Abstract
Anthropogenic disturbance of farmed animals can be detrimental by adversely affecting behaviours and metabolic rate, potentially reducing their commercial value. However, relatively little is known about the normal behavioural time budgets and associated metabolism of many such species, particularly for example pectinid bivalves, which use anaerobic metabolism during periods of short-burst activity. In the present study, we used the accelerometry technique to measure scallop overall dynamic body acceleration in combination with respirometry in order to obtain and compare the behavioural time budgets and associated metabolism of 10 scallops, Pecten maximus, in an aquaculture hatchery and 10 in the wild. Scallops in the wild typically spent only 0.1 per cent of the time moving (less than 2 min d−1), yet, on average, the estimated metabolism of such movement represented 16.8 per cent of daily energy expenditure. Furthermore, owing to their reliance on anaerobic pathways during such activity, movement resulted in the wild scallops having a raised metabolic rate for, on average, an estimated 7.8 per cent of the time, during which oxygen debts accumulated during movement were paid off. Hatchery scallops also typically spent only 0.1 per cent of the time moving but estimated metabolism of such movement represented 41.8 per cent of daily energy expenditure. Estimated mean daily metabolism of scallops in the hatchery was significantly higher than scallops in the wild (169.1 versus 120.7 mg O2 d−1) because anthropogenic disturbance in the hatchery caused energetically costly non-feeding behaviours. Consequently, hatchery scallops also spent a far greater amount of time with a raised metabolic rate (an estimated 26.6% of the time) than wild scallops. While short-term bursts of movement in pectinid bivalves may appear innocuous, they result in large expenditures of energy and an oxygen debt that is paid off over long periods of time that together limit further movement. These findings have implications for the farming industry; mitigating anthropogenic disturbances to farmed colonies may minimize non-feeding behaviours and hence maximize growth rates by reducing the costs of such movements and increasing the opportunity to feed.
Keywords: accelerometry, overall dynamic body acceleration, bivalve, metabolic rate, excess post-exercise oxygen consumption, anthropogenic
1. Introduction
Research on the detailed ecology of edible organisms is needed to aid both their conservation because of overexploitation [1] and their sustainable farming. Fished and farmed species, for instance, can suffer considerable anthropogenic disturbance, for example on account of working practices that expose them to unnatural vibrations, light and shadows. Indeed, disturbance associated with observing tanks of non-experimental, commercially grown aquatic species can cause some animals to perform unnatural or anti-predator behaviours that can be energetically costly. Repeated disturbance is known to have negative (e.g. decreased growth rate) or even fatal effects on species [2] and thus an important energy-based issue to investigate in commercially important aquatic organisms concerns whether anthropogenic disturbance causes increased levels of anti-predator behaviour [3] that in turn significantly increases daily energy expenditure.
Aquatic living resources are exploited by mankind worldwide. There are about 400 pectinid species, with around 33 species exploited commercially. The commercial pectinid scallops have a combined value of about US$ 1.7 billion worldwide [4]. The ecological energy budgets of scallops have been determined in relation to seasonal changes in temperature [5], food availability [6] and reproductive stage [7], but not in concert with the analysis of scallop movement, although there has been research into the dynamics and energetics of a single scallop movement—swimming [8–11]. Scallop movement and orientation in response to water flow have been studied under laboratory conditions but not to the level of detail required to provide a behavioural time budget [12]. Indeed, excluding studies on scallop escape reactions and subsequent recovery, the amount of time that scallops spend quiescent versus moving has not been quantified. For example, the daily cost of scallop valve movement (which includes active behaviours such as valve closure, digging and jumping [13,14]) has not been measured, and thus whether it constitutes a significant proportion of daily energy expenditure is unclear. Thus, relatively little is known about the normal behavioural time budgets and associated metabolism of scallops, either in aquaculture or in the wild.
The accelerometry technique, which measures body motion to estimate behaviours and associated metabolic rate, is starting to establish itself as a valuable method for obtaining behavioural time and/or energy budgets of a range of animals [15–20]. Gleiss' theoretical paper gives detailed physical explanations as to why the method should work [21], but key is that strong relationships are found across a range of animals between the rate of energy expenditure and acceleration measurements. The method generally involves firstly calibrating a derivative of acceleration (typically ODBA; overall dynamic body acceleration; [16]) with metabolic rate. To date, the latter has been measured as the rate of oxygen consumption (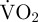 ) during the behaviour(s) of interest via respirometry [21,22], which assumes that metabolism is fuelled by entirely aerobic processes [23]. However, anaerobic metabolism occurs in the muscles of many aerobic species during unsustainable, short-term movement such as fast running [24,25], fast swimming [26,27] and combative behaviours [28,29], and can sometimes even represent the source of the great majority of energy expenditure during these behaviours [30].
) during the behaviour(s) of interest via respirometry [21,22], which assumes that metabolism is fuelled by entirely aerobic processes [23]. However, anaerobic metabolism occurs in the muscles of many aerobic species during unsustainable, short-term movement such as fast running [24,25], fast swimming [26,27] and combative behaviours [28,29], and can sometimes even represent the source of the great majority of energy expenditure during these behaviours [30].
Anaerobic metabolism also predominates during scallop movement [3,31], which occurs over short time periods usually less than 1–10 s in duration [32]. Subsequent to such short-burst activity, a recovery period after movement is required for restoration of the phosphoarginine pool and ATP pool (energy reservoirs) [33,34], with recuperation relying upon ATP production by mitochondria within the muscle fibres [34]. These recovery periods are represented by oxygen consumption after exercise over and above oxygen consumption representing routine metabolic rate, i.e. oxygen uptake known as excess post-exercise oxygen consumption (EPOC [35]). When measuring metabolic rate as the rate of oxygen consumption, where anaerobic metabolism is prominent, including EPOC is often considered to at least approximately account for the anaerobic metabolic component, such that measured oxygen consumption during the movement plus the subsequent EPOC is a reasonable representation of metabolism during that movement [30]. When movement durations are short, EPOC comprises a large percentage of the total oxygen consumed as a result of the movement because the oxygen consumed during the brief period of movement is small [30].
In the present study, we relate metabolic rate with ODBA in scallops, Pecten maximus (Linnaeus 1758), estimating metabolic rate as total oxygen consumption (total VO2) during the movement of interest plus the subsequent EPOC. This enabled the generation of ODBA-based prediction equations to estimate scallop, P. maximus, energetics. Further, we document the ODBA signatures that indicate each of the major scallop behaviours. These metabolism and behaviour calibrations allowed us to compare in detail the behavioural time budgets and associated metabolic rates of scallops, P. maximus, in a hatchery and in the wild. We hypothesize that anthropogenic disturbance will cause hatchery scallops to display a different behavioural time budget to wild scallops and expend more energy overall. We further hypothesize that since anaerobic metabolism in scallops is used for short bursts of movement but is unsustainable and reduces the energy reserves available to escape (swim) from predators, scallops will generally fully recover from a movement event, denoted by a return of 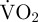 to resting levels, before undertaking another movement event.
to resting levels, before undertaking another movement event.
2. Material and methods
All research detailed below was conducted in accordance with institutional, national and international guidelines relating to the use of bivalves in research.
Ten scallops in the Rade de Brest were caught by divers near l'Écloserie du Tinduff (48.3024° N, −4.4477° W), Port Du Tinduff, 29470, France. They were placed in a single hatchery tank filled with natural, unfiltered sea water (3 × 1.4 × 0.55 m deep) at l'Écloserie du Tinduff. The tank had a coarse shell and gravel bottom and the sea water in the tank was continuously exchanged with fresh sea water from the Rade de Brest. Thus, scallops in the hatchery had a natural seston diet. An infrared camera recorded all scallop movements while in the hatchery.
