of a sensitive middle ear in air is to funnel sound energy into the inner ear, minimizing impedance mismatch through compliant sound receiving structures, the main task of a sensitive underwater ear is to maximize the ear's response to the small particle motion components. Amphibious animals must find a compromise between these tasks [5].
(a). Specializations of the turtle ear
The turtle tympanic disc and large, air-filled middle ear cavity (figure 1) may be specializations for underwater hearing. Laser measurements show that the tympanic disc vibrates with 40 dB larger amplitudes than adjacent head regions to underwater sound, with a frequency optimum close to best frequency in the audiogram (400–600 Hz). The disc vibrations are reduced by partial filling of the middle ear cavity with water. Furthermore, the peak frequency matches the predicted resonance frequency of an air bubble with the same volume as the middle ear cavity. Thus, we hypothesize that the air-filled cavities resonate in the underwater sound field and the pulsations of the enclosed air drive the tympanic disc. Therefore, the ear is driven by sound pressure both in air and water. Sound pressure thresholds in air are 5–12 dB lower than in water, and since the sound energy in air at equal sound pressure is more than 30 dB higher than in water, owing to the characteristic impedance difference, the ear responds to much lower sound energy in water and is more efficient in water. The difference in pressure sensitivity is probably owing to increased load on the outer surface of the tympanic disc underwater.
The proposed mechanism resembles the swimbladder–inner ear coupling in otophysine fish, where coupling of the air-filled swimbladder to the inner ear by the Weberian ossicles enhances sound sensitivity by 40 dB or more [25]. The turtle ear also closely resembles the ear of the clawed frog (Xenopus laevis), which has a cartilaginous tympanic disc and an air-filled middle ear cavity that adapt the ear for underwater hearing [23,26–28]. In Xenopus, the ear's peak frequency is higher (about 2.4 kHz) because the middle ear cavity is smaller. Nevertheless, the peak disc velocity of 0.3 mm s−1 Pa−1 measured in the present study is comparable to that of Xenopus, ranging from 0.05 to 0.25 mm s−1 Pa−1 [26]. Therefore, the sensitivity of the turtle to underwater sound is broadly comparable to the sensitivity of both Xenopus [24] and otophysine fish [25], allowing for differences in experimental design and the generally higher thresholds in ABR experiments (see below).
The convergence belies the common misconception that a tympanic ear is less efficient in water. Turtle and Xenopus ears clearly show that a tympanic ear with minor modifications, e.g. in shape and extent of the extracolumella, can function very efficiently under water [28]. A comparison of these species with another amphibian species, the American alligator [29], is instructive. The alligator ear resembles that of birds, with a delicate tympanum. It, too, has air in the middle ear, which may drive a tympanic response underwater, as suggested by the alligator's relatively good sensitivity to underwater sound. However, the delicate tympanum of the alligator may be less efficiently coupled with the extracolumella if driven by middle ear air pulsation, as proposed for terrestrial frogs [26].
For the turtle in air, impedance mismatch from the heavy tympanic disc may reduce sound sensitivity, resulting in ABR thresholds of 50–60 dB SPL at 400–600 Hz. Since ABR thresholds are approximately 20 dB above the lowest sensory thresholds [30–32], an estimate of the lowest sensory thresholds would be near 40 dB SPL in air, consistent with previous neurophysiological and behavioural studies [12,16,33]. Thus, the estimated best neural thresholds are approximately 30 dB above thresholds in other tympanate animals such as anurans, lizards, birds and mammals. Furthermore, the apparent tympanum, that is, the skin covering the tympanic disc, may not be functional, as was suggested previously [34,35], since the sensitivity is unchanged after its removal (figure 3a, insert).
