Abstract
Epidemiological evidence suggests protective effect for higher consumption of vegetables against cardiovascular disease risk. Impaired bioavailability of nitric oxide (NO), a critical regulator of vascular homeostasis, in the vasculature represents a major problem for CVD. Classically, vascular endothelium is suggested to be a sole source of bioactive NO in the vasculature. However, emerging literature associates nitrate-nitrite-NO pathway, in which endogenous nitrate undergoes reduction to nitrite and then to NO in various tissues including blood, with bioactive NO production in the body. Indeed, NO generated from nitrate-nitrite-NO pathway has recently been implicated in the maintenance of NO homeostasis in the body. Endogenous nitrate originates mostly from NO oxidation in biological milieu and from dietary nitrate exposure. Consumption of vegetables accounts for ~ 80–85 % of daily nitrate exposure in humans, therefore establishing inorganic nitrate as a promising factor in cardiovascular health benefits of vegetables. However, at this point of time, the benefit/hazard ratio of inorganic nitrate and its active metabolite nitrite remains less clear and must be studied in prospective controlled studies. This brief review discusses the potential role of inorganic dietary nitrate in cardiovascular health benefits of vegetables.
Keywords: Cardiovascular, diet, nitrate, nitric oxide, vegetables
Introduction
Epidemiological evidence suggests that increased consumption of vegetables reduce risk of cardiovascular disease (CVD), the number one killer in the Western world (1–5). Although these benefits were traditionally postulated to the antioxidant factors in vegetables, studies over the past two decades have proposed many non-antioxidant factors also as likely candidates. Of late, one such factor that has garnered much scientific and medical interest is inorganic nitrate. Vegetables are rich source of inorganic nitrate and account for ~ 80–85 % of daily dietary nitrate exposure in the average population (6–9). Several recent experimental and clinical studies show that dietary nitrate supplementation at doses that are commonly found in vegetable-rich diets exert beneficial effects on the cardiovascular system (10–16). These beneficial effects of nitrate are largely thought to be mediated by its reduction to nitric oxide (NO), a critical regulator of vascular homeostasis, in the body (10–16). Current data suggests that nitrate undergoes reduction to nitrite and then to NO through a nitrate-nitrite-NO pathway, which recently received appreciation as an alternative pathway to classical L-arginine-nitric oxide synthase (NOS) pathway for NO production in the body (17–21). In this short review, we discuss a potential role for inorganic nitrate in cardiovascular health benefits of vegetables. Nutritional epidemiologists often join vegetables with fruits when discussing their cardiovascular health benefits, because these foods in general share similar nutrients, phytochemicals and functional aspects. However, in the context of the present review, it is unwise to join these food together since the inorganic nitrate content of fruits and accordingly their contribution to endogenous nitrate levels is several folds lesser (or negligible) compared with vegetables (6, 7).
NO synthesis from classical L-arginine-NOS pathway
NO synthesis from the classical L-arginine-nitric oxide (NOS) pathway involves L-arginine oxidation by three different isoforms of NOS enzyme [i.e., endothelial (eNOS), neuronal (nNOS) and inducible (iNOS)] (22–24). The three NOS isoforms exhibit different characteristics and produce NO at different rates. The eNOS is associated with plasma membrane whereas nNOS and iNOS are found predominantly in the cytosol (22–24). In addition, eNOS and nNOS are constitutively expressed as latent enzymes in healthy cells and require a higher calcium concentration for their enzymatic activity, whereas iNOS is mainly expressed in the presence of infection or inflammation and its activity is calcium-independent (22–24). Although all three NOS isoforms play distinct roles, eNOS is thought to be mainly involved in the regulation of the cardiovascular system because it is the major source of basal and stimulated NO synthesis in the vasculature (22–24). The eNOS has a bidomain (oxygenase and reductase) structure and functions as a dimer (22–24). The catalysis of L-arginine oxidation by eNOS requires availability of molecular oxygen and several cofactors [nicotinamide adenine dinucleotide phosphate (NADPH), flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), tetrahydrobiopterin (BH4), and calcium / calmodulin]. The catalytic mechanisms of eNOS involve stepwise transfer of electrons from NADPH to FAD to FMN to heme-containing oxygenase domain where oxygen is reduced and incorporated into the guanidine group of L-arginine, leading to NO production. The eNOS can be stimulated by a variety of mechanical forces (eg: shear stress, hypoxia), humoral factors ranging from growth factors to peptide hormones (eg: acetylcholine, vascular endothelial growth factor, bradykinin, estrogen) and calcium-mobilizing agents (eg: angiotensin-II, epinephrine) (22–24). For example, acetylcholine leads to an increase in intracellular calcium in endothelial cells, activation of calmodulin, alignment of the oxygenase and reductase domains of the eNOS, and ultimately to efficient NO synthesis (25).