The scallops were maintained within the tank for 6 days and then each was instrumented with a custom-built accelerometer logger (JUV Elektronik, Borstel, Germany), replaced in the tank and left untouched for 7 days (‘observation experiments’). Recording time was limited by battery capacity, which supplied sufficient voltage for around 36 h. The accelerometer logger recorded for the final 24 h of the observation experiments at which point with the accelerometer logger still recording, each scallop was placed alone inside a respirometry chamber submerged in the tank described above for 12 h, during which time accelerometer logger behaviour and metabolic rate-calibration data were obtained. Soon afterwards, the accelerometer logger was removed. Then scallop dimensions and mass were measured.
A further 10 scallops were caught in the same area in the Rade de Brest, immediately instrumented with accelerometer loggers and returned to the location where they had been caught. The accelerometer loggers started recording data 6 days after deployment and they were removed after about 36 h of recording. Scallop dimensions and mass were measured at this time. These wild scallops were not involved in respirometry experiments.
2.1. Accelerometer loggers
The accelerometer loggers were set to record acceleration in three axes (0–6 g), gape angle (°) via a calibrated Hall sensor [36], depth (m) and temperature (°C) at 16 Hz with 22-bit resolution onto a 1 Gb RA memory card. This recording frequency is sufficiently high to use measures of scallop acceleration as a proxy for metabolic rate [37] and to ascertain unique signatures for each scallop behaviour. Preset accelerometer loggers were heat-sealed into a polyethylene film. After epibionts were removed from the outer shell surface, a sealed accelerometer logger was glued to the outer shell of the upper (left/flat) valve of each scallop using glue (Araldite 90 s epoxy, Huntsman Advanced Materials) and waterproof tape (Tesa tape No. 4651, Hamburg, Germany).
The accelerometer loggers employed were smaller and lighter than those used in previous studies measuring ODBA [16,19]. Including the memory card, the accelerometer loggers were 4.0 × 1.7 × 1.1 cm maximum dimensions and the Hall sensor, including the associated wire, had maximum dimensions of 3.0 × 0.4 × 0.3 cm. Including the memory card, Hall sensor and one-half AA 3.6 V lithium battery, the package deployed had a mass of 21.1 g. The air inside the polyethylene film made the accelerometer loggers neutrally buoyant in sea water.
Data from the accelerometer loggers were downloaded onto a PC using custom-made software. The x-axis of the accelerometer logger measured sway, the y-axis measured surge and the z-axis measured heave (see [17] for more details). From the downloaded accelerometer logger data, an approximation of absolute acceleration (g) resulting only from dynamic acceleration in each of the three dimensions was extracted from each axis following removal of the static acceleration using a running mean of 3 s (cf. [38]). These values were then summed to produce ODBA (see [16,38] for more details). Valve movements were recorded and quantified from the valve gape data.
2.2. Observation experiments
Scallops in the hatchery were continuously exposed to indirect artificial light and periodic anthropogenic shadows and vibrations caused by the workings of a scallop hatchery manned 24 h d−1. Ten scallops were equipped with accelerometer loggers on 14 May 2010 which recorded data in observation experiments from 09.00 h on 20 May for 24 h. Mean hatchery scallop ash-free dry tissue mass (dry mass) = 10.9 g, range 9.0–13.6 g; see table 1; shell height (maximum distance from umbo to shell edge) 108.0 mm ± s.e.m. 2.1, maximum shell width 129.3 ± 1.5, sea water temperature 15.3–16.6°C, salinity 32–33‰.
Table 1.
Summary of the 24 h behavioural time budgets and associated metabolic rate of hatchery and wild scallops. Standard error of the mean are provided for measured values, while standard error of the estimate are provided for estimated values.
| dry mass (g) | mean ODBA during movement within a 24 h period (g) | mean estimated 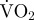 per 24 h (mg O2 d−1) per 24 h (mg O2 d−1) |
mean estimated duration of raised metabolic rate per 24 h period (h) | time spent on movement per 24 h period (s) | proportion of time spent on movement (%) | proportion of time spent on movement + EPOC (%) | |
|---|---|---|---|---|---|---|---|
| hatchery scallop ID | |||||||
| 1 | 12.0 | 0.5185 | 254.1 | 0.29 | |||
| 2 | 10.9 | 0.5881 | 110.3 | 0.13 | |||
| 3 | 9.1 | 0.6334 | 194.3 | 0.22 | |||
| 4 | 10.0 | 0.5564 | 99.7 | 0.12 | |||
| 5 | 9.0 | 0.7875 | 55.3 | 0.06 | |||
| 6 | 10.2 | 0.6898 | 91.4 | 0.11 | |||
| 7 | 11.0 | 0.7078 | 70.2 | 0.08 | |||
| 8 | 13.6 | 0.8505 | 50.0 | 0.06 | |||
| 9 | 10.5 | 0.7510 | 73.0 | 0.08 | |||
| 10 | 12.6 | 0.7093 | 119.4 | 0.14 | |||
| mean ± s.e.m./s.e.e. | 10.9 ± 0.5 | 0.6792 ± 0.0332 | 169.1 ± 10.7 | 6.38 ± 0.90 | 111.8 ± 20.5 | 0.13 ± 0.02 | 26.6 ± 3.8 |
| wild scallop ID | |||||||
| 1 | 10.7 | 0.2115 | 237.8 | 0.28 | |||
| 2 | 11.6 | 0.4058 | 50.4 | 0.06 | |||
| 3 | 12.3 | 0.1935 | 88.7 | 0.10 | |||
| 4 | 12.6 | 0.2616 | 71.4 | 0.08 | |||
| 5 | 13.6 | 0.1971 | 87.4 | 0.10 | |||
| 6 | 11.2 | 0.3452 | 55.9 | 0.06 | |||
| 7 | 9.8 | 0.3169 | 84.7 | 0.10 | |||
| 8 | 12.4 | 0.2012 | 98.1 | 0.11 | |||
| 9 | 10.1 | 0.1936 | 58.0 | 0.07 | |||
| 10 | 9.3 | 0.1821 | 48.1 | 0.06 | |||
| mean ± s.e.m./s.e.e. | 11.4 ± 0.4 | 0.2509 ± 0.0249 | 120.7 ± 4.8 | 1.88 ± 0.32 | 88.1 ± 17.6 | 0.10 ± 0.02 | 7.8 ± 1.3 |
The 10 scallops studied in the wild were instrumented on 1 July 2010, with the accelerometer loggers programmed to record data 6 days after deployment at 09.00 h for approximately 36 h. Observation experiments in the wild when the accelerometer logger recorded data were for 24 h (or 36 h where specifically stated) from the 09.00 h start time of data logging. Mean wild scallop dry mass = 11.4 g, range 9.3–13.6 g; shell height 111.8 mm ± s.e.m. 1.4, maximum shell width 131.1 ± 1.2, sea water temperature 15.1–16.2°C, salinity 32–33‰, depth 7.0–13.1 m.