(b). Comparison of the different auditory brainstem response measurements
ABRs are a useful method to evaluate turtle hearing. The audiogram shape in air and the responses to clicks are similar to earlier results [36]. The four ABR experiments (air, water, disc vibration and whole-body vibration) show that air, water and disc vibration generate similar curves, with peak sensitivity at 400–500 Hz (figure 5, median values). The shape of the audiogram may partly be explained by the middle ear cavity resonance, while the similarity of the disc vibration and air audiogram curves suggests that the inner ear is most responsive around 400–500 Hz. This could reflect the position of the 400–500 Hz region in the middle of the basilar papilla; excitation here would stimulate a maximal number of sensory cells. Alternatively, it may reflect increased sensitivity or hair cell density in this region. O'Neill & Bearden [37] showed that the inner ear basilar membrane motion largely follows the mechanics of the middle ear, while Ruggero & Temchin [38], contrasting the steep cut-off frequency of the audiogram with the almost flat frequency response of the columella in air, hypothesized that the cut-off was largely caused by the inner ear.
Figure 5.
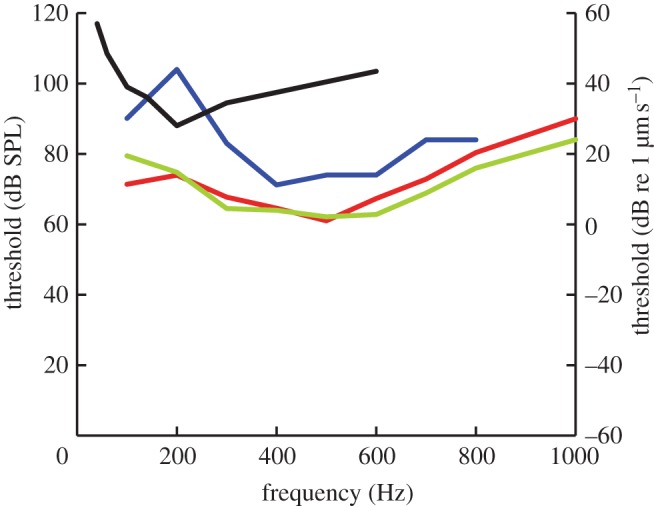
Summary. Audiograms (median values) from figures 3 and 4 for comparison. Note that the vibrograms are plotted relative to the right axis as vibration velocities (dB re 1 µm s−1), while both airborne and underwater sound thresholds are plotted relative to the left axis in dB SPL (i.e. re 20 µPa, r.m.s.). Red line, sound–air; blue line, sound–water; black line, body vibration; green line, tympanic disc vibration.
By comparing thresholds to sound and disc vibration, we can calculate the vibration transfer function of the eardrum, assuming thresholds measured in the two paradigms are comparable. The lowest threshold in air at 61 dB SPL corresponds to a tympanic disc vibration of approximately 3.6 µm s−1 (0.5 nm displacement at 500 Hz). Direct measurements of disc transfer function using light scattering spectroscopy [35] showed peak amplitudes of 15–50 nm at 500 Hz and 90 dB SPL sound pressure, so these measurements are comparable. Whole-body vibrograms had a best frequency of 100–200 Hz, lower than both sound and disc vibration audiograms. This difference suggests that auditory sensitivity is not driven by particle motion, as is underwater hearing in fish [39] and lungfish [40]. Also, auditory sensitivity in turtles is probably not based on sound-induced vibrations in the skull, as in the Royal Python [41]. Instead, the different best frequencies suggest another pathway of vibration stimulation to the inner ear, or, more likely, stimulation of a different sensory epithelium. Turtles are less sensitive to whole-body vibration than pythons, for which sensitivity in a similar set-up was approximately 5 dB re 1 µm s−1, or about 20 dB lower than the turtle threshold [41].
(c). Comparisons with other reptiles
Whether the mechanism we have described here also applies to hearing in the completely aquatic sea turtles (Chelonidae and Dermochelydae) is an open question. The sea turtle middle ear is more massive than the present turtle ear, but the audiogram of Chelonia mydas shows a best frequency at 400 Hz and a similar shape to Trachemys, with comparable sensitivities to direct vibration of the tympanum [42]. The Chelonia ear also has a middle ear cavity and a long columella. If the cavity is air-filled, its pulsations could drive the columella, as we report for Trachemys. An alternative suggestion, proposed by Lenhardt [43], is that the ear should be specialized for bone conduction, since the thick tympanum should enhance bone conduction cues. This is unlikely, since the vibration input would be small, given that particle velocities are small. Therefore, an ear based on bone conduction would only respond to very intense sound levels or sound at close range.
(d). Evolution of the turtle ear
The tympanic ear originated independently in turtles [9]. Specializations for underwater hearing may reflect the primitive condition in turtles if they originated in aquatic habitats. A marine origin is inferred for turtles considered closely related to extinct marine sauropterygian diapsids [44]. Near-shore marine sediments preserving Odontochelys (the oldest known fossil turtle, lower Triassic) support that inference [44], as does the upper Triassic Proganochelys, also found in marine sediments [45]. Interestingly, Proganochelys had a large ear opening and middle ear cavities that are open like in modern lizards [45], and not closed-like modern turtles. Closed middle ear cavities are not seen until the Eocene [11]. On the other hand, front limb proportions, shell shape and histology suggest that other near-crown stem turtles were terrestrial [46,47]. In addition, Eunotosaurus and successive out-groups lack obvious aquatic adaptations and are only known from terrestrial sediments [48]. The present finding that underwater hearing sensitivity is dominated by the properties of air in the middle ear allows us to use anatomical measures to estimate the frequency response of the ears of some of these extinct species [49,50].
(e). Function of turtle underwater hearing
The behavioural significance of hearing in turtles remains to be discovered, but navigation, prey detection and predator avoidance are probably important in this group, as in many aquatic vertebrates. A recent fascinating study showed that Australian long-necked turtles, Chelodina, have a complex underwater sound repertoire [51]. The very similar ear of the clawed frog Xenopus appears useful in underwater sound communication [27]. It remains to be seen whether underwater sound communication occurs among members of the Cryptodira. The sensitive aquatic ear of turtles strongly suggests the possibility of undetected instances of underwater sound communication.
Acknowledgements
Experiments were performed according to the guidelines approved by the Marine Biological Laboratory (Woods Hole, MA, USA), the University of Maryland Institutional Animal Care and Use Committees (IACUC) and the Danish National Animal Experimentation Board (Dyreforsøgstilsynet).
Supported by awards from the Danish National Science Foundation 09-065990 and Carlsberg Foundation 2009-01-0684 (JCD), the Velux Foundation (Denmark) and NIH DC00436 (CEC), and by NIH P30 DC0466 to the University of Maryland Center for Comparative and Evolutionary Biology of Hearing. We thank Julie Arruda for help with CT scanning and imaging and Tobias Wang for providing turtles.
References
- 1.Christensen-Dalsgaard J. 2005. Directional hearing in nonmammalian tetrapods. In Sound source localization (eds Popper A., Fay R.), pp. 67–123 New York, NY: Sound Source Localization; 10.1007/0-387-28863-5 (doi:10.1007/0-387-28863-5) [DOI] [Google Scholar]
- 2.Christensen-Dalsgaard J., Carr C. E. 2008. Evolution of a sensory novelty: tympanic ears and the associated neural processing. Brain Res. Bull. 75, 365–370 10.1016/j.brainresbull.2007.10.044 (doi:10.1016/j.brainresbull.2007.10.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coles R., Gower D. M., Boyd P. J., Lewis D. B. 1982. Acoustic transmission through the head of the common mole, Talpa europaea. J. Exp. Biol. 101, 337–341 [DOI] [PubMed] [Google Scholar]
- 4.Manley G. A. 2010. An evolutionary perspective on middle ears. Hear. Res. 263, 3–8 10.1016/j.heares.2009.09.004 (doi:10.1016/j.heares.2009.09.004) [DOI] [PubMed] [Google Scholar]
- 5.Wever E. G. 1978. The reptile ear, its structure and function. Princeton, NJ: Princeton University Press [Google Scholar]
- 6.Hedges S. B., Poling L. 1999. A molecular phylogeny of reptiles. Science 283, 998–1001 10.1126/science.283.5404.998 (doi:10.1126/science.283.5404.998) [DOI] [PubMed] [Google Scholar]
- 7.Rieppel O., Reisz R. R. 1999. The origin and early evolution of turtles. Annu. Rev. Ecol. Syst. 30, 1–22 10.1146/annurev.ecolsys.30.1.1 (doi:10.1146/annurev.ecolsys.30.1.1) [DOI] [Google Scholar]
- 8.Lyson T. R., Bever G. S., Bhullar B.-A. S., Joyce W. G., Gauthier J. A. 2010. Transitional fossils and the origin of turtles. Biol. Lett. 6, 830–833 10.1098/rsbl.2010.0371 (doi:10.1098/rsbl.2010.0371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clack J. A. 1997. The evolution of tetrapod ears and the fossil record. Brain Behav. Evol. 50, 198–212 10.1159/000113334 (doi:10.1159/000113334) [DOI] [PubMed] [Google Scholar]
- 10.Wever E. G., Vernon J. A. 1956. Sound transmission in the turtle's ear. Proc. Natl Acad. Sci. USA 42, 292–299 10.1073/pnas.42.5.292 (doi:10.1073/pnas.42.5.292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hetherington T. 2008. Comparative anatomy and function of hearing in aquatic amphibians, reptiles, and birds. In Sensory evolution on the threshold: adaptations in secondarily aquatic vertebrates (eds Thewissen J., Nummela S.), pp. 183–209 Berkeley, CA: University of California Press [Google Scholar]
- 12.Crawford A., Fettiplace R. 1980. The frequency selectivity of auditory nerve fibres and hair cells in the cochlea of the turtle. J. Physiol. 306, 79–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Art J., Fettiplace R. 1987. Variation of membrane properties in hair cells isolated from the turtle cochlea. J. Physiol. 385, 207–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricci A. J., Crawford A. C., Fettiplace R. 2003. Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron 40, 983–990 10.1016/S0896-6273(03)00721-9 (doi:10.1016/S0896-6273(03)00721-9) [DOI] [PubMed] [Google Scholar]
- 15.Holt J. C., Lysakowski A., Goldberg J. M. 2006. Mechanisms of efferent-mediated responses in the turtle posterior crista. J. Neurosci. 26, 13 180–13 193 10.1523/JNEUROSCI.3539-06.2006 (doi:10.1523/JNEUROSCI.3539-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson W. C. 1966. Hearing in the turtle. J. Aud. Res. 6, 453–464 [Google Scholar]
- 17.Willis K., Potter K., Christensen-Dalsgaard J., Carr C. E. 2011. Allometry of the middle ear in Trachemys scripta elegans. Assoc. Res. Otolaryngol. 44, 44 [Google Scholar]
- 18.Au W. W. L., Hastings M. C. 2008. Principles of marine bioacoustics. New York, NY: Springer Verlag [Google Scholar]
- 19.Alexander R. 1966. Physical aspects of swimbladder function. Biol. Rev. 41, 141–176 10.1111/j.1469-185X.1966.tb01542.x (doi:10.1111/j.1469-185X.1966.tb01542.x) [DOI] [PubMed] [Google Scholar]
- 20.Berlin C., Hood L., Barlow E., Morehouse C., Smith E. 1991. Derived guinea pig compound VIIIth nerve action potentials to continuous pure tones. Hear. Res. 52, 271–280 10.1016/0378-5955(91)90017-4 (doi:10.1016/0378-5955(91)90017-4) [DOI] [PubMed] [Google Scholar]
- 21.Brandt C., Andersen T., Christensen-Dalsgaard J. 2008. Demonstration of a portable system for auditory brainstem recordings based on pure tone masking difference. In 1st Int. Symp. on Auditory and Audiological Research, pp. 241–247 Holbæk, Denmark: Centertryk. [Google Scholar]
- 22.Christensen-Dalsgaard J., Carr C. E., Madsen P., Brandt C., Willis K., Ketten D., Edds-Walton P., Fay R. 2010. Specialisation for underwater hearing in the red-eared Slider Turtle, Trachemys scripta elegans. Assoc. Res. Otolaryngol. Abs. 51, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen-Dalsgaard J., Breithaupt T., Elepfandt A. 1990. Underwater hearing in the clawed frog, Xenopus laevis. Tympanic motion studied with laser vibrometry. Naturwissenschaften 77, 135–137 10.1007/BF01134478 (doi:10.1007/BF01134478) [DOI] [PubMed] [Google Scholar]
- 24.Elliott T. M., Christensen-Dalsgaard J., Kelley D. B. 2007. Tone and call responses of units in the auditory nerve and dorsal medullary nucleus of Xenopus laevis. J. Comp. Physiol. A 193, 1243–1257 10.1007/s00359-007-0285-z (doi:10.1007/s00359-007-0285-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popper A. N., Fay R. R. 2011. Rethinking sound detection by fishes. Hear. Res. 273, 25–36 10.1016/j.heares.2009.12.023 (doi:10.1016/j.heares.2009.12.023) [DOI] [PubMed] [Google Scholar]
- 26.Christensen-Dalsgaard J., Elepfandt A. 1995. Biophysics of underwater hearing in the clawed frog, Xenopus laevis. J. Comp. Physiol. A 176, 317–324 10.1007/BF00219057 (doi:10.1007/BF00219057) [DOI] [PubMed] [Google Scholar]
- 27.Christensen-Dalsgaard J., Elliott T. M. 2009. Amphibian underwater hearing: biophysics and neurophysiology. Bioacoustics 17, 60–62 [Google Scholar]
- 28.Mason M. J., Wang M., Narins P. 2009. Structure and function of the middle ear apparatus of the aquatic frog, Xenopus laevis. Proc. Inst. Acoust. 31, 13–21 [PMC free article] [PubMed] [Google Scholar]
- 29.Higgs D. M., Brittan-Powell E. F., Soares D., Souza M. J., Carr C. E., Dooling R. J., Popper A. N. 2002. Amphibious auditory responses of the American alligator (Alligator mississipiensis). J. Comp. Physiol. A 188, 217–223 10.1007/s00359-002-0296-8 (doi:10.1007/s00359-002-0296-8) [DOI] [PubMed] [Google Scholar]
- 30.Gorga M. P., Kaminski J. R., Beauchaine K. A., Jesteadt W. 1988. Auditory brainstem responses to tone bursts in normally hearing subjects. J. Speech Hear. Res. 31, 87–97 [DOI] [PubMed] [Google Scholar]
- 31.Brittan-Powell E. F., Dooling R. J., Gleich O. 2002. Auditory brainstem responses in adult budgerigars (Melopsittacus undulatus). J. Acoust. Soc. Am. 112, 999–1008 10.1121/1.1494807 (doi:10.1121/1.1494807) [DOI] [PubMed] [Google Scholar]
- 32.Köppl C., Gleich O. 2007. Evoked cochlear potentials in the barn owl. J. Comp. Physiol. A 193, 601–612 10.1007/s00359-007-0215-0 (doi:10.1007/s00359-007-0215-0) [DOI] [PubMed] [Google Scholar]
- 33.Manley G. A. 1970. Comparative studies of auditory physiology in reptiles. J. Comp. Psychol. 67, 363–381 [Google Scholar]
- 34.Adrian E., Craik K., Sturdy R. 1938. The electrical response of the auditory mechanism in cold-blooded vertebrates. Proc. R. Soc. Lond. B 125, 435–455 10.1098/rspb.1938.0036 (doi:10.1098/rspb.1938.0036) [DOI] [Google Scholar]
- 35.Moffatt A., Capranica R. 1978. Middle-ear sensitivity in anurans and reptiles measured by light-scattering spectroscopy. J. Comp. Physiol. A 127, 97–107 10.1007/BF01352294 (doi:10.1007/BF01352294) [DOI] [Google Scholar]
- 36.Corwin J. T., Bullock T., Schweitzer J. 1982. The auditory brain stem response in five vertebrate classes. Electroencephalogr. Clin. Neurophysiol. 54, 629–641 10.1016/0013-4694(82)90117-1 (doi:10.1016/0013-4694(82)90117-1) [DOI] [PubMed] [Google Scholar]
- 37.O'Neill M. P., Bearden A. 1995. Laser-feedback measurements of turtle basilar membrane motion using direct reflection. Hear. Res. 84, 125–138 10.1016/0378-5955(95)00018-Y (doi:10.1016/0378-5955(95)00018-Y) [DOI] [PubMed] [Google Scholar]
- 38.Ruggero M., Temchin A. 2002. The roles of the external, middle, and inner ears in determining the bandwidth of hearing. Proc. Natl Acad. Sci. USA 99, 13 206–13 210 10.1073/pnas.202492699 (doi:10.1073/pnas.202492699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sand O., Karlsen H. E. 2000. Detection of infrasound and linear acceleration in fishes. Phil. Trans. R. Soc. Lond. B 355, 1295–1298 10.1098/rstb.2000.0687 (doi:10.1098/rstb.2000.0687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christensen-Dalsgaard J., Brandt C., Wilson M., Wahlberg M., Madsen P. T. 2011. Hearing in the African lungfish (Protopterus annectens): pre-adaptation to pressure hearing in tetrapods? Biol. Lett. 7, 139–141 10.1098/rsbl.2010.0636 (doi:10.1098/rsbl.2010.0636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christensen C. B., Christensen-Dalsgaard J., Brandt C., Madsen P. T. 2012. Hearing with an atympanic ear: good vibration and poor sound-pressure detection in the royal python, Python regius. J. Exp. Biol. 215, 331–342 10.1242/jeb.062539 (doi:10.1242/jeb.062539) [DOI] [PubMed] [Google Scholar]
- 42.Ridgway S., Wever E., McCormick J., Palin J., Anderson J. 1969. Hearing in the giant sea turtle, Chelonia mydas. Proc. Natl Acad. Sci. USA 64, 884–890 10.1073/pnas.64.3.884 (doi:10.1073/pnas.64.3.884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenhardt M. 1982. Bone conduction hearing in turtles. J. Aud. Res. 22, 153–160 [PubMed] [Google Scholar]
- 44.Li C., Wu X.-C., Rieppel O., Wang L.-T., Zhao L.-J. 2008. An ancestral turtle from the Late Triassic of southwestern China. Nature 456, 497–501 10.1038/nature07533 (doi:10.1038/nature07533) [DOI] [PubMed] [Google Scholar]
- 45.Gaffney E. S. 1990. The comparative osteology of the Triassic turtle Proganochelys. Bull. AMNH 194, 1–263 [Google Scholar]
- 46.Joyce W. G., Gauthier J. A. 2004. Palaeoecology of Triassic stem turtles sheds new light on turtle origins. Proc. R. Soc. Lond. B 271, 1–5 10.1098/rspb.2003.2523 (doi:10.1098/rspb.2003.2523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheyer T. M., Sander P. M. 2007. Shell bone histology indicates terrestrial palaeoecology of basal turtles. Proc. R. Soc. B 274, 1885–1893 10.1098/rspb.2007.0499 (doi:10.1098/rspb.2007.0499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gow C. 1997. A reassessment of Eunotosaurus africanus Seeley (Amniota: Parareptilia). Palaeontol. Africana 33, 33–42 [Google Scholar]
- 49.Walsh S. A., Barrett P. M., Milner A. C., Manley G. A., Witmer L. M. 2009. Inner ear anatomy is a proxy for deducing auditory capability and behaviour in reptiles and birds. Proc. R. Soc. B 276, 1355–1360 10.1098/rspb.2008.1390 (doi:10.1098/rspb.2008.1390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gleich O., Dooling R. J., Manley G. A. 2005. Audiogram, body mass, and basilar papilla length: correlations in birds and predictions for extinct archosaurs. Naturwissenschaften 92, 595–598 10.1007/s00114-005-0050-5 (doi:10.1007/s00114-005-0050-5) [DOI] [PubMed] [Google Scholar]
- 51.Giles J. C., Davis J. A., McCauley R. D., Kuchling G. 2009. Voice of the turtle: the underwater acoustic repertoire of the long-necked freshwater turtle, Chelodina oblonga. J. Acoust. Soc. Am. 126, 434–443 10.1121/1.3148209 (doi:10.1121/1.3148209). [DOI] [PubMed] [Google Scholar]