Abstract
Although pancreatic ribonucleases are extensively studied proteins, little information is available on nucleic acids coding for these enzymes. Here, for the first time, the structure of a gene coding for such an enzyme, the well known bovine pancreatic ribonuclease, is reported. The coding region of this gene is devoid of introns, whereas the 5' untranslated sequence of the pancreatic transcript contains an intron of 735 nucleotides. This intervening sequence is endowed with signals (CAAT and TATA boxes) which might act as regulatory elements. The structural organization of this gene suggests that the sequence coding for the bovine pancreatic ribonuclease might be expressed under the control of two different promoters.
Full text
PDF







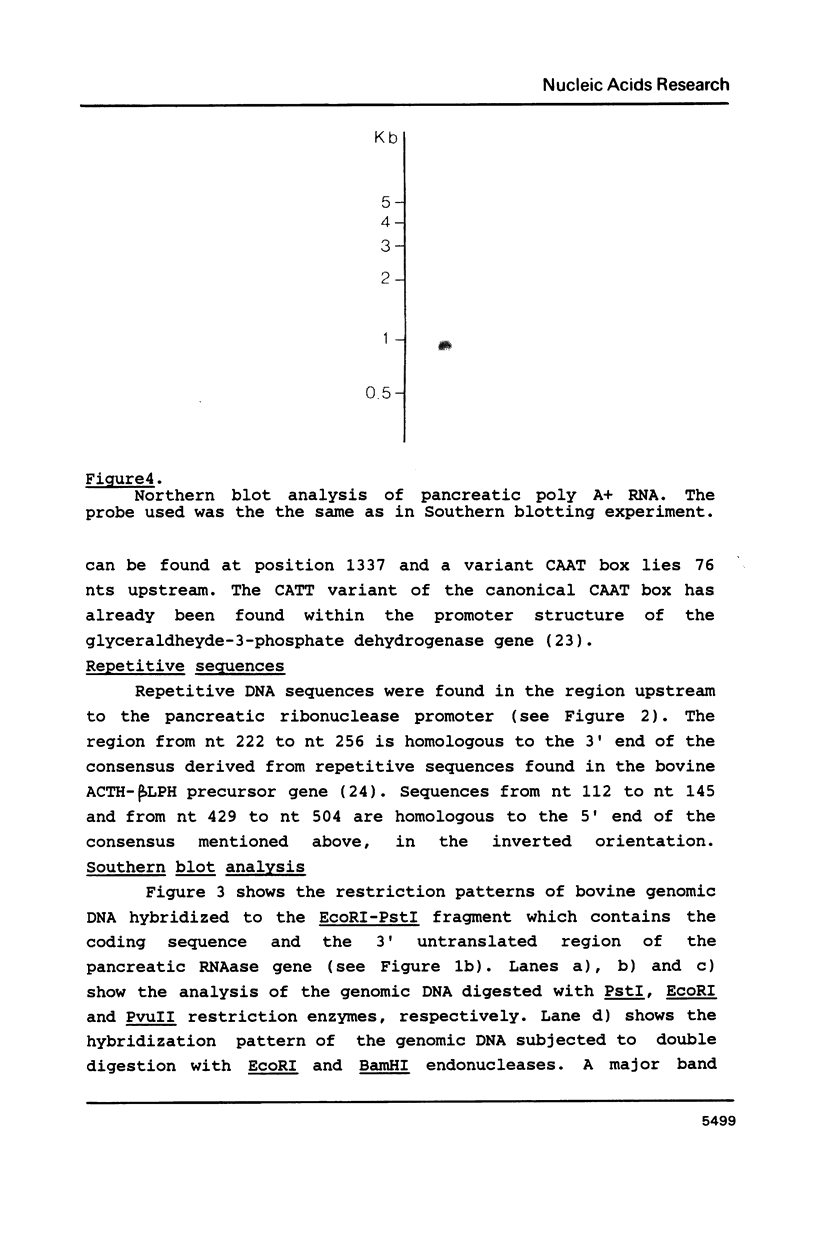



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Bingham E. W., Kalan E. B. Ribonuclease B of bovine milk. Arch Biochem Biophys. 1967 Aug;121(2):317–324. doi: 10.1016/0003-9861(67)90082-3. [DOI] [PubMed] [Google Scholar]
- Boulet A. M., Erwin C. R., Rutter W. J. Cell-specific enhancers in the rat exocrine pancreas. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3599–3603. doi: 10.1073/pnas.83.11.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Carsana A., Furia A., Calabria R., Palmieri M. In vitro synthesis of pig pancreas ribonuclease. Biochim Biophys Acta. 1985 Jul 24;825(3):299–302. doi: 10.1016/0167-4781(85)90017-x. [DOI] [PubMed] [Google Scholar]
- D'Alessio G., Di Donato A., Furia A., Leone E., Libonati M., Parente A., Suzuki H. Bull semen RNAase revisited. J Mol Biol. 1981 Feb 25;146(2):269–274. doi: 10.1016/0022-2836(81)90437-x. [DOI] [PubMed] [Google Scholar]
- D'Alessio G., Malorni M. C., Parente A. Dissociation of bovine seminal ribonuclease into catalytically active monomers by selective reduction and alkylation of the intersubunit disulfide bridges. Biochemistry. 1975 Mar 25;14(6):1116–1122. doi: 10.1021/bi00677a004. [DOI] [PubMed] [Google Scholar]
- D'Alessio G., Parente A., Guida C., Leone E. Dimeric structure of seminal ribonuclease. FEBS Lett. 1972 Nov 1;27(2):285–288. doi: 10.1016/0014-5793(72)80642-2. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Elson M., Glitz D. G. Characterization of a ribonuclease from bovine brain. Biochemistry. 1975 Apr 8;14(7):1471–1476. doi: 10.1021/bi00678a019. [DOI] [PubMed] [Google Scholar]
- Furia A., Palmieri M., Libonati M. Bovine seminal ribonuclease precursor synthesized in vitro. Biochim Biophys Acta. 1983 Dec 22;741(3):303–307. doi: 10.1016/0167-4781(83)90149-5. [DOI] [PubMed] [Google Scholar]
- Kumagai H., Yoshihara K., Umemoto M., Igarashi K., Hirose S., Ohgi K., Irie M. Studies on salivary gland ribonucleases. III. Purification and properties of three ribonucleases from bovine parotid gland. J Biochem. 1983 Mar;93(3):865–874. doi: 10.1093/jb/93.3.865. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Davie E. W., Strydom D. J., Riordan J. F., Vallee B. L. Sequence of the cDNA and gene for angiogenin, a human angiogenesis factor. Biochemistry. 1985 Sep 24;24(20):5494–5499. doi: 10.1021/bi00341a032. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Stary S. J., Swift G. H. Rat pancreatic ribonuclease messenger RNA. The nucleotide sequence of the entire mRNA and the derived amino acid sequence of the pre-enzyme. J Biol Chem. 1982 Dec 25;257(24):14582–14585. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Niwata Y., Ohgi K., Sanda A., Takizawa Y., Irie M. Purification and properties of bovine kidney ribonucleases. J Biochem. 1985 Mar;97(3):923–934. doi: 10.1093/oxfordjournals.jbchem.a135134. [DOI] [PubMed] [Google Scholar]
- Palmieri M., Carsana A., Furia A., Libonati M. Sequence analysis of a cloned cDNA coding for bovine seminal ribonuclease. Eur J Biochem. 1985 Oct 15;152(2):275–277. doi: 10.1111/j.1432-1033.1985.tb09194.x. [DOI] [PubMed] [Google Scholar]
- Robert B., Daubas P., Akimenko M. A., Cohen A., Garner I., Guenet J. L., Buckingham M. A single locus in the mouse encodes both myosin light chains 1 and 3, a second locus corresponds to a related pseudogene. Cell. 1984 Nov;39(1):129–140. doi: 10.1016/0092-8674(84)90198-3. [DOI] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Soares M. B., Turken A., Ishii D., Mills L., Episkopou V., Cotter S., Zeitlin S., Efstratiadis A. Rat insulin-like growth factor II gene. A single gene with two promoters expressing a multitranscript family. J Mol Biol. 1986 Dec 20;192(4):737–752. doi: 10.1016/0022-2836(86)90025-2. [DOI] [PubMed] [Google Scholar]
- Stone E. M., Rothblum K. N., Alevy M. C., Kuo T. M., Schwartz R. J. Complete sequence of the chicken glyceraldehyde-3-phosphate dehydrogenase gene. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1628–1632. doi: 10.1073/pnas.82.6.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler E. E., Periasamy M., Strehler-Page M. A., Nadal-Ginard B. Myosin light-chain 1 and 3 gene has two structurally distinct and differentially regulated promoters evolving at different rates. Mol Cell Biol. 1985 Nov;5(11):3168–3182. doi: 10.1128/mcb.5.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Parente A., Farina B., Greco L., La Montagna R., Leone E. Complete amino-acid sequence of bovine seminal ribonuclease, a dimeric protein from seminal plasma. Biol Chem Hoppe Seyler. 1987 Oct;368(10):1305–1312. doi: 10.1515/bchm3.1987.368.2.1305. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Von Tigerstrom R. G., Manchak J. M. Isolation and characterization of two alkaline ribonucleases from calf serum. Biochim Biophys Acta. 1976 Jan 19;418(2):184–194. doi: 10.1016/0005-2787(76)90068-x. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Tsukada T., Notake M., Nakanishi S., Numa S. Structural analysis of repetitive DNA sequences in the bovine corticotropin-beta-lipotropin precursor gene region. Nucleic Acids Res. 1982 Mar 11;10(5):1459–1469. doi: 10.1093/nar/10.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pagter-Holthuizen P., Jansen M., van Schaik F. M., van der Kammen R., Oosterwijk C., Van den Brande J. L., Sussenbach J. S. The human insulin-like growth factor II gene contains two development-specific promoters. FEBS Lett. 1987 Apr 20;214(2):259–264. doi: 10.1016/0014-5793(87)80066-2. [DOI] [PubMed] [Google Scholar]