2.3. Respirometry experiments
A closed-circuit respirometry system was used to measure scallop metabolic rate via calculations of 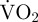 . The respirometer chamber was cylindrical, flat-bottomed (internal diameter 15 cm, height 26.1 cm) and contained sterilized sand to a depth of 8 cm. The respirometer chamber contained 3.2 l of sea water when no scallop was present and water flow in the chamber was 2 l min−1, allowing stable measurement of oxygen concentration by a dissolved oxygen probe (YSI 6562 Rapid Pulse Sensor) once per second [39,40]. Scallops were placed in the respirometry chamber at approximately 09.00 h on 21 May 2010 by which time they had made neither valve movements for at least 15 min nor swum for at least 6 days. Metabolic rate of scallops and controls (no scallop) was determined in darkness (see [39,40] and references therein for details). Respirometry measurements were made over 12 h by repeatedly measuring for approximately 1 h before opening the chamber valves to reoxygenate the water and then closing the valves to continue oxygen measurement. The oxygen level within the respirometer chamber was never allowed to drop to less than 75 per cent of saturation [6]. On three occasions, after each scallop in the respirometer chamber had made no valve movement(s) for 30 min, a steady routine metabolic rate (the metabolic rate of a quiescent, undisturbed animal which can include feeding behaviour but specifically no valve adduction or rapid (<0.5 s per event) valve abduction [41]) was measured over 1 h. For each scallop, the three measurements of routine metabolic rate were combined, averaged and the standard deviation calculated.
. The respirometer chamber was cylindrical, flat-bottomed (internal diameter 15 cm, height 26.1 cm) and contained sterilized sand to a depth of 8 cm. The respirometer chamber contained 3.2 l of sea water when no scallop was present and water flow in the chamber was 2 l min−1, allowing stable measurement of oxygen concentration by a dissolved oxygen probe (YSI 6562 Rapid Pulse Sensor) once per second [39,40]. Scallops were placed in the respirometry chamber at approximately 09.00 h on 21 May 2010 by which time they had made neither valve movements for at least 15 min nor swum for at least 6 days. Metabolic rate of scallops and controls (no scallop) was determined in darkness (see [39,40] and references therein for details). Respirometry measurements were made over 12 h by repeatedly measuring for approximately 1 h before opening the chamber valves to reoxygenate the water and then closing the valves to continue oxygen measurement. The oxygen level within the respirometer chamber was never allowed to drop to less than 75 per cent of saturation [6]. On three occasions, after each scallop in the respirometer chamber had made no valve movement(s) for 30 min, a steady routine metabolic rate (the metabolic rate of a quiescent, undisturbed animal which can include feeding behaviour but specifically no valve adduction or rapid (<0.5 s per event) valve abduction [41]) was measured over 1 h. For each scallop, the three measurements of routine metabolic rate were combined, averaged and the standard deviation calculated.
Movement was defined as valve adduction at any speed (all <0.5 s per event) or rapid (<0.5 s per event) valve abduction (active and rapid relaxation of the smooth adductor muscle [42,43]) occurring after the glide period [32] of spinning and swimming. Movement also included the glide period during spinning and swimming. EPOC (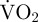 after exercise over and above
after exercise over and above 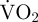 representing routine metabolic rate) started immediately after a period of movement and the endpoint of EPOC was defined as when
representing routine metabolic rate) started immediately after a period of movement and the endpoint of EPOC was defined as when 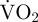 returned to within +1 s.d. of routine
returned to within +1 s.d. of routine 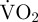 . Periods without movement were defined as periods without valve adduction, rapid valve abduction or gliding. During periods without movement when there is no EPOC,
. Periods without movement were defined as periods without valve adduction, rapid valve abduction or gliding. During periods without movement when there is no EPOC, 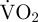 should be an accurate measure of routine metabolic rate. In contrast, during movement a large proportion of scallop metabolism is anaerobic and thus cannot be measured as
should be an accurate measure of routine metabolic rate. In contrast, during movement a large proportion of scallop metabolism is anaerobic and thus cannot be measured as 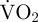 during that movement. However, measuring the EPOC associated with movement reasonably accounts for the anaerobic element of metabolism [30]. Therefore, in the present study, the estimated metabolic rate of a scallop during a period of movement was measured as: [(
during that movement. However, measuring the EPOC associated with movement reasonably accounts for the anaerobic element of metabolism [30]. Therefore, in the present study, the estimated metabolic rate of a scallop during a period of movement was measured as: [(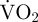 during movement × movement duration) + ((
during movement × movement duration) + ((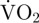 during period of EPOC − routine
during period of EPOC − routine 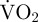 ) × EPOC duration)]/movement duration. Daily metabolism was calculated by summing estimates of routine metabolic rate during periods without movement and estimates of metabolic rate during movement over a 24 h period. Estimated total VO2 per day associated with a type of movement was calculated as the estimated metabolic rate during such movement multiplied by the total duration of that movement over the day.
) × EPOC duration)]/movement duration. Daily metabolism was calculated by summing estimates of routine metabolic rate during periods without movement and estimates of metabolic rate during movement over a 24 h period. Estimated total VO2 per day associated with a type of movement was calculated as the estimated metabolic rate during such movement multiplied by the total duration of that movement over the day.
2.4. Data analysis
Using the infrared camera recordings, scallop movements were classified as the following:
— a ‘cough’—a rapid valve adduction associated with the expulsion of faeces and other substances from the mantle cavity [14,42];
— digging—creating a self-formed depression in the seafloor sediment, using only infrequent rapid valve adductions (valve abduction is very gradual (>4 s) between each digging adduction), so that the scallop lies recessed with the flat valve roughly in the plane of the substrate [13];
— a ‘turn’—a rapid valve adduction causing less than or equal to about one full rotation on the substrate;
— spinning—rapid valve adduction followed by abduction events (2–3 s−1) causing multiple rotations on the substrate;
— swimming—rapid valve adduction followed by abduction events (2–3 s−1) causing the scallop to lift off the substrate.
The infrared camera recording of each type of movement in the hatchery confirmed a unique ODBA trace as a function of time (cf. [17]; OriginPro v. 7.5, OriginLab Corporation, USA). Then, by manually identifying and quantifying the duration of each scallop behaviour from the ODBA traces, the behavioural time budgets of the scallops were calculated.
All data included in statistical analyses met the necessary parametric assumptions; certain variables were transformed to make them normally distributed. A relationship to predict routine metabolic rate from dry mass was generated from a single linear regression using data from the 10 instrumented scallops in a hatchery. This equation for any individual scallop of known mass was used to estimate the routine metabolic rate of scallops in the hatchery and in the wild during periods without movement. A relationship to predict metabolic rate during movement included ODBA (mean ODBA per movement) and, as much of any individual variation in the metabolic rate–ODBA relationship is likely to be due to differences in mass, dry mass was included as a covariate. Thus, a linear effects model (estimated metabolic rate ∼ ODBA + dry mass) was generated from the data for the 10 hatchery scallops to produce an appropriate prediction equation of metabolic rate during movement for any individual scallop of known mass. The 95% prediction intervals for the model were calculated [44,45]. This equation was used to estimate the metabolic rate of scallops in the hatchery and in the wild during movement.
The accelerometer signal included a small amount of inherent noise in the surge axis, which was subtracted from the recordings by using a running mean over three points. Consequently, periods without movement were easily recognizable as represented by negligible ODBA values.
