Abstract
The reproductive costs associated with the upregulation of immunity have been well-documented and constitute a fundamental trade-off between reproduction and self-maintenance. However, recent experimental work suggests that parents may increase their reproductive effort following immunostimulation as a form of terminal parental investment as prospects for future reproduction decline. We tested the trade-off and terminal investment hypotheses in a wild population of house wrens (Troglodytes aedon) by challenging the immune system of breeding females with lipopolysaccharide, a potent but non-lethal antigen. Immunized females showed no evidence of reproductive costs; instead, they produced offspring of higher phenotypic quality, but in a sex-specific manner. Relative to control offspring, sons of immunized females had increased body mass and their sisters exhibited higher cutaneous immune responsiveness to phytohaemagglutinin injection, constituting an adaptive strategy of sex-biased allocation by immune-challenged females to enhance the reproductive value of their offspring. Thus, our results are consistent with the terminal investment hypothesis, and suggest that maternal immunization can induce pronounced transgenerational effects on offspring phenotypes.
Keywords: immune challenge, life-history trade-off, maternal effect, sex allocation, terminal investment
1. Introduction
The trade-off between present and future reproduction is fundamental to life-history evolution [1,2] and influences parental investment strategies because increased investment in current offspring reduces residual reproductive value [3–5]. Aside from trade-offs between present and future offspring, research foci have broadened to include trade-offs across life-history functions as it is generally thought that the optimal balance among growth, reproduction and self-maintenance maximizes individual fitness [6]. Resource-allocation theory suggests that an individual's investment in immunity should necessarily reduce its ability to invest in reproduction, and vice versa, when resources are in short supply, and this trade-off has been the subject of considerable study across a variety of taxa [7–10]. Indeed, recent reviews and meta-analyses generally conclude that both the maintenance and activation of the immune system are physiologically taxing, and can lead to reproductive costs [11–13].
Despite the apparent ubiquity of the trade-off between reproductive effort and immunity, exceptions have been reported [14–17]. Recent work suggests that, even when the immune system is activated during breeding, individuals may continue to allocate scarce resources to reproduction if the immunostimulation provides a cue to impending illness and increased mortality risk and, consequently, a reduction in residual reproductive value [15,18–21]. Such findings are consistent with the terminal investment hypothesis, which predicts that individuals with a low probability of future reproduction (i.e. low residual reproductive value) should increase their current reproductive effort relative to those with higher residual reproductive value [22,23]. The terminal investment hypothesis predicts that if an individual perceives its prospects for survival and future reproduction have been jeopardized, then it should boost its current reproductive effort because it will probably be that individual's last chance to reproduce. Thus, terminal investment is expected from parasitized individuals if the immunostimulation provides a cue to their reduced survival prospects [18–21]. For example, Weil et al. [20] elicited an immune response in male Siberian hamsters (Phodopus sungorus) in concert with decreasing day-length, and found that immune-challenged males maintained larger testes, larger seminal vesicles and higher circulating testosterone than control males as day-length shortened, indicative of an oncoming winter. Immune-challenged males produced more sperm than controls, demonstrating increased reproductive effort despite the simulated infection [20]. Thus, terminal investment might be expected from seasonally breeding individuals that are less likely than others to survive until their next reproductive opportunity.
In this study, we tested the trade-off and terminal investment hypotheses by inducing an immune response in breeding female house wrens (Troglodytes aedon). We injected females with lipopolysaccharide (LPS) near the end of their breeding season, failed their current clutch of eggs and monitored their subsequent reproductive success. We predicted that, if immune-challenged females trade reproduction for immunity, then their subsequent reproductive success should be lower than that of vehicle-injected controls, as measured by their likelihood of producing a replacement clutch and the condition of their offspring. In contrast, the terminal investment hypothesis predicts that immune-challenged females should be more likely than control females to produce a replacement clutch, and that their offspring should be in better condition than those of control females. We also determined whether our treatment had an effect on females' allocation towards sons and daughters because sex-allocation theory predicts that maternal condition or reproductive effort should influence allocation towards each sex [24,25]. In our study population, the effect of mass on future fecundity is sex-specific: heavier sons out-reproduce lighter sons as adults, whereas the less-variable fecundity of daughters is less strongly related to their mass and environment as nestlings (E. K. Bowers 2009–2011, unpublished data). Thus, we predicted that, if immune-challenged females trade reproductive effort for self-maintenance, then they should bias their subsequent allocation towards daughters rather than sons as their ability to produce high-quality offspring is reduced, because poor-quality daughters should have higher fitness than poor-quality sons; however, if immunized females increase reproductive effort consistent with the terminal investment hypothesis, then they should preferentially invest in sons to produce high-quality males of high reproductive value.
2. Methods
(a). Study species and site
House wrens are small (10–12 g), migratory songbirds that are sexually monomorphic in size and plumage. Males return to the study area from their over-wintering grounds in April and select and defend a nest cavity in which they begin nest construction. The later-arriving females select a mate and complete nest construction before laying a clutch of four to eight eggs. Approximately half of the females that complete a successful breeding attempt in May attempt a second brood in the study area [5], with peak egg production in early May for early-season broods and early July for late-season broods. House wrens are well-suited to study sex allocation because females routinely differentially invest in sons and daughters in association with a variety of social and environmental conditions (reviewed in Bowers et al. [26]). See Johnson [27] for more on house wren biology.
Our study population breeds at the Mackinaw study area, a secondary deciduous forest bordering the Mackinaw River in McLean County, IL, USA (40°40′ N, 88°53′ W). This site has 700 nest-boxes distributed along north–south-oriented transects (fig. 1 in DeMory et al. [28]); details on nest-box materials and dimensions are described elsewhere [29].
(b). Procedures and experimental design
Beginning in mid-June 2010, we checked nest-boxes regularly for female settlement. After females completed their late-season clutches, we captured them 3.1 ± 0.1 (mean ± s.e.) days into incubation and randomly assigned them to a control or experimental treatment. We injected control females (n = 27) intra-abdominally with 50 μl of phosphate-buffered saline (PBS) and experimental females (n = 28) with 50 μl of PBS containing 0.1 mg × kg body mass−1 LPS (from Salmonella enterica serotype typhimurium; Sigma, prod no. L7261). LPS is derived from the outer coat of Gram-negative bacteria and is commonly used to challenge the immune system in ecoimmunological studies [16,18−20,30]. After injection, we failed the female's nest by collecting the eggs and subsequently determined whether they laid a replacement clutch (i.e. re-nested). Forced re-nesting allows time for females to develop an immune response [31]. There were no differences between control and experimental females in their pre-injection clutch size (t53 = 0.02, p = 0.981), body mass (t53 = 1.29, p = 0.204) or the proportion that had already bred on the study area prior to our experiment (proportion that attempted to produce a brood: χ2 = 0.05, p = 0.826; proportion that successfully fledged young: χ2 = 0.23, p = 0.630).
For females that re-nested, we monitored the growth of their nestlings and, 11 days after hatching began, weighed nestlings to the nearest 0.1 g on an electronic balance and measured their tarsus length to the nearest 0.1 mm with dial callipers. At this time, we also drew a blood sample (approx. 50 µl) from each nestling's left brachial vein, storing them on ice until return to the laboratory, where we separated the plasma and red blood cells by centrifugation at 1610g for 60 s. Because nestlings cannot be sexed using external morphology, we preserved their nucleated red blood cells for later DNA extraction and sexing using the polymerase chain reaction (details in Bowers et al. [26]). A portion of the plasma was used that day for a bactericidal assay by incubating approximately 200 colony-forming bacteria with and without nestling plasma overnight, and using the proportion of colonies killed on the plates containing plasma relative to those without as our measure of bactericidal activity (further details in Forsman et al. [32]). We froze the remaining plasma at −20°C until later determination of LPS antibody levels, which we quantified using ELISA. We coated 96-well, polystyrene plates with 100 µl of a 20 µl × ml−1 solution of LPS in PBS and incubated them overnight at 4°C. We then washed each well three times for 3 min with 200 µl of a buffer solution (PBS-T) containing 1 per cent PBS, 0.05 per cent bovine serum albumen and 0.01 per cent Tween buffer. Plasma samples were diluted 1 : 25 in 100 µl PBS-T, incubated for 1 h at room temperature, and washed as before. Antibodies were detected with 100 µl of a 1 : 100 dilution of anti-bird IgG (Bethyl Laboratories, prod no. A140-110P) in PBS-T and incubated and washed as before. We then washed the plate with 100 µl ddH2O and added 100 µl of ABTS solution (Southern Biotech, prod no. 0202-01) to each well. Twenty minutes after adding ABTS, we read the plate at 405 nm using a Powerwave 340 plate reader (BioTek Inc.). Because our primary aim was to measure bactericidal activity, we did not have sufficient plasma remaining to measure antibody levels in all nestlings; thus, our sample size for antibody levels (n = 41) is smaller than for other measures of nestling condition. After drawing blood samples from nestlings, we administered the phytohaemagglutinin (PHA) skin test as a measure of cutaneous immune responsiveness by injecting the left wing web with 50 μl of PBS containing 1 mg × ml−1 of PHA (Sigma, prod no. L8754). PHA is a plant-derived mitogen that induces inflammation and swelling upon injection, and large swellings indicate a robust immune response. We measured swelling from the difference between the mean of three pre- and post-injection measurements of wing-web thickness with a digital thickness gauge (no. 547–500, Mitutoyo America Corp.).
(c). Data analysis
We used SAS (version 9.2) for all analyses and all tests are two-tailed. We obtained parsimonious models through stepwise elimination of non-significant (p > 0.1) effects from full models, beginning with removal of two-way interactions. Thus, analyses presented are for reduced models, with interactions reported only where significant.
Of the 55 females injected, 29 produced a replacement clutch following failure of their pre-injection clutch (14 control; 15 experimental). We first analysed how the treatment affected control and experimental females by comparing the probability that females would re-nest using logistic regression (PROC GENMOD), and the time elapsed between injection/nest-failure and re-nesting using survival analysis (PROC PHREG). We also analysed the clutch sizes of pre- and post-injection clutches using repeated-measures ANOVA (PROC MIXED), the hatching success of post-injection clutches (as expressed by the number of eggs hatched relative to the clutch size) and brood sex ratios using a generalized linear-mixed model (GLMM) with a binary response and logit link (PROC GLIMMIX). Because time-of-season influences whether females produce second broods and the length of time it takes to do so [5], we included this variable as a fixed effect in each analysis. We also collected blood samples from a subset of control (n = 4) and experimental (n = 6) females upon completion of their replacement nests (mean ± s.e. = 28.2 ± 1.5 days post-injection) to determine whether LPS or total antibody levels differed between treatments using a two-sample t-test. Unfortunately, adult return rates in 2011 were low, and the only LPS-injected females that returned were those that produced replacement clutches in 2010 (no LPS-injected females that failed to re-nest following our treatment returned); thus, because of the self-selected sample, we did not analyse fecundity in the next breeding season.
We analysed the effect of maternal immunization on nestlings (n = 116 from 25 successful re-nesting attempts) by analysing their mass, tarsus length, a body condition index (residual of a log[mass] × log[tarsus] linear regression), PHA response (arcsin-transformed), bactericidal ability (square-root-transformed) and LPS antibody levels (log-transformed) using mixed-model ANOVA with nest ID as a random effect. Although nestling morphological data met assumptions of the statistical test, the immunological data were heteroscedastic; thus, we best satisfied the assumption of homoscedasticity by transforming our immunological data [33,34]. For example, the residual variance among PHA responses was greater among offspring of saline-injected females than those of LPS-injected females (Bartlett's test on raw data: p = 0.01; transformed data: p = 0.08), and also tended to be higher among sons than among daughters (Bartlett's test on raw data: p = 0.15; transformed data: p = 0.53). We tested whether females differentially allocated resources to sons or daughters by including nestling sex in each analysis. In addition to testing for a treatment × sex interaction, we initially included hatching date, maternal body mass and brood size as covariates in each model along with all two-way interactions, which were removed in backward-iterative fashion if non-significant (p > 0.1). For follow-up tests, we used the slice option to compare sons and daughters within control and experimental broods. We used the Satterthwaite degrees of freedom estimation, which can result in non-integer degrees of freedom.
3. Results
(a). No effect of treatment on maternal females
Immunization with LPS did not influence whether control and experimental females produced a replacement clutch (control: 14 of 27 females re-nested; experimental: 15 of 28 females re-nested; Wald logistic regression: χ2 = 0.00, p = 0.998), but females became less likely to re-nest as the breeding season progressed, regardless of treatment (parameter estimate = −0.21 ± 0.06, Wald: χ2 = 13.17, p < 0.001). Females took an average of 5.8 ± 0.3 days to initiate replacement clutches, but treatment did not influence the time they took to do so (Wald survival analysis: χ2 = 0.01, p = 0.913). As with the probability of re-nesting, females took longer to re-nest as the breeding season progressed, independent of treatment (parameter estimate = −0.117 ± 0.03, Wald: χ2 = 18.1, p < 0.001).
Clutch size declined from pre-injection to post-injection clutches (pre-injection least-squares (LS) mean = 6.00 ± 0.15 eggs, post-injection LS mean = 5.06 ± 0.15 eggs; F1,27 = 25.8, p < 0.001), but treatment did not influence the change in clutch size (TRT × clutch number: F1,27 = 1.23, p = 0.277), and females in each treatment group fledged the same number of offspring (LS mean = 4.6 ± 0.3 for each group). There was also no difference in hatching success (χ2 = 0.09, p = 0.767) or in the sex ratio of control and experimental post-injection broods (proportion sons: control = 0.58 ± 0.07, experimental = 0.57 ± 0.06; GLMM: F1,113 = 0.01, p = 0.909). Among the females for which we measured LPS antibodies following injection (n = 4 control, 6 experimental), there was no difference in LPS antibody levels (control mean = 1.38 ± 1.11, experimental mean = 1.33 ± 1.07; t8 = 0.33, p = 0.746) or total antibodies (t6 = 0.34, p = 0.747). Of the original 55 females, 12 returned to breed the next year, and females in each treatment group were equally likely to return (control: six of 27; experimental: six of 28).
(b). Effects on nestling condition and immune function
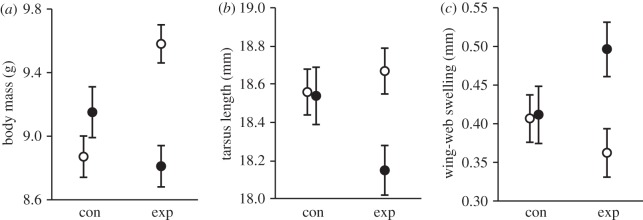
The effects of maternal LPS injection on offspring phenotype were almost entirely sex-specific, as revealed by interactions between treatment and sex in their effects on mass, tarsus length, body condition index and PHA response (tables 1 and 2). Follow-up tests showed that sons of experimental females were heavier and had a longer tarsus than their sisters (mass: F1 = 9.10, p = 0.003, figure 1a; tarsus: F1 = 8.77, p = 0.004, figure 1b), and were also heavier and had higher condition indices than sons of control females (mass: F1 = 7.98, p = 0.006, figure 1a; condition index: F1 = 10.91, p = 0.001). However, control sons and daughters did not differ in mass (F1 = 0.95, p = 0.334), tarsus length (F1 = 0.02, p = 0.883) or condition index (F1 = 2.17, p = 0.144).
Table 1.
Effects of maternal lipopolysaccharide injection on nestling mass, tarsus length and residual body mass. See text for details of follow-up tests.
| source of variation | F | d.f. | p |
|---|---|---|---|
| body mass | |||
| treatment | 0.25 | 1,24.4 | 0.622 |
| sex | 1.57 | 1,88 | 0.214 |
| treatment × sex | 7.38 | 1,88 | 0.008 |
| tarsus length | |||
| treatment | 0.32 | 1,24.4 | 0.577 |
| sex | 4.24 | 1,88 | 0.043 |
| treatment × sex | 3.37 | 1,88 | 0.069 |
| residual body mass | |||
| treatment | 1.29 | 1,24.3 | 0.267 |
| sex | 0.03 | 1,88 | 0.867 |
| treatment × sex | 4.18 | 1,88 | 0.044 |
Table 2.
Effects of maternal lipopolysaccharide (LPS) injection on nestling phytohaemagglutinin (PHA) response, bactericidal activity and LPS antibody titre. Estimates and confidence interval (lower and upper 95% confidence limits, CL) are back-transformed.
| source of variation | estimate | lower CL | upper CL | F | d.f. | p |
|---|---|---|---|---|---|---|
| PHA response | ||||||
| treatment | 0.04 | −0.10 | 0.18 | 0.46 | 1,22.5 | 0.503 |
| sex | 0.68 | 0.25 | 0.99 | 4.63 | 1,95.3 | 0.034 |
| treatment × sex | −0.16 | −0.30 | −0.02 | 5.34 | 1,93.9 | 0.023 |
| hatching date | 0.014 | 0.004 | 0.025 | 3.60 | 1,22.3 | 0.010 |
| sex × hatching date | −0.011 | −0.021 | −0.001 | 4.40 | 1,95.5 | 0.040 |
| intercept | −0.56 | −1.00 | −0.36 | |||
| bactericidal activity | ||||||
| treatment | 0.0001 | −0.025 | 0.031 | 0.01 | 1,22.2 | 0.916 |
| sex | 15.08 | 0.96 | 46.10 | 7.04 | 1,99.6 | 0.009 |
| hatching date | 0.0001 | −0.00003 | 0.0005 | 0.03 | 1,22.5 | 0.861 |
| sex × hatching date | −0.0004 | −0.0011 | −0.00003 | 7.37 | 1,99.8 | 0.008 |
| intercept | −1.54 | −17.06 | 2.72 | |||
| LPS antibody levels | ||||||
| treatment | 0.028 | −0.047 | 0.109 | 0.61 | 1,11.8 | 0.450 |
| sex | 15.2 | 0.68 | 155.7 | 6.29 | 1,30.3 | 0.018 |
| hatching date | 0.009 | 0.002 | 0.018 | 0.71 | 1,15.1 | 0.021 |
| sex × hatching date | −0.014 | −0.025 | −0.002 | 6.22 | 1,30.3 | 0.018 |
| intercept | −0.82 | −0.97 | −0.06 | |||
Figure 1.
(a) Nestling mass (b) tarsus length and (c) phytohaemagglutinin response in relation to maternal immune challenge and sex. Least-squares means ± s.e. are shown. Open circles denote sons, whereas filled circles denote daughters.
Although sons and daughters of control females did not differ in their PHA responses (F1 = 0.25, p = 0.616), experimental daughters mounted stronger PHA responses than experimental sons (F1 = 8.59, p = 0.004; table 2 and figure 1c), and also tended to mount stronger PHA responses than control daughters (F1 = 2.81, p = 0.101; figure 1c). Treatment and sex did not interact to influence nestling bactericidal activity or LPS antibody levels, but nestling sex and hatching date interacted to influence PHA response, bactericidal activity and antibody levels among nestlings (table 2), as these measures increased as the breeding season progressed among sons, but not among daughters (figure 2).
Figure 2.
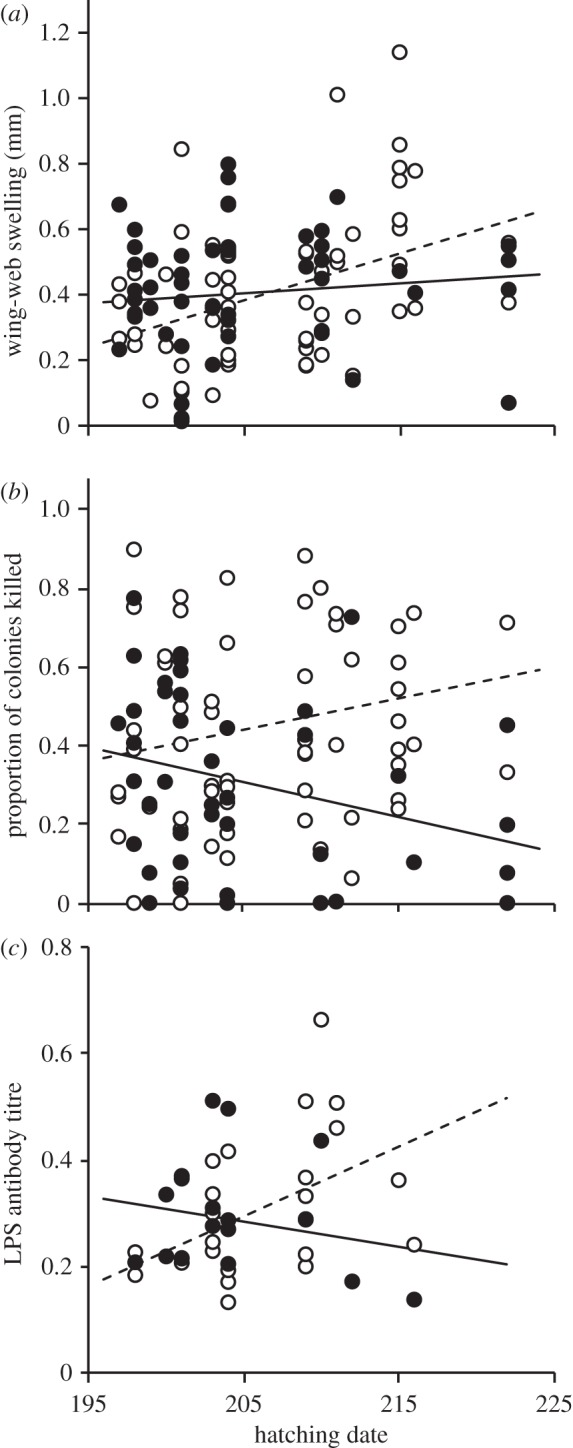
Nestling (a) phytohaemagglutinin response, (b) bacterial killing ability and (c) lipopolysaccharide (LPS) antibody titre in relation to hatching date and sex. Hatching dates are reported as date numbers (195 = 14 July). See table 2 for parameter estimates. Open circles with dashed line denote sons, whereas filled circles with solid line denote daughters.
4. Discussion
LPS- and vehicle-injected females did not differ in their likelihood of re-nesting, the time they took to do so, the size of their replacement clutches or their likelihood of breeding on the study area the following summer, suggesting that the immunostimulation did not cause females to trade reproductive effort for self-maintenance. These findings are consistent with a previous study of a similar-sized songbird, the pied flycatcher (Ficedula hypoleuca), that received a dosage of LPS comparable with what we used ([31], see also Williams et al. [14]). The LPS antibody levels of control and experimental nestlings also did not differ, consistent with that previous study [31], and post-injection LPS antibodies did not differ between control and experimental females when recaptured at their replacement nests. Lack of an effect on LPS antibody levels is not unexpected, considering that the compound is derived from bacteria that animals are continuously exposed to in nature [31]. However, LPS injection is known to cause an immunostimulatory response and to exact physiological costs at concentrations comparable with the one we used [16,30]. Nonetheless, we observed no direct reproductive or survival costs to LPS-injected females associated with the immune challenge.
Our LPS treatment did, however, influence subsequent reproductive performance consistent with the terminal investment hypothesis, as evidenced by increased phenotypic quality of offspring produced by LPS-injected females relative to vehicle-injected females. Sons of experimental females were heavier than offspring of control females, and daughters of experimental females mounted stronger cutaneous immune responses. In most species studied to date, nestling mass prior to leaving the nest is positively correlated with survival and recruitment as a breeder [35–37], and with subsequent reproductive performance [38]. In our study population, the effect of mass on future fecundity is also sex-specific; heavier sons out-reproduce lighter sons as adults, whereas the less-variable fecundity of daughters is less strongly related to their mass and environment as nestlings (E. K. Bowers 2009–2011, unpublished data). Cutaneous immune response, as assessed by the PHA assay, is also a positive predictor of nestling survival in other species ([39–41], but see Butler et al. [42]). Thus, the increased body mass and condition index of sons and increased PHA response of daughters produced by LPS-injected females should afford them a substantial fitness advantage in that they are more likely to survive and reproduce than offspring of control females.
Our results suggest that investment in sons is optimized via size, and in daughters via immune function. For example, male house wrens experience intense intrasexual competition for territories and sexually receptive females, which are choosy of their potential mates [28,43–46], and we recently showed that, although male and female nestlings do not differ in average body mass or size, those fitness-related traits are more variable for sons than daughters with respect to natal environmental conditions [26]. Thus, when hatching among earlier-laid eggs of the clutch provides individuals with a sibling-competitive advantage, maternal females bias sons towards earlier-laid eggs of the clutch and daughters among later-laid eggs [26]. Indeed, if reproductive success is more variable for males than for females, and more strongly dependent upon body condition, large sons should have higher reproductive value than daughters of similar size [47,48]. However, Leimar [49] has shown that, when maternal quality is passed to offspring, high-quality daughters may have higher fitness than high-quality sons if the quality of the sons' mates is variable or uncertain. In other words, high-quality sons may have higher mating success than their sisters, but if they mate indiscriminately and pair with medium- or poor-quality females, then the condition of their offspring may not be as good as those produced by their high-quality sisters [49]. Indeed, individual quality can manifest itself in different forms, which likely differ for each sex. Thus, we suggest that the quality of sons is determined largely by their size, whereas the quality of daughters is determined by their immune responsiveness, particularly given the fact that transgenerational priming of the immune system occurs between females and their offspring ([31,50] and this study). Similarly, a recent study of zebra finches (Taeniopygia guttata) reported that mothers injected with sheep red blood cells (SRBCs) preferentially allocated SRBC-specific antibodies to their daughters [51]. Thus, producing large, heavy sons and daughters with enhanced immune responsiveness appears to be an optimal sex-allocation strategy, particularly for immune-challenged mothers in pathogen-rich environments.
Apart from the sex-specific treatment effect on the cutaneous immune response of offspring, sons and daughters diverged in bacterial killing ability, cutaneous immune response and LPS antibody levels as the breeding season progressed, indicating that the sexes allocate resources to immunity differently during development. Similarly, Love et al. [52] documented that male zebra finch nestlings have greater cutaneous immune responses than female nestlings, a species in which the sexes also do not differ in size or plumage as nestlings; surprisingly, the sexes converged in this trait as adults. Although earlier hypotheses attributed sex differences in immunity to differences in size or plumage characteristics [52], that house wrens and zebra finches are sexually monomorphic in size and plumage as nestlings, but differ in immune function, suggests that selection on immunity differs for males and females.
Although some studies have not detected differences in measures of male and female immunity [53,54], phenotypic divergence between the sexes during ontogeny is expected when sons and daughters face differing selection pressures [55]. For example, Badyaev et al. [56] recently documented that ectoparasitic nest mites can reduce survivorship of sons to a greater extent than that of daughters in house finches (Carpodacus mexicanus), and although the cause of increased sensitivity of males to parasites is unclear, it may be a general pattern [57–59]. Thus, increased sensitivity of either sex towards parasites or disease may select for increased immunocompetence during ontogeny, particularly when offspring remain in the nest for an extended period of time [52]. A question that arises, then, is why sons and daughters do not always differ in immune function under normal conditions, as was the case for control broods in this study (figure 1c)? Recent experimental studies from our population demonstrate that, although sons in benign or beneficial rearing conditions grow faster and obtain greater mass and size than daughters in similar conditions, those sons have weaker measures of immune function (comparable with the data in figure 1). On the other hand, sons in poor- or adverse-rearing conditions grow slowly and are lighter and smaller than daughters in similar conditions, yet they develop strong immune function. Indeed, morphological and immunological measures for female nestlings are generally less variable than for males and less subject to environmental variation (E. K. Bowers 2009–2011, unpublished data). Thus, we suggest that a sex-specific trade-off between growth and immunity may explain, at least in part, why sons and daughters may not noticeably differ in average measures of immunity. This sexually dimorphic variability in immune function may help to explain greater developmental sensitivity of sons than daughters [26,57–59], and is also consistent with the hypothesis that the quality of sons, particularly in polygynous mating systems, is mediated through size and daughters through immune function. Further studies of sex-biased allocation to offspring and sex-specific variability in the immune system across taxa and contexts may provide insight into the evolution of sexual dimorphism in this trait.
Acknowledgements
We thank the 2010 and 2011 Wren Crews for field assistance; L. A. Vogel for helpful discussion; the ParkLands Foundation (Merwin Nature Preserve); the Sears and Butler families for the use of their properties for this study, and three anonymous referees for constructive comments on the manuscript. Financial support was provided by NSF grant nos. IBN-0316580 and IOS-0718140; the School of Biological Sciences, Illinois State University; the Sigma Xi Society; the Audubon Society of Champaign County, IL; and the Beta Lambda Chapter of the Phi Sigma Biological Honor Society. This work was performed in accordance with (i) Illinois State University IACUC number 05-2010 and (ii) USF&WS banding permit 09211.
References
- 1.Stearns S. C. 1992. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Roff D. A. 2002. Life history evolution. Sunderland, MA: Sinauer Associates Inc [Google Scholar]
- 3.Williams G. C. 1966. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 100, 687–690 10.1086/282461 (doi:10.1086/282461) [DOI] [Google Scholar]
- 4.Trivers R. L. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man, 1871–1971 (ed. Campbell B.), pp. 136–179 Chicago, IL: Aldine [Google Scholar]
- 5.Bowers E. K., Sakaluk S. K., Thompson C. F. 2012. Experimentally increased egg production constrains future reproduction of female house wrens. Anim. Behav. 83, 495–500 10.1016/j.anbehav.2011.11.026 (doi:10.1016/j.anbehav.2011.11.026) [DOI] [Google Scholar]
- 6.Zera A. J., Harshman L. G. 2001. The physiology of life-history trade-offs in animals. Ann. Rev. Ecol. Syst. 32, 95–126 10.1146/annurev.ecolsys.32.081501.114006 (doi:10.1146/annurev.ecolsys.32.081501.114006) [DOI] [Google Scholar]
- 7.Sheldon B. C., Verhulst S. 1996. Ecological immunology: costly parasite defenses and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321 10.1016/0169-5347(96)10039-2 (doi:10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 8.Schmid-Hempel P. 2003. Variation in immune defense as a question of evolutionary ecology. Proc. R. Soc. Lond. B 270, 357–366 10.1098/rspb.2002.2265 (doi:10.1098/rspb.2002.2265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uller T., Isaksson C., Olsson M. 2006. Immune challenge reduces reproductive output and growth in a lizard. Funct. Ecol. 20, 873–879 10.1111/j.1365-2435.2006.01163.x (doi:10.1111/j.1365-2435.2006.01163.x) [DOI] [Google Scholar]
- 10.Leman J. C., Weddle C. B., Gershman S. N., Kerr A. M., Ower G. D., St. John J. M., Vogel L. A., Sakaluk S. K. 2009. Lovesick: immunological costs of mating to male sagebrush crickets. J. Evol. Biol. 22, 163–171 10.1111/j.1420-9101.2008.01636.x (doi:10.1111/j.1420-9101.2008.01636.x) [DOI] [PubMed] [Google Scholar]
- 11.Lochmiller R. L., Deerenberg C. 2000. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88, 87–98 10.1034/j.1600-0706.2000.880110.x (doi:10.1034/j.1600-0706.2000.880110.x) [DOI] [Google Scholar]
- 12.Martin L. B., Weil Z. M., Nelson R. J. 2008. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Phil. Trans. R. Soc. B 363, 321–339 10.1098/rstb.2007.2142 (doi:10.1098/rstb.2007.2142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowles S. C. L., Nakagawa S., Sheldon B. C. 2009. Elevated reproductive effort increases blood parasitaemia and decreases immune function in birds: a meta-regression approach. Funct. Ecol. 23, 405–415 10.1111/j.1365-2435.2008.01507.x (doi:10.1111/j.1365-2435.2008.01507.x) [DOI] [Google Scholar]
- 14.Williams T. D., Christians J. K., Aiken J. J., Evanson M. 1999. Enhanced immune function does not suppress reproductive output. Proc. R. Soc. Lond. B 266, 753–757 10.1098/rspb.1999.0701 (doi:10.1098/rspb.1999.0701) [DOI] [Google Scholar]
- 15.Lozano G. A., Ydenberg R. C. 2002. Transgenerational effects of maternal immune challenge in tree swallows (Tachycineta bicolor). Can. J. Zool. 80, 918–925 10.1139/z02-063 (doi:10.1139/z02-063) [DOI] [Google Scholar]
- 16.Lee K. A., Martin L. B., II, Wikelski M. C. 2005. Responding to inflammatory challenges is less costly for a successful avian invader, the house sparrow (Passer domesticus), than its less-invasive congener. Oecologia 145, 244–251 10.1007/s00442-005-0113-5 (doi:10.1007/s00442-005-0113-5) [DOI] [PubMed] [Google Scholar]
- 17.Verhulst S., Riedstra B., Wiersma P. 2005. Brood size and immunity costs in zebra finches Taeniopygia guttata. J. Avian Biol. 36, 22–30 10.1111/j.0908-8857.2005.03342.x (doi:10.1111/j.0908-8857.2005.03342.x) [DOI] [Google Scholar]
- 18.Bonneaud C., Mazuc J., Chastel O., Westerdahl H., Sorci G. 2004. Terminal investment induced by immune challenge and fitness traits associated with major histocompatibility complex in the house sparrow. Evolution 58, 2823–2830 10.1111/j.0014-3820.2004.tb01633.x (doi:10.1111/j.0014-3820.2004.tb01633.x) [DOI] [PubMed] [Google Scholar]
- 19.Velando A., Drummond H., Torres R. 2006. Senescent birds redouble reproductive effort when ill: confirmation of the terminal investment hypothesis. Proc. R. Soc. B 273, 1443–1448 10.1098/rspb.2006.3480 (doi:10.1098/rspb.2006.3480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weil Z. M., Martin L. B., Workman J. L., Nelson R. J. 2006. Immune challenge retards seasonal reproductive regression in rodents: evidence for terminal investment. Biol. Lett. 2, 393–396 10.1098/rsbl.2006.0475 (doi:10.1098/rsbl.2006.0475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanssen S. A. 2006. Cost of an immune challenge and terminal investment in a long-lived bird. Ecology 87, 2440–2446 10.1890/0012-9658(2006)87[2440:COAICA]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[2440:COAICA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 22.Williams G. C. 1966. Adaptation and natural selection: a critique of some current evolutionary thought. Princeton, NJ: Princeton University Press [Google Scholar]
- 23.Clutton-Brock T. H. 1984. Reproductive effort and terminal investment in iteroparous animals. Am. Nat. 123, 212–229 10.1086/284198 (doi:10.1086/284198) [DOI] [Google Scholar]
- 24.Trivers R. L., Willard D. E. 1973. Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92 10.1126/science.179.4068.90 (doi:10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- 25.West S. A. 2009. Sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- 26.Bowers E. K., Sakaluk S. K., Thompson C. F. 2011. Adaptive sex allocation in relation to hatching synchrony and offspring quality in house wrens. Am. Nat. 177, 617–629 10.1086/659630 (doi:10.1086/659630) [DOI] [PubMed] [Google Scholar]
- 27.Johnson L. S. 1998. House wren (Troglodytes aedon). In The birds of North America, no. 380 (eds Poole A., Gill F.), pp. 1–32 Washington, DC: The American Ornithologists’ Union [Google Scholar]
- 28.DeMory M. L., Thompson C. F., Sakaluk S. K. 2010. Male quality influences male provisioning in house wrens independent of attractiveness. Behav. Ecol. 21, 1156–1164 10.1093/beheco/arq123 (doi:10.1093/beheco/arq123) [DOI] [Google Scholar]
- 29.Lambrechts M. M., et al. 2010. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol. 45, 1–26 10.3161/000164510X516047 (doi:10.3161/000164510X516047) [DOI] [Google Scholar]
- 30.Bonneaud C., Mazuc J., Gonzalez G., Haussy C., Chastel O., Faivre B., Sorci G. 2003. Assessing the cost of mounting an immune response. Am. Nat. 161, 367–379 10.1086/346134 (doi:10.1086/346134) [DOI] [PubMed] [Google Scholar]
- 31.Grindstaff J. L., Hasselquist D., Nilsson J-Å., Sandell M., Smith H. G., Stjernman M. 2006. Transgenerational priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc. R. Soc. B 273, 2551–2557 10.1098/rspb.2006.3608 (doi:10.1098/rspb.2006.3608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forsman A. M., Vogel L. A., Sakaluk S. K., Grindstaff J. L., Thompson C. F. 2008. Immune-challenged house wren broods differ in the relative strengths of their responses among different axes of the immune system. J. Evol. Biol. 21, 873–878 10.1111/j.1420-9101.2008.01503.x (doi:10.1111/j.1420-9101.2008.01503.x) [DOI] [PubMed] [Google Scholar]
- 33.Sokal R. R., Rohlf F. J. 1995. Biometry, 3rd edn. New York, NY: W. H. Freeman and Company [Google Scholar]
- 34.Grafen A., Hails R. 2002. Modern statistics for the life sciences. Oxford, UK: Oxford University Press [Google Scholar]
- 35.Alatalo R. V., Lundberg A. 1986. Heritability and selection on tarsus length in the pied flycatcher (Ficedula hypoleuca). Evolution 40, 574–583 10.2307/2408578 (doi:10.2307/2408578) [DOI] [PubMed] [Google Scholar]
- 36.Young B. E. 1996. An experimental analysis of small clutch size in tropical house wrens. Ecology 77, 472–488 10.2307/2265623 (doi:10.2307/2265623) [DOI] [Google Scholar]
- 37.Both C., Visser M. E., Verboven N. 1999. Density-dependent recruitment rates in great tits: the importance of being heavier. Proc. R. Soc. Lond. B 266, 465–469 10.1098/rspb.1999.0660 (doi:10.1098/rspb.1999.0660) [DOI] [Google Scholar]
- 38.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348 10.1016/S0169-5347(99)01639-0 (doi:10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 39.Cichoń M., Dubiec A. 2005. Cell-mediated immunity predicts the probability of local recruitment in nestling blue tits. J. Evol. Biol. 18, 962–966 10.1111/j.1420-9101.2005.00910.x (doi:10.1111/j.1420-9101.2005.00910.x) [DOI] [PubMed] [Google Scholar]
- 40.Moreno J., Merino S., Sanz J. J., Arriero E., Morales J., Tomás G. 2005. Nestling cell-mediated immune response, body mass and hatching date as predictors of local recruitment in the pied flycatcher Ficedula hypoleuca. J. Avian Biol. 36, 251–260 10.1111/j.0908-8857.2005.03413.x (doi:10.1111/j.0908-8857.2005.03413.x) [DOI] [Google Scholar]
- 41.López-Rull I., Celis P., Salaberria C., Puerta M., Gil D. 2011. Post-fledging recruitment in relation to nestling plasma testosterone and immunocompetence in the spotless starling. Funct. Ecol. 25, 500–508 10.1111/j.1365-2435.2010.01783.x (doi:10.1111/j.1365-2435.2010.01783.x) [DOI] [Google Scholar]
- 42.Butler M. W., Garvin J. C., Wheelwright N. T., Freeman-Gallant C. R. 2009. Ambient temperature, but not paternity, is associated with immune response in savannah sparrows (Passerculus sandwichensis). Auk 126, 536–542 10.1525/auk.2009.08179 (doi:10.1525/auk.2009.08179) [DOI] [Google Scholar]
- 43.Kendeigh S. C. 1941. Territorial and mating behavior of the house wren. Ill. Biol. Monogr. 18, 1–120 10.5962/bhl.title.50316 (doi:10.5962/bhl.title.50316) [DOI] [Google Scholar]
- 44.Belles-Isles J.-C., Picman J. 1987. Suspected adult intraspecific killing by house wrens. Wilson Bull. 99, 497–498 [Google Scholar]
- 45.Johnson L. S., Kermott L. H. 1990. Possible causes of territory takeovers in a north-temperate population of house wrens. Auk 107, 781–784 [Google Scholar]
- 46.Eckerle K. P., Thompson C. F. 2006. Mate choice in house wrens: nest cavities trump male characteristics. Behaviour 143, 253–271 10.1163/156853906775900694 (doi:10.1163/156853906775900694) [DOI] [Google Scholar]
- 47.Sheldon B. C. 1998. Recent studies of avian sex ratios. Heredity 80, 397–402 10.1046/j.1365-2540.1998.00374.x (doi:10.1046/j.1365-2540.1998.00374.x) [DOI] [Google Scholar]
- 48.Krist M. 2006. Should mothers in poor condition invest more in daughter than in son? Ethol. Ecol. Evol. 18, 241–246 10.1080/08927014.2006.9522711 (doi:10.1080/08927014.2006.9522711) [DOI] [Google Scholar]
- 49.Leimar O. 1996. Life-history analysis of the Trivers and Willard sex-ratio problem. Behav. Ecol. 7, 316–325 [Google Scholar]
- 50.Grindstaff J. L., Brodie E. D., III, Ketterson E. D. 2003. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. R. Soc. Lond. B 270, 2309–2319 10.1098/rspb.2003.2485 (doi:10.1098/rspb.2003.2485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martyka R., Rutkowska J., Cichoń M. 2011. Sex-specific effects of maternal immunization on yolk antibody transfer and offspring performance in zebra finches. Biol. Lett. 7, 50–53 10.1098/rsbl.2010.0549 (doi:10.1098/rsbl.2010.0549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Love O. P., Salvante K. G., Dale J., Williams T. D. 2008. Sex-specific variability in the immune system across life-history stages. Am. Nat. 172, E99–E112 10.1086/589521 (doi:10.1086/589521) [DOI] [PubMed] [Google Scholar]
- 53.Parejo D., Silva N., Avilés J. M. 2007. Within-brood size differences affect innate and acquired immunity in roller Coracias garrulus nestlings. J. Avian Biol. 38, 717–725 10.1111/j.2007.0908-8857.04081.x (doi:10.1111/j.2007.0908-8857.04081.x) [DOI] [Google Scholar]
- 54.Saino N., de Ayala R. M., Martinelli R., Boncoraglio G. 2008. Male-biased brood sex ratio depresses average phenotypic quality of barn swallow nestlings under experimentally harsh conditions. Oecologia 156, 441–453 10.1007/s00442-008-0971-8 (doi:10.1007/s00442-008-0971-8) [DOI] [PubMed] [Google Scholar]
- 55.Badyaev A. V. 2002. Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol. Evol. 17, 369–378 10.1016/S0169-5347(02)02569-7 (doi:10.1016/S0169-5347(02)02569-7) [DOI] [Google Scholar]
- 56.Badyaev A. V., Hamstra T. L., Oh K. P., Acevedo Seaman D. A. 2006. Sex-biased maternal effects reduce ectoparasite-induced mortality in a passerine bird. Proc. Natl Acad. Sci. USA 103, 14 406–14 411 10.1073/pnas.0602452103 (doi:10.1073/pnas.0602452103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potti J., Merino S. 1996. Parasites and the ontogeny of sexual size dimorphism in a passerine bird. Proc. R. Soc. Lond. B 263, 9–12 10.1098/rspb.1996.0002 (doi:10.1098/rspb.1996.0002) [DOI] [Google Scholar]
- 58.Tschirren B., Fitze P. S., Richner H. 2003. Sexual dimorphism in susceptibility to parasites and cell-mediated immunity in great tit nestlings. J. Anim. Ecol. 72, 839–845 10.1046/j.1365-2656.2003.00755.x (doi:10.1046/j.1365-2656.2003.00755.x) [DOI] [Google Scholar]
- 59.Dubiec A., Cichoń M., Deptuch K. 2006. Sex-specific development of cell-mediated immunity under experimentally harsh conditions. Proc. R. Soc. B 273, 1759–1764 10.1098/rspb.2006.3510 (doi:10.1098/rspb.2006.3510) [DOI] [PMC free article] [PubMed] [Google Scholar]