NO synthesis from nitrate-nitrite-NO pathway
NO generation from endogenous nitrate was recently discovered as an alternative pathway to classical L-arginine-eNOS pathway for bioactive NO production in the body (Figure 1) (17–21). Interestingly, endogenous nitrite and nitrate originates predominantly from the oxidation of endogenously produced NO in the biological fluids (19). In addition, significant portion of body nitrate also originates from diet, in particular from consumption of plant foods (19). Current data suggests that nitrate reduction to NO in the body involves its initial reduction to nitrite and then to NO (Figure 1) (17–21). Nitrate reduction to nitrite has been largely thought to be carried out by commensal bacteria present on the dorsal surface of the tongue and possibly in the gastrointestinal tract (17–21). The bacteria uses nitrate as an alternative electron acceptor to produce energy, thereby effectively reduces it to nitrite. Indeed, use of antibacterial mouth wash (11) or spitting of saliva (12) after dietary nitrate load has been reported to attenuate expected rise in systemic nitrite levels. In addition, more recently, allopurinol-sensitive tissue nitrate reductase enzymes that reduce nitrate to nitrite have been suggested to contribute to nitrate reduction to nitrite in the body, but the magnitude of this pathway is not clear (13). Once formed, nitrite can be further reduced to NO by a variety of sources, including but not limited to deoxyhemoglobin and xanthine oxidoreductase, in the body (17–21). It is important to note that at this point of time the relative contribution of these pathways to NO production from the nitrate-nitrite-NO pathway and to vascular NO levels remains unknown. Interestingly, unlike NO production from the eNOS whose activity is oxygen-dependent, NO production from the nitrate-nitrite-NO pathway has been suggested to be increased with diminished oxygen (17–21). In this context, NO generation from the nitrate-nitrite-NO pathway should be viewed as an alternative source to NOS-dependent NO production in the body (17–21). The nitrate and nitrate reduction mechanisms are the subject of detailed discussions in earlier reviews (17–21, 26) and are not discussed further here.
Figure 1.
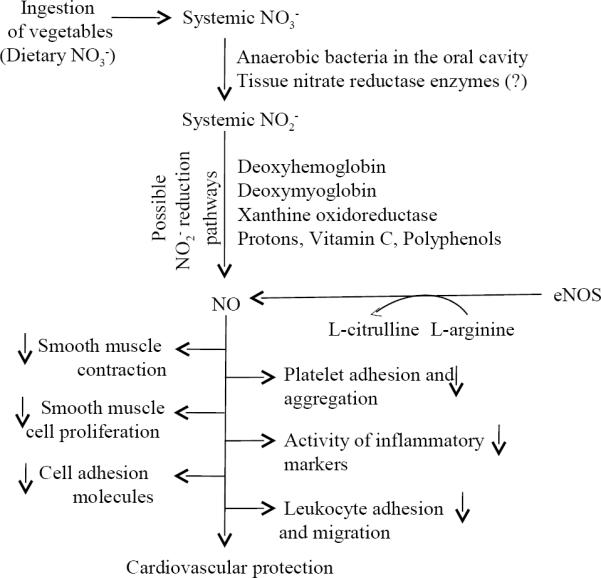
Schematic presentation of inorganic nitrate (NO3−) mediation of cardiovascular protection by vegetable-rich diets. After ingestion of vegetables, NO3− gets absorbed into circulation, undergoes reduction to nitrite (NO2−) and then to nitric oxide (NO) leading to possible cardiovascular protection. NO3− and NO2− reduction pathways and classical L-arginineendothelial nitric oxide synthase (eNOS) pathway for NO production in the body are depicted.
NO, vascular homeostasis and cardiovascular disease
NO influences vascular homeostasis in many ways (27–30). For example, NO enters vascular smooth muscle cells where it increases cyclic guanosine 3',5'-monophosphate (cGMP) production by activating soluble guanylyl cyclase enzyme, leading to smooth muscle relaxation (vasodilation). In addition, NO inhibits platelet aggregation and platelet adhesion to the endothelium. Moreover, NO suppresses vascular smooth muscle cell proliferation, adhesion and migration of leukocytes / monocytes into the arterial wall, activity of inflammatory factors, and expression of certain adhesion molecules. Therefore, NO represents the most important regulator of vascular homeostasis and loss of its function has been implicated in a variety of cardiovascular diseases including hypertension and atherosclerosis (31–33). Diminished synthesis and/or increased depletion as from increased levels of superoxide anions have been predominantly implicated in reduction in NO bioavailability in the vasculature (31–33). Superoxide anions react with NO in a near diffusion controlled reaction to form peroxynitrite leading to depletion of NO in the vasculature (31–33).
Cardiovascular protection by dietary nitrate
Nitrate has been used to treat cardiovascular disease conditions (angina and digital ischemia) since medieval times as evidenced by a translation of medieval Buddhist manuscripts 19. However, it is only in the past several years that knowledge regarding the potential cardiovascular beneficial effects of nitrate, in particular that present in the vegetables, has advanced enormously. Current data suggests that nitrate in our diet influences cardiovascular system through increasing vascular NO bioavailability via the nitrate-nitrite-NO pathway (17–21) (Figure 1). In this context, studies over the past two decades demonstrated that antioxidants and other interventions that can improve NO bioavailability in the vasculature, presumably through their interaction with the vascular endothelium, can lead to improved clinical outcome in cardiovascular disease patients (32, 33). Dietary nitrate deficiency (10), dietary nitrate load (12) and/or supplementation with inorganic nitrate salts (eg: sodium nitrate, potassium nitrate) (14–16) have been used to investigate cardiovascular effects of dietary nitrate in animals and humans.
Animal studies
Bryan and colleagues demonstrated that mice fed with low nitrite and nitrate diet (i.e., dietary nitrite and nitrate deficiency) displayed exacerbated myocardial injury (up by 59%) and post-myocardial mortality rates (up by 13%) compared to a control group of mice which received standard diet (10). This study also demonstrated that nitrate supplementation for 7 days (1 g/L in drinking water) afforded significant protection against myocardial ischemic-injury in mice fed with standard diet as well as low nitrite and nitrate diet (10). In another study, Jansson et al., demonstrated that intravenous administration of nitrate (sodium nitrate, 10 mg per kg) reduced mean arterial blood pressure (~ 10 % vs placebo group which received sodium chloride) in control rats as well as in Nω-nitro-L-arginine methylester (L-NAME, a non-specific NOS inhibitor that inhibits endogenous NO production) pre-treated rats13. In this study, nitrate administration also improved, albeit by a non-significant level, the aortic blood flow in rats (13). More recently, Lundberg and colleagues demonstrated that supplementation with sodium nitrate (85 mg/L, 1mM) at a dose that is readily achievable through diet reduced blood pressure and circulating levels of triglycerides and improved glucose homeostasis (i.e., features of metabolic syndrome) in eNOS deficient mice (16). Overall, the results from these animal studies show that dietary nitrate exposure may reverse or improve pathological changes that are paralleled by loss of endothelium-derived NO bioavailability, leading to improved cardiovascular parameters.
Human studies
Few human studies have investigated the cardiovascular effects of dietary nitrate and the results from these studies are consistent with those of the animal studies. Larsen et al., demonstrated that dietary supplementation with sodium nitrate (0.1 mmol/Kg of body weight/day, for 3 days) in doses equivalent to a diet rich in vegetables reduces blood pressure (reduction in mm of Hg: diastolic ~ 3.5 mm Hg, mean arterial pressure ~ 3.2 and no change in systolic) in healthy humans (14). In addition, in another study, Webb et al., demonstrated that ingestion of a dietary nitrate load (500 mL of beet root juice, ~ 45 mmol/L or ~ 2.79 g/L of inorganic nitrate) lowers blood pressure (3 hr post-ingestion, reduction in mm of Hg: systolic ~ 10.4 ± 3, diastolic ~ 8.1 ± 2.1, mean arterial pressure ~ 8.0 ± 2.1), inhibits platelet aggregation (2.5 hr post-ingestion, adenosine diphosphate 30 μM-induced aggregation was reduced by ~ 20 %) and improves ischemia-induced endothelial dysfunction (~ 30 % increase in flow mediated dilation compared to control subjects following acute ischemia) in healthy volunteers (12). Moreover, using a randomized crossover study design, Kapil et al., recently demonstrated that nitrate ingestion in the form of either supplementation (i.e., potassium nitrate capsules) or by dietary elevation (i.e., 250 mL of beetroot juice, 5.5 mmol of nitrate) reduces blood pressure in healthy volunteers (15).
Dietary nitrate and cancer risk
A major health concern with dietary nitrate is the risk of development of cancer, because of its proposed association with the in vivo formation of N-nitrosamines, a class of carcinogenic substances (37). However, experimental studies and epidemiological studies failed to consistently show increased N-nitrosamines formation / risk for cancer with increasing consumption of dietary nitrate (6, 38–41). For example, Pannala et al., demonstrated no significant change in plasma 3-nitrotyrosine concentrations in healthy volunteers following high dietary nitrate (< 3.65 mg/kg body weight or ~ 250 mg nitrate) ingestion (41). Indeed, in 2003, Joint FAO/WHO Expert Committee on Food Additives reviewed studies investigating possible association between nitrate intake and cancer risk and concluded that there was no evidence that nitrate was carcinogenic to humans (42). Most importantly, epidemiological evidence mostly indicates that abundant consumption of vegetables reduces the risk of cancer (43, 44). Collectively, these studies suggest that dietary nitrate does not exert carcinogenic activity in humans and would not be harmful to human health via this mechanism.
Vegetables as a source of dietary nitrate intake
Vegetables are the main source of dietary nitrate exposure and represent about 80–85 % of nitrates consumed per day (see Table-1 for the classification of vegetables on the basis of their nitrate content) (6–9). In the United States, average dietary nitrate intake was found to be ~ 40–100 mg/day whereas reported International estimates of daily nitrate intake are between 53 to 350 mg/day (9, 42). This variability could be due to several factors including number of servings, species, fertilizer application, maturity, and storage conditions. For example, the average nitrate content of spinach collected from different markets in Delhi (India) varied from 710 to 4293 mg/kg fresh weight (45). After ingestion of vegetables, nitrate gets rapidly absorbed in the small intestine, enters circulation, mixes with NO-derived nitrate and readily distributes throughout the body (46, 47). For instance, ingestion of beetroot juice (500 mL, mean nitrate concentration ~ 45 mmol/L) rapidly increased plasma nitrate levels (~ 16 fold) in healthy volunteers (12). The half life of nitrate after ingestion is about 5–7 hours and ~ 60–70 % of the ingested nitrate is rapidly excreted unchanged in urine (46, 47). Unlike nitrate, nitrite content of the vegetables are very low (< 10 mg per kg) and rarely exceed 100 mg/kg (6–9). However, nitrite levels of up to 400 mg/kg have been found in vegetables that have been damaged, poorly stored, or stored for extended periods as well as pickled or fermented (48).
Table 1.
Classification of vegetables according to inorganic nitrate content (mg/100 gm fresh weight)
| Class I (< 20) | Artichoke, asparagus, broad bean, Brussels sprouts, eggplant, garlic, onion, green bean, mushroom, pea, pepper, potato, squash, tomato |
| Class II (20 to < 50) | Broccoli, carrot, cauliflower, cucumber, pumpkin, chicory |
| Class III (50 to < 100) | Cabbage, dill, turnip, Savoy cabbage |
| Class IV (100 to < 250) | Celeriac, Chinese cabbage, endive, escarole, fennel, kohlrabi, leaf chicory, leek, parsley |
| Class V (> 250) | Celery, chervil, cress, Lamb's lettuce, lettuce, radish, red beetroot, rocket (rucola), spinach, Swiss chard |
data from references6–9.
Nitrate vs other constituents of vegetables in cardiovascular protection
Studies over the past two decades have demonstrated that, apart from inorganic nitrate, several constituents of vegetables, in particular polyphenols and vitamin C, can also improve cardiovascular health (34–36). These effects are attributed to improvement in endothelial function, achieved mainly through increased endothelial NO production and/or bioavailability (34–36). It is now clear that endothelial NO production is oxygen-dependent, meaning that endothelial NO-driven processes decline with depletion of normal oxygen levels. In addition, cardiovascular diseases share a common pathophysiology involving depletion of normal oxygen levels, coupled to diminished blood supply due to atherosclerosis (i.e., thickening or narrowing of the arteries due to the development of plaques in the arterial wall) and/or thrombosis (i.e., arterial blood clotting) (49–52). For example, thickening of coronary arteries can restrict blood supply to myocardium leading to myocardial infarction or acute heart attack. Moreover, as discussed above, NO production from nitrate-nitrite-NO pathway increases with decrease in oxygen. In this regard, indeed, a growing number of studies demonstrate that NO generation from the nitrate-nitrite-NO pathway may contribute to hypoxic vasodilation (53–55). Furthermore, emerging data indicates that polyphenols and vitamin C are able to reduce nitrite to NO that, in turn, may exert cardiovascular protection (Figure 1) (56, 57). For example, Rocha et al., recently demonstrated that quercetin, a polyphenolic antioxidant flavonoid widely available in vegetables, potentiated vasodilating effects of nitrite through enhancing NO production from nitrite under acidic conditions (57). Putting together, these findings suggest an intriguing possibility that inorganic nitrate may play a major role in the apparent protective effects of vegetables against cardiovascular diseases. Indeed, this would explain the findings from recent large cohort studies which demonstrated that green leafy vegetables, which are rich source of inorganic nitrate, and vitamin C-rich fruits and vegetables contribute most to the cardiovascular protective effects of total fruit and vegetable intake (2, 3).
Conclusion
From the studies presented above, it appears that inorganic nitrate may play a major role in the cardiovascular health benefits of vegetables, presumably through enhancing NO bioavailability in the vasculature. However, at this point of time, the relative contribution of this pathway to total cardiovascular benefit of vegetable-rich diets as compared to other potential mechanisms remains unknown.
Acknowledgements
All the authors contributed equally to the MS. We thank Dr Barbora Piknova (Molecular Medicine Branch, NIDDK) for her valuable inputs.
Footnotes
Declaration of interest: A.N.S. is a co-inventor of a patent held by the National Institutes of Health for the use of nitrite salts for the treatment of cardiovascular diseases
References
- 1.Ness AR, Powles JW. Fruit and vegetables, and cardiovascular disease: a review. Int J Epidemiol. 1997;26:1–13. doi: 10.1093/ije/26.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Joshipura KJ, Ascherio A, Manson JE, et al. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282:1233–1239. doi: 10.1001/jama.282.13.1233. [DOI] [PubMed] [Google Scholar]
- 3.Joshipura KJ, Hu FB, Manson JE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 4.Bazzano LA, Serdula MK, Liu S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr Atheroscler Rep. 2003;5:492–499. doi: 10.1007/s11883-003-0040-z. [DOI] [PubMed] [Google Scholar]
- 5.Miller ER, 3rd, Erlinger TP, Appel LJ. The effects of macronutrients on blood pressure and lipids: an overview of the DASH and OmniHeart trials. Curr Atheroscler Rep. 2006;8:460–465. doi: 10.1007/s11883-006-0020-1. [DOI] [PubMed] [Google Scholar]
- 6.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]
- 7.Susin J, Kmecl V, Gregorcic A. A survey of nitrate and nitrite content of fruit and vegetables grown in Slovenia during 1996–2002. Food Addit Contam. 2006;23:385–390. doi: 10.1080/02652030600573715. [DOI] [PubMed] [Google Scholar]
- 8.Santamaria P. Nitrate in vegetables: toxicity, content, intake and EC regulation. J Sci Food Agric. 2006;86:10–17. [Google Scholar]
- 9.Pennington JAT. Dietary exposure models for nitrates and nitrites. Food Control. 1998;9:385–395. [Google Scholar]
- 10.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Webb AJ, Patel N, Loukogeorgakis S, et al. Acute blood pressure lowering, vasoprotective, and anti-platelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansson EA, Huang L, Malkey R, et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4:411–417. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 14.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 15.Kapil V, Milsom AB, Okorie M, et al. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 16.Carlstrom M, Larsen FJ, Nystrom T, et al. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc Natl Acad Sci USA. 2010;107:17716–17720. doi: 10.1073/pnas.1008872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dejam A, Hunter CJ, Schechter AN, Gladwin MT. Emerging role of nitrite in human biology. Blood Cells Mol Dis. 2004;32:423–429. doi: 10.1016/j.bcmd.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Kapil V, Webb AJ, Ahluwalia A. Inorganic nitrate and the cardiovascular system. Heart. 2010;96:1703–1709. doi: 10.1136/hrt.2009.180372. [DOI] [PubMed] [Google Scholar]
- 19.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 20.Lundberg JO, Weitzberg E. NO generation from inorganic nitrate and nitrite: Role in physiology, nutrition and therapeutics. Arch Pharm Res. 2009;32:1119–1126. doi: 10.1007/s12272-009-1803-z. [DOI] [PubMed] [Google Scholar]
- 21.Bryan NS. Cardioprotective actions of nitrite therapy and dietary considerations. Front Biosci. 2009;14:4793–4808. doi: 10.2741/3568. [DOI] [PubMed] [Google Scholar]
- 22.Moncada S. Nitric oxide: discovery and impact on clinical medicine. J R Soc Med. 1999;92:164–169. doi: 10.1177/014107689909200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villanueva C, Giulivi C. Subcellular and cellular locations of nitric oxide synthase isoforms as determinants of health and disease. Free Radic Biol Med. 2010;49:307–316. doi: 10.1016/j.freeradbiomed.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatterjee A, Black SM, Catravas JD. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul Pharmacol. 2008;49:134–140. doi: 10.1016/j.vph.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 26.van Faassen EE, Bahrami S, Feelisch M, et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 28.Radomski MW, Moncada S. Regulation of vascular homeostasis by nitric oxide. Thromb Haemost. 1993;70:36–41. [PubMed] [Google Scholar]
- 29.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Llorens S, Jordán J, Nava E. The nitric oxide pathway in the cardiovascular system. J Physiol Biochem. 2002;58:179–188. doi: 10.1007/BF03179855. [DOI] [PubMed] [Google Scholar]
- 31.Cai Hua, Harrison David G. Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 32.Hadi HAR, Carr CS, Suwaidi JA. Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vasc Health Risk Manag. 2005;1:183–198. [PMC free article] [PubMed] [Google Scholar]
- 33.Hirata Y, Nagata D, Suzuki E, Nishimatsu H, Suzuki J, Nagai R. Diagnosis and treatment of endothelial dysfunction in cardiovascular disease. Int Heart J. 2010;51:1–6. doi: 10.1536/ihj.51.1. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt CA, Dirsch VM. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide. 2009;21:77–91. doi: 10.1016/j.niox.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Davis N, Katz S, Wylie-Rosett J. The effect of diet on endothelial function. Cardiol Rev. 2007;15:62–66. doi: 10.1097/01.crd.0000218824.79018.cd. [DOI] [PubMed] [Google Scholar]
- 36.Stoclet JC, Chataigneau T, Ndiaye M, et al. Vascular protection by dietary polyphenols. Eur J Pharmacol. 2004;500:299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 37.Spiegelhalder B, Eisenbrand G, Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 38.National Toxicology Program Technical Report on the toxicology and carcinogenesis studies of sodium nitrite (CAS No. 7632-00-0) in F344/N rats and B6C3F1 mice (drinking water studies). NIH Publication No. 01-3954. 2001. pp. 7–273. [PubMed] [Google Scholar]
- 39.van Loon AJ, Botterweck AA, Goldbohm RA, Brants HA, van Klaveren JD, van den Brandt PA. Intake of nitrate and nitrite and the risk of gastric cancer: a prospective cohort study. Br J Cancer. 1998;78:129–135. doi: 10.1038/bjc.1998.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Y, Jiang H, Bryan NS. Nitrite and nitrate: cardiovascular risk-benefit and metabolic effect. Curr Opin Lipidol. 2011;22:11–15. doi: 10.1097/MOL.0b013e328341942c. [DOI] [PubMed] [Google Scholar]
- 41.Pannala AS, Mani AR, Spencer JP, et al. The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free Radic Biol Med. 2003;34:576–584. doi: 10.1016/s0891-5849(02)01353-9. [DOI] [PubMed] [Google Scholar]
- 42.Nitrate (and potential endogenous formation of N-nitroso compounds) 2003. Joint Food and Agricultural Organization of the United States/World Health Organization committee on Food additives. (WHO Food Additive series No: 50). [Google Scholar]
- 43.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 44.Terry P, Terry JB, Wolk A. Fruit and vegetable consumption in the prevention of cancer: an update. J Intern Med. 2001;250:280–290. doi: 10.1046/j.1365-2796.2001.00886.x. [DOI] [PubMed] [Google Scholar]
- 45.Anjana SUIM. Abrol YP. Are nitrate concentrations in leafy vegetables within safe limits? Curr Sci. 2007;92:355–360. [Google Scholar]
- 46.Walker R. The metabolism of dietary nitrites and nitrates. Biochem Soc Trans. 1996;24:780–785. doi: 10.1042/bst0240780. [DOI] [PubMed] [Google Scholar]
- 47.Tannenbaum SR. Nitrate and nitrite: origin in humans. Science. 1994;205:1333–1335. doi: 10.1126/science.472750. [DOI] [PubMed] [Google Scholar]
- 48.Evaluation of certain food additives and contaminants. 1995. pp. 29–35. (WHO Technical Report Series No. 859). [PubMed] [Google Scholar]
- 49.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 50.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 51.Chesebro JH, Rauch U, Fuster V, Badimon JJ. Pathogenesis of thrombosis in coronary artery disease. Haemostasis. 1997;27:12–18. doi: 10.1159/000217477. [DOI] [PubMed] [Google Scholar]
- 52.Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol. 2005;14:170–175. doi: 10.1016/j.carpath.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Crawford JH, Isbell TS, Huang Z, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dejam A, Hunter CJ, Tremonti C, et al. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 55.Gladwin MT. Evidence mounts that nitrite contributes to hypoxic vasodilation in the human circulation. Circulation. 2008;117:594–597. doi: 10.1161/CIRCULATIONAHA.107.753897. [DOI] [PubMed] [Google Scholar]
- 56.Sibmooh N, Piknova B, Rizzatti F, Schechter AN. Oxidation of iron-nitrosyl-hemoglobin by dehydroascorbic acid releases nitric oxide to form nitrite in human erythrocytes. Biochemistry. 2008;47:2989–2996. doi: 10.1021/bi702158d. [DOI] [PubMed] [Google Scholar]
- 57.Rocha BS, Gago B, Barbosa RM, Laranjinha J. Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation. Toxicology. 2009;265:41–48. doi: 10.1016/j.tox.2009.09.008. [DOI] [PubMed] [Google Scholar]