The duration of a movement event plus the subsequent EPOC is hereafter termed the duration of raised metabolic rate (i.e. the duration that metabolic rate is above the routine metabolic rate). A prediction equation of the duration of raised metabolic rate from ODBA for a scallop was generated by including the square root of the duration of raised metabolic rate, square root of total ODBA (where total ODBA is mean ODBA × duration) during the movement and dry mass for the 10 hatchery scallops in a linear effects model (duration of raised metabolic rate ∼ total ODBA + dry mass). The equation was also used to estimate the total duration of swimming required for an average scallop to have a constantly raised metabolic rate. In modelling this hypothetical situation, we assume that the multiple swim behaviours exhibited during the 24 h to create a constant raised metabolic rate have the average behaviour-specific mean ODBA recorded in the present study (swim ODBA = 1.3559 g) and the average behaviour-specific mean duration (swim = 5 s) for a scallop of 11 g dry mass. The estimated average duration of raised metabolic rate for this behaviour was then calculated, and then 24 h was divided by this value to calculate the number of periods of raised metabolic rate of this duration, and hence total duration of movement, required for a metabolic rate to be raised continually over 24 h.
The mean metabolic rate across individual scallops for each type of movement was calculated by applying the metabolic rate prediction equation to the mean ODBA values for each behaviour. These average ODBA values were derived by averaging within individuals and those individual means then averaged across individuals. Means are reported including ±1 s.e.m., or for estimates of metabolic rate and duration of raised metabolic rate, ±1 s.e.e. Student's t-tests and paired t-tests were used to test differences between measured means. Two-sample z-tests were used to compare estimated means between hatchery and wild scallops (cf. [46]).
3. Results
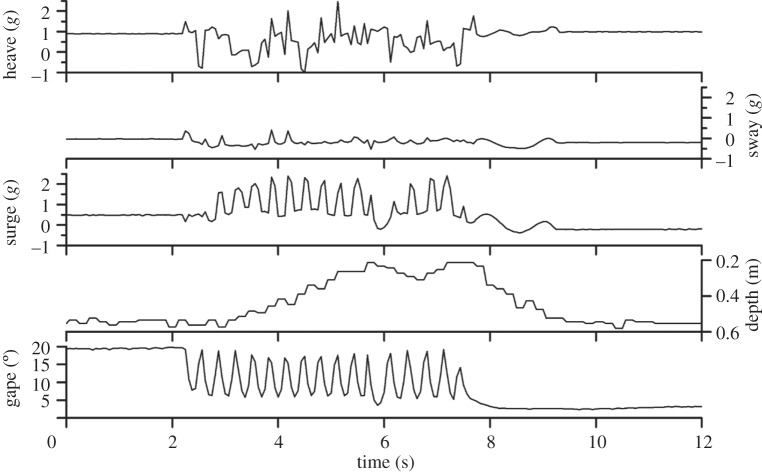
The mean whole wet mass of a scallop with epibionts on the outer shell surface (n = 20) was 278.8 ± 7.9 g (range 224.8–332.8 g) and was significantly greater than for the same scallops with the epibionts removed and instrumented with an accelerometer logger (mean 210.0 ± 6.6 g, range 168.9–279.9 g, t = 9.76, p ≤ 0.001). Each scallop behaviour provided a consistently unique acceleration signature both in terms of individual axes and ODBA (figures 1 and 2), yet was relatively consistent within and across individuals. Thus, it was straightforward to identify each scallop behaviour that occurred based on the acceleration data recorded. The infrared camera recordings showed that the coughing, digging, turning and spinning behaviours occurred daily in all hatchery scallops before, during and after they were instrumented with an accelerometer logger. No hatchery scallops swam in the 5 days before they were instrumented and none in the 5 days, when instrumented, before the accelerometer logger started recording. All scallops performed coughing, digging, turning and spinning behaviours in the respirometer. On occasion, three spinning scallops produced enough ODBA (>1.36g) to swim upwards (mean swimming ODBA = 1.36g) but did not perform enough rapid valve adduction followed by abduction events to lift off the substrate. Reoxygenating the water inside the respiromerty chamber did not influence the metabolic rate of the scallops. Between 0 and 4 h, after removal from the respirometer chamber, all scallops swam in hatchery experiments (figure 1).
Figure 1.
Measures of hatchery scallop valve gape, depth and tri-axial acceleration. Scallop swimming is clearly evident as a result of 17 valve adduction and 16 abduction events at a rate of 2–3 s−1. Note the time lag between the start of valve movement and the scallop lifting off the substrate. On the x-axis, the trough in scallop elevation in the water column at around 7 s is a result of the relative reduction in valve movement speed just before and just after 6 s.
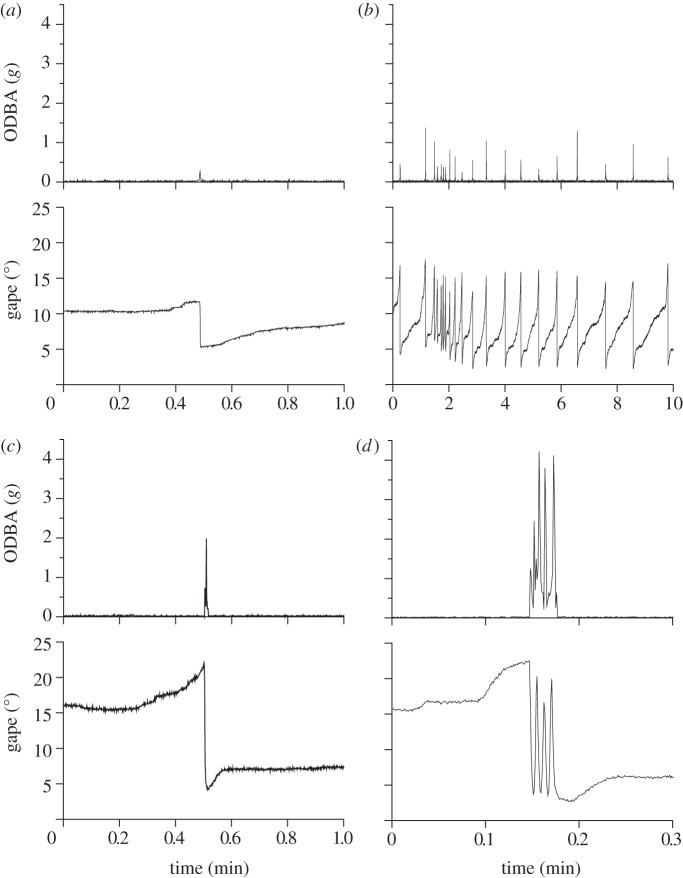
Figure 2.
Examples of valve gape and ODBA of various scallop behaviours: (a) coughing, (b) digging, (c) turning and (d) spinning behaviours.
3.1. Metabolic rate prediction equations
Mean routine metabolic rate of the 10 hatchery scallops in the respirometer was 0.066 mg O2 min−1 ± 0.003 at 16°C. Based on the respirometry data, the linear regression equation for predicting routine metabolic rate of a scallop from dry mass was significant (p < 0.001; figure 3):
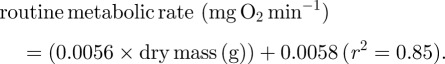 |
3.1 |
Figure 3.
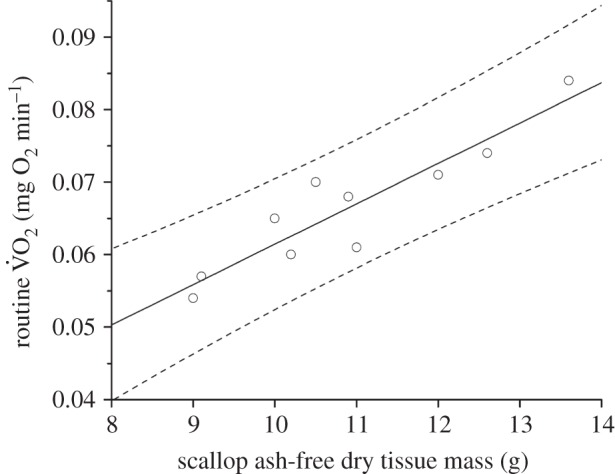
Routine metabolic rate (measured as rate of oxygen consumption, 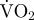 ; mg O2 min−1) against hatchery scallop ash-free dry tissue mass (g). The best-fit regression line (solid line) and 95% prediction intervals (dashed lines) are shown.
; mg O2 min−1) against hatchery scallop ash-free dry tissue mass (g). The best-fit regression line (solid line) and 95% prediction intervals (dashed lines) are shown.
The equation generated for predicting metabolic rate of a scallop during movement from ODBA (figure 4) and dry mass was also significant (p < 0.001):
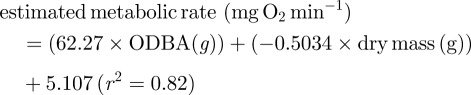 |
3.2 |
as was the equation generated for predicting the duration of raised metabolic rate of a scallop from total ODBA (figure 5) and dry mass (p < 0.001):
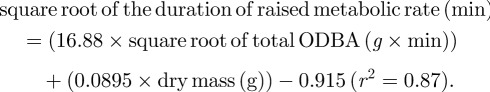 |
3.3 |
In the respirometry chamber, after one or multiple valve movements, measurements of 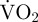 always returned to routine
always returned to routine 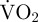 for at least 5 min before another valve movement.
for at least 5 min before another valve movement.
Figure 4.
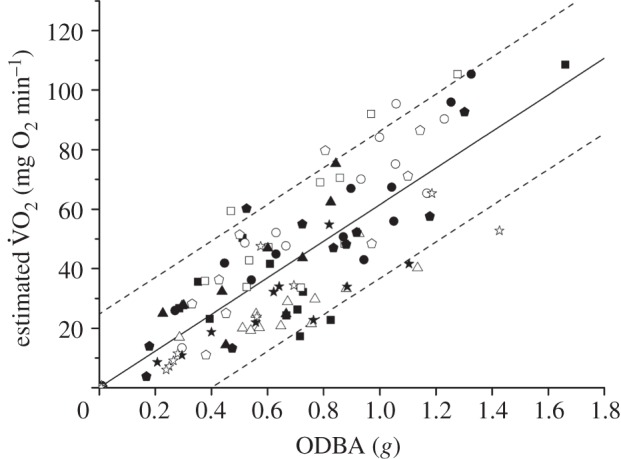
Estimated metabolic rate (measured as rate of oxygen consumption, 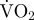 ; mg O2 min−1) against ODBA (g) for each hatchery scallop (denoted by different symbols) during a range of movements. The best-fit regression line (solid line) and 95% prediction intervals (dashed lines) are shown.
; mg O2 min−1) against ODBA (g) for each hatchery scallop (denoted by different symbols) during a range of movements. The best-fit regression line (solid line) and 95% prediction intervals (dashed lines) are shown.
Figure 5.
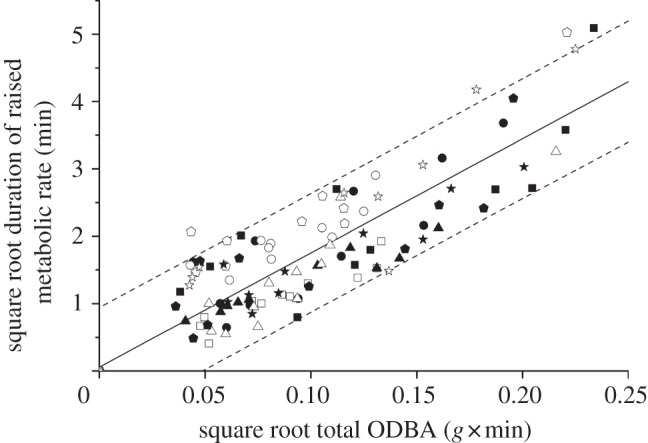
Square root of the duration of raised metabolic rate (min) associated with movement against the square root of total ODBA (mean ODBA for a movement × duration of movement) during the movement for each hatchery scallop (denoted by different symbols) during a range of movements. The best-fit regression line (solid line) and 95% prediction intervals (dashed lines) are shown.
3.2. Comparing scallops in the hatchery and in the wild
The morphology of the hatchery and wild scallops was similar. There was no significant difference between the dry mass of the two groups of scallops after the experiments (mean dry mass: 10.9 ± 0.5 g and 11.4 ± 0.4 g, for hatchery and wild scallops, respectively; t18 = −0.73, p = 0.473). Furthermore, there was no significant difference in the estimated total routine metabolism per 24 h (mean = 96.0 mg O2 ± 3.8 and 99.9 mg O2 ± 3.5 for the hatchery and wild scallops, respectively; z = −0.74, n = 20, p = 0.460 (table 2)). For each 24 h, for both groups of scallops, routine behaviour was estimated to be undertaken during a total of approximately 23 h and 58 min and movement behaviours for approximately 2 min.
Table 2.
Detailed 24 h behavioural time budgets and associated metabolic rates of hatchery and wild scallops. Standard error of the mean are provided for measured values, while standard error of the estimate are provided for estimated values.
| cough mean ODBA (g) | dig mean ODBA (g) | turn mean ODBA (g) | spin mean ODBA (g) | swim mean ODBA (g) | mean estimated routine 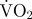 (mg O2 min−1) (mg O2 min−1) |
mean estimated 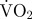 associated with coughing (mg O2 min−1) associated with coughing (mg O2 min−1) |
mean estimated 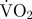 associated with digging (mg O2 min−1) associated with digging (mg O2 min−1) |
mean estimated 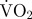 associated with turning (mg O2 min−1) associated with turning (mg O2 min−1) |
mean estimated 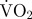 associated with spinning (mg O2 min−1) associated with spinning (mg O2 min−1) |
mean estimated 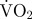 associated with swimming (mg O2 min−1) associated with swimming (mg O2 min−1) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| hatchery scallop mean ± s.e.m./s.e.e. | 0.1941 ± 0.0144 | 0.6519 ± 0.0417 | 0.6959 ± 0.0437 | 0.9660 ± 0.0410 | n.a. | 0.067 ± 0.003 | 11.7 ± 1.0 | 40.2 ± 2.7 | 43.0 ± 2.9 | 59.8 ± 2.6 | n.a. |
| wild scallop mean ± s.e.m./s.e.e. | 0.1625 ± 0.0126 | 0.6707 ± 0.0680 (n = 8) | 0.7298 ± 0.0528 (n = 9) | n.a. | 1.3559 ± 0.0455 (n = 2) | 0.069 ± 0.002 | 9.5 ± 0.8 | 41.2 ± 4.2 (n = 8) | 44.8 ± 3.2 (n = 9) | n.a. | 84.2 ± 2.4 (n = 2) |
| proportion of time spent coughing (%) | proportion of time spent digging (%) | proportion of time spent turning (%) | proportion of time spent spinning (%) | proportion of time spent swimming (%) | estimated total VO2 associated with routine metabolic cost per day (mg O2) | estimated total VO2 associated with coughing per day (mg O2) | estimated total VO2 associated with digging per day (mg O2) | estimated total VO2 associated with turning per day (mg O2) | estimated total VO2 associated with spinning per day (mg O2) | estimated total VO2 associated with swimming per day (mg O2) | |
| Hatchery scallop mean ± s.e.m./s.e.e. | 0.025 ± 0.004 | 0.056 ± 0.019 | 0.008 ± 0.002 | 0.042 ± 0.007 | 0 | 96.0 ± 3.8 | 4.1 ± 0.6 | 29.7 ± 9.0 | 4.9 ± 1.2 | 34.7 ± 6.0 | 0 |
| Wild scallop mean ± s.e.m./s.e.e. | 0.087 ± 0.019 | 0.011 ± 0.003 | 0.004 ± 0.001 | 0 | 0.001 ± 0.001 | 99.9 ± 3.5 | 11.2 ± 2.2 | 6.0 ± 1.7 | 2.2 + 0.5 | 0 | 1.4 ± 0.9 |
For 92.9 ± 0.8% of the time, over 24 h in the hatchery and 95.4 ± 0.7% of the time over 36 h in the wild, equation (3.3) predicted that 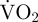 would return to routine metabolic rate before the next movement event. There was no significant difference in the time spent in movement per day for scallops in the hatchery and in the wild (mean: 111.8 ± 20.5 s and 88.1 ± 17.6 s for hatchery and wild, respectively; t18 = 0.88, p = 0.392 (table 1)).
would return to routine metabolic rate before the next movement event. There was no significant difference in the time spent in movement per day for scallops in the hatchery and in the wild (mean: 111.8 ± 20.5 s and 88.1 ± 17.6 s for hatchery and wild, respectively; t18 = 0.88, p = 0.392 (table 1)).
However, while all scallops in the hatchery exhibited the cough, digging and turning behaviours, all scallops in the wild also exhibited the cough behaviour but two of them did not exhibit the digging behaviour and one scallop did not exhibit the turning behaviour (tables 2 and 3). Furthermore, there were differences in metabolism during movement in general between the two groups, which affected daily metabolism. Estimated daily metabolism was significantly higher for scallops in the hatchery (mean: 169.1 ± 10.7 versus 120.7 ± 4.8 mg O2 d−1; z = 4.13, n = 20, p < 0.001), as was the estimated duration of raised metabolic rate per day (mean: 6.38 ± 0.90 h versus 1.88 ± 0.32 h; z = 4.69, n = 20, p < 0.001). It was estimated that scallops in the hatchery expended more energy in movement than those in the wild (73.4 ± 10.3 mg O2 d−1 and 20.8 ± 3.8 mg O2 d−1, respectively; z = 4.80, n = 20, p < 0.001). Scallops in the hatchery used an estimated mean of 41.8 ± 3.2% of metabolic expenditure per day on movement while those in the wild used a mean of 16.8 ± 2.2%.
Table 3.
Details of movement behaviours exhibited over 24 h by hatchery and wild scallops. Standard error of the mean are provided for measured values.
| duration of one cough (s) | duration of one dig (s)a | number of digs and time to create one self-formed depression in sediment | duration of one turn (s) | duration of one spinning event (s) | duration of one swim (s) | |
|---|---|---|---|---|---|---|
| hatchery scallop mean ± s.e.m. | 0.31 ± 0.03 | 0.26 ± 0.01 | 22 ± 3 (29 min ± 8) | 0.22 ± 0.01 | 1.44 ± 0.08 | n.a. |
| wild scallop mean ± s.e.m. | 0.32 ± 0.03 | 0.25 ± 0.01 (n = 8) | 18 ± 2 (25 min ± 7) (n = 8) | 0.24 ± 0.01 (n = 9) | n.a. | 5.04 ± 0.14 (n = 2) |
| number of cough events per day | number of digging adduction events per day | number of self-formed depressions made per day | number of turn events per day | number of spinning events per day | number of swims per day | |
| hatchery scallop mean ± s.e.m. | 69 ± 10 | 181 ± 58 | 8 ± 3 | 30 ± 6 | 25 ± 4 | 0 |
| wild scallop mean ± s.e.m. | 233 ± 39 | 36 ± 9 | 2 ± 1 | 13 ± 4 | 0 | 0.2 (i.e. <1) ± 0 |
aOne rapid valve adduction associated with digging.
There was no significant difference in the estimated metabolic rate during a cough, a dig or a turn between scallops in the hatchery and in the wild (table 2; z = −0.43 to 1.80, n = 20, p = 0.072–0.849). Scallops in the wild coughed for more of the time (t18 = 3.19, p = 0.005) and spent more energy in total coughing (z = 3.16, n = 20, p = 0.002) than did scallops in the hatchery. Scallops in the hatchery spent significantly more time and energy digging and turning compared with scallops in the wild (time digging: t18 = 2.33, p = 0.032; time turning: t18 = 2.20, p = 0.041; energy digging: z = 2.59, n = 20, p = 0.010; energy turning: z = 2.06, n = 20, p = 0.040). All 10 scallops in the hatchery spun during the course of 1 day (table 2) but no spinning behaviour was recorded in any scallops in the wild during the entirety of the 36 h recording period. Only two scallops in the wild swam when the accelerometer logger was recording; both just once and for a short period (ca 5 s). Estimated metabolic rate during these swims was 84.2 ± 2.4 mg O2 min−1 (n = 2) and the estimated total VO2 associated with swimming (total VO2 during swimming + EPOC) per day was 7.1 ± 0.4 mg O2 (n = 2; cf. table 2). Visual inspection of the data suggested no influence of tidal cycles or circadian rhythms on the movement and associated metabolic rate of scallops in the hatchery or in the wild.
Calculations made using equation (3.3) estimated that if a scallop of 11 g dry mass swam for a total of 218 s d−1, composed of many short swims averaging 5 s, it would have a constantly raised metabolic rate.
4. Discussion
The present study shows that although scallops undertake active behaviours infrequently and lasting just fractions of a second to a few seconds at a time, those behaviours nonetheless incur large energetic costs. In total, the time scallops moved in the present study was typically less than 2 min d−1 yet on average an estimated 29.3 per cent of daily energy expenditure was spent on such movement. Furthermore, owing to their reliance on anaerobic pathways during such activity, movement resulted in the scallops having a raised metabolic rate for on average an estimated 17.2 per cent of the time, during which rapidly accumulated oxygen debts were paid off. Validity of these results is provided by comparison with previous studies. For example, in the respirometry experiments of the present study, maximum ODBA was produced by a spin involving six rapid valve adduction–abduction events at a rate of 2–3 s−1 although this resulted in a relatively small subsequent EPOC of less than 30 min (figure 5). This finding is similar to the results of Livingstone et al. [33], who recorded a recovery period of approximately 30 min after five rapid valve adduction, followed by abduction events at a rate of 2–3 s−1 in the giant scallop, Placopecten magellanicus (cf. recovery period of scallops after valve movements causing complete exhaustion [33,47,48]).
4.1. The accelerometry method
The present study is the first to apply the accelerometry technique for investigating the behavioural energetics of a bivalve; by far, the smallest species studied by this method to date. As found in previous studies calibrating metabolic rate with ODBA [16,49], the relationship for scallops was strong (figure 4). Thus, within the body mass and temperature range included in the present study, using equations (3.1) and (3.2), the detailed behaviour and the estimated metabolic rate of a group of scallops can be obtained (estimates for individual animals will often be inaccurate [22]). Furthermore, equation (3.3) (figure 5) enables estimates of the time for complete recovery from oxygen debt subsequent to movement, which is a key metabolic parameter for scallops as periods of EPOC constrain their behaviours.
Instrumentation of a scallop with an accelerometer logger did not seem to limit their behavioural options because scallops in the present study were able to perform all the movement types previously reported [8,13,14,50]. Accelerating the accelerometer is a cost to the scallop but accelerating the epibionts is likely an even greater cost to the animal, or at the least similar to the cost of the accelerometer, because epibiont mass removed was greater than logger mass. Indeed, we specifically picked scallops with significant biofouling such that its removal would counter the mass and volume of the logger, and significant biofouling is the norm for scallops at least in the Rade de Brest area. All the instrumented scallops had a similar mass and thus drag on scallops was assumed to be relatively constant. Future studies incorporating smaller accelerometer loggers (such loggers are becoming ever further miniaturized [51]) could provide insight into issues of energetic costs during movement for biofouled scallops versus non-biofouled scallops. Lack of swimming in the hatchery indicates a lack of escape behaviour or attempts at migration, suggesting that the animals were not highly distressed. Furthermore, the instrumentation of the accelerometer logger did not prevent the most energetic behaviour of scallops because two scallops in the wild swam and after removal from the respirometer chamber, all scallops in hatchery experiments swam, presumably in response to the anthropogenic disturbance.
4.2. Comparing scallops in the hatchery and in the wild
Measuring the behavioural time budgets and associated metabolic rate of an animal in different environments provides valuable data to empirically determine if the variation in the environment affects their ecology. The present scallop data are the most detailed behaviour and energetics data yet obtained using the accelerometry technique.
There was neither difference in the time spent on movement in the two environments nor in the estimated metabolic cost of the coughing, digging and turning behaviours. However, the behavioural budgets of the periods spent moving differed. Wild scallops performed either digging or turning, or both behaviours, so some daily change in orientation, possibly towards optimum feeding conditions [12,52] was possible since the infrared camera recording showed that both digging and turning can result in a change in orientation. Hatchery scallops also exhibited digging and turning behaviours, but spent more time and energy on these behaviours than the wild scallops. Furthermore, spinning was exhibited by every scallop in the hatchery but was not recorded at all in the wild. This stark contrast is probably owing to anthropogenic disturbance (vibrations, artificial light and shadows) in the working scallop hatchery, resulting in anti-predatory behaviour [3]. In contrast, scallops in the wild spent more time and energy on the relatively low-energy cough, indicating that they were feeding more. Indeed, more time and energy was spent on the cough than any other movement type in the wild, while in the hatchery, more time and energy was spent on digging, turning and spinning. Hatchery scallops likely exhibited lower feeding rates not only because of time spent on anti-predator behaviours but also because these behaviours reduced the energy available for feeding; hatchery scallops spent a far greater amount of time with a raised metabolic rate (an estimated 26.6% versus 7.8%). We conclude that the estimated metabolic costs per day were significantly higher for scallops in the hatchery compared with the wild because of the type, and not the duration, of movement performed per day.
These findings have implications for the farming industry; mitigating anthropogenic disturbances to farmed colonies may minimize energetically costly non-feeding behaviours and hence maximize growth rates by reducing the costs of such activities and increasing the opportunity to feed.
4.3. Recovery and excess post-exercise oxygen consumption
Our results highlight that scallops increase oxygen uptake immediately after movement [33] and almost always fully metabolically recover from that movement before another movement event (based on equation (3.3)). While anthropogenic touch stimulation can cause scallops to perform movement during periods of EPOC [42,53], in the present study, where there was no touch stimulation, the scallops rarely moved until their oxygen debt had been repaid. None of the scallops in the present study displayed apparent exhaustion after valve movement(s) in the form of a complete lack of oxygen uptake [3,41].
Full recovery may be strategic because during EPOC, behavioural options are limited because of the reduced amount and rate of energy expenditure possible. For example, scallops exhibiting EPOC will be more limited in their ability to swim away from predators or dig themselves into sediment.
Anthropogenic stimulation of scallops to swim until exhaustion clearly results in a rapid accumulation of oxygen debt [33,54]. It was estimated that if the average scallop (11 g dry mass) swam for a total of 218 s (3.6 min) per day (cf. typical time moving—coughing, digging, turning, spinning and swimming—totalling less than 2 min d−1; tables 1–3), it would have a constantly raised metabolic rate. While likely a theoretical scenario, a constantly raised metabolic rate may represent the limiting factor on the amounts of scallop movement that can be undertaken.
5. Conclusions
The accelerometry technique has provided unprecedented information about the typical behavioural time budgets and associated metabolic rate of a commercially important invertebrate both in the hatchery and in the wild. While short-term bursts of scallop movement may appear innocuous, they result in large expenditures of energy and an oxygen debt that is paid off over long periods of time that together limit further scallop movement. So, for an animal that relies on unsustainable, short-term activity for movement, undertaking a small movement has serious implications, perhaps most importantly that for a significant period of time subsequently, these animals are likely less able to escape predation and less able to feed. The latter issue probably has consequences for somatic growth rates and gamete production in farmed colonies where anthropogenic disturbance can cause animals to elicit a range of energetically costly behaviours. While this finding may well be generalizable to other ectothermic species, the total activity budget of an endotherm is much smaller than that of an ectotherm overall as a proportion of total metabolic costs owing to the dominance of basal metabolic costs in endotherms. Thus, anthropogenic disturbances increasing the occurrence of energetically costly anaerobic activities are likely to impact the bottom line of an alligator, frog, turtle, salmon or scallop farm much more than a cattle farm.
Acknowledgements
The authors gratefully acknowledge helpful comments from three anonymous referees. We are most indebted to Jens-Uwe Voigt for developing the latest version of the ‘daily diary’ logger. We thank Emily Shepard for help with data analysis, Victoria Hobson for advice on waterproofing the loggers, Erwan Amice and the IUEM dive team for collecting and deploying scallops, l'Écloserie du Tinduff for the use of its facilities, Morgana Tagliarolo for help with measuring dry mass, Jacques Clavier for help in setting up respirometry experiments, GIS–Eurôpole Mer 2007–2012 for funding A.A. Robson and CHIVAS for buying equipment.
References
- 1.Jackson J. B. C., et al. 2001. Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–638 10.1126/science.1059199 (doi:10.1126/science.1059199) [DOI] [PubMed] [Google Scholar]
- 2.Wikelski M., Cooke S. J. 2006. Conservation physiology. Trends Ecol. Evol. 21, 38–46 10.1016/j.tree.2005.10.018 (doi:10.1016/j.tree.2005.10.018) [DOI] [PubMed] [Google Scholar]
- 3.Thompson R. J., Livingstone D. R., De Zwaan A. 1980. Physiological and biochemical aspects of the valve snap and valve closure responses in the giant scallop Placopecten magellanicus. I. Physiology. J. Comp. Physiol. B: Biochem., Syst., Environ. Physiol. 137, 97–104 [Google Scholar]
- 4.Lowther A., Vannuccini S. 2005. Aquaculture topics and activities. Trends in aquaculture production. Rome, Italy: FAO Fisheries and Aquaculture Department [Google Scholar]
- 5.Vahl O. 1978. Seasonal-changes in oxygen-consumption of Iceland scallop (Chlamys-Islandica (O F Muller)) from 70-Degrees-N. Ophelia 17, 143–154 [Google Scholar]
- 6.MacDonald B. A., Thompson R. J. 1986. Influence of temperature and food availability on the ecological energetics of the giant scallop Placopecten Magellanicus. III. Physiological ecology, the gametogenic cycle and scope for growth. Mar. Biol. 93, 37–48 10.1007/BF00428653 (doi:10.1007/BF00428653) [DOI] [Google Scholar]
- 7.Sundet J. H., Vahl O. 1981. Seasonal-changes in dry-weight and biochemical-composition of the tissues of sexually mature and immature Iceland scallops, Chlamys-Islandica. J. Mar. Biol. Assoc. UK 61, 1001–1010 10.1017/S0025315400023110 (doi:10.1017/S0025315400023110) [DOI] [Google Scholar]
- 8.Buddenbrock W. v. 1911. Untersuchungen über die Schwimmbewegungen und die Statocysten der Gattung Pecten. Sitzungsberichte der Heidelberger Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Klasse 28, 1–24 [Google Scholar]
- 9.Cheng J.-Y., Davison I. G., Demont M. E. 1996. Dynamics and energetics of scallop locomotion. J. Exp. Biol. 199, 1931–1946 [DOI] [PubMed] [Google Scholar]
- 10.Anderson E. J., MacGillivray P. S., Demont M. E. 1997. Scallop shells exhibit optimization of riblet dimensions for drag reduction. Biol. Bull. 192, 341–344 10.2307/1542744 (doi:10.2307/1542744) [DOI] [PubMed] [Google Scholar]
- 11.Guderley H., Labbe-Giguere S., Janssoone X., Bourgeois M., Perez H. M., Tremblay I. 2009. Thermal sensitivity of escape response performance by the scallop Placopecten magellanicus: impact of environmental history. J. Exp. Mar. Biol. Ecol. 377, 113–119 10.1016/j.jembe.2009.07.024 (doi:10.1016/j.jembe.2009.07.024) [DOI] [Google Scholar]
- 12.Sakurai I., Seto M. 2000. Movement and orientation of the Japanese scallop Patinopecten yessoensis (Jay) in response to water flow. Aquaculture 181, 269–279 10.1016/S0044-8486(99)00242-2 (doi:10.1016/S0044-8486(99)00242-2) [DOI] [Google Scholar]
- 13.Baird R. H., Gibson F. A. 1956. Underwater observations on escallop (Pecten maximus L.) beds. J. Mar. Biol. Assoc. UK 35, 555–562 10.1017/S0025315400010419 (doi:10.1017/S0025315400010419) [DOI] [Google Scholar]
- 14.Thomas G. E., Gruffydd L. D. 1971. The types of escape reactions elicited in the scallop Pecten maximus by selected sea-star species. Mar. Biol. 10, 87–93 10.1007/BF02026771 (doi:10.1007/BF02026771) [DOI] [Google Scholar]
- 15.Tsuda Y., Kawabe R., Tanaka H., Mitsunaga Y., Hiraishi T., Yamamoto K., Nashimoto K. 2006. Monitoring the spawning behaviour of chum salmon with an acceleration data logger. Ecol. Freshw. Fish. 15, 264–274 10.1111/j.1600-0633.2006.00147.x (doi:10.1111/j.1600-0633.2006.00147.x) [DOI] [Google Scholar]
- 16.Wilson R. P., White C. R., Quintana F., Halsey L. G., Liebsch N., Martin G. R., Butler P. J. 2006. Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. J. Anim. Ecol. 75, 1081–1090 10.1111/j.1365-2656.2006.01127.x (doi:10.1111/j.1365-2656.2006.01127.x) [DOI] [PubMed] [Google Scholar]
- 17.Shepard E. L. C., et al. 2008. Identification of animal movement patterns using tri-axial accelerometry. Endang. Spec. Res. 10, 47–60 10.3354/esr00084 (doi:10.3354/esr00084) [DOI] [Google Scholar]
- 18.Green J. A., Halsey L. G., Wilson R. P., Frappell P. B. 2009. Estimating energy expenditure of animals using the accelerometry technique: activity, inactivity and comparison with the heart-rate technique. J. Exp. Biol. 212, 471–482 10.1242/jeb.026377 (doi:10.1242/jeb.026377) [DOI] [PubMed] [Google Scholar]
- 19.Halsey L. G., White C. R. 2010. Measuring energetics and behaviour using accelerometry in Cane Toads Bufo marinus. PLoS ONE 5, e10170. 10.1371/journal.pone.0010170 (doi:10.1371/journal.pone.0010170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payne N., Gillanders B., Seymour R., Webber D., Snelling E., Semmens J. 2011. Accelerometry estimates field metabolic rate in giant Australian cuttlefish Sepia apama during breeding. J. Anim. Ecol. 80, 422–430 10.1111/j.1365-2656.2010.01758.x (doi:10.1111/j.1365-2656.2010.01758.x) [DOI] [PubMed] [Google Scholar]
- 21.Gleiss A. C., Wilson R. P., Shepard E. L. C. 2011. Making overall dynamic body acceleration work: on the theory of acceleration as a proxy for energy expenditure. Methods Ecol. Evol. 2, 23–33 10.1111/j.2041-210X.2010.00057.x (doi:10.1111/j.2041-210X.2010.00057.x) [DOI] [Google Scholar]
- 22.Halsey L. G., Shepard E. L. C., Wilson R. P. 2011. Assessing the development and application of the accelerometry technique for estimating energy expenditure. Comp. Biochem. Physiol.–Part A: Mol. Integr. Physiol. 158, 305–314 10.1016/j.cbpa.2010.09.002 (doi:10.1016/j.cbpa.2010.09.002) [DOI] [PubMed] [Google Scholar]
- 23.Lighton J. R. B., Halsey L. G. 2011. Flow-through respirometry applied to chamber systems: pros and cons, hints and tips. Comp. Biochem. Physiol.–Part A: Mol. Integr. Physiol. 158, 265–275 [DOI] [PubMed] [Google Scholar]
- 24.Bennett A. F., Ruben J. A. 1979. Endothermy and activity in vertebrates. Science 206, 649–654 10.1126/science.493968 (doi:10.1126/science.493968) [DOI] [PubMed] [Google Scholar]
- 25.Williams T. M., Dobson G. P., Mathieu-Costello O., Morsbach D., Worley M. B., Phillips J. A. 1997. Skeletal muscle histology and biochemistry of an elite sprinter, the African cheetah. J. Comp. Physiol. B: Biochem., Syst., Environ. Physiol. 167, 527–535 10.1007/s003600050105 (doi:10.1007/s003600050105) [DOI] [PubMed] [Google Scholar]
- 26.Reidy S. P., Kerr S. R., Nelson J. A. 2000. Aerobic and anaerobic swimming performance of individual Atlantic cod. J. Exp. Biol. 203, 347–357 [DOI] [PubMed] [Google Scholar]
- 27.Selch T. M., Chipps S. R. 2007. The cost of capturing prey: measuring largemouth bass (Micropterus salmoides) foraging activity using glycolytic enzymes (lactate dehydrogenase). Can. J. Fish Aquat. Sci. 64, 1761–1769 10.1139/f07-133 (doi:10.1139/f07-133) [DOI] [Google Scholar]
- 28.Ruben J. A. 1976. Aerobic and anaerobic metabolism during activity in snakes. J. Comp. Physiol. B: Biochem., Syst., Environ. Physiol. 109, 147–157 10.1007/BF00689414 (doi:10.1007/BF00689414) [DOI] [Google Scholar]
- 29.Feder M. E., Arnold S. J. 1982. Anaerobic metabolism and behavior during predatory encounters between snakes (Thamnophis elegans) and salamanders (Plethodon jordani). Oecologia 53, 93–97 10.1007/BF00377141 (doi:10.1007/BF00377141) [DOI] [PubMed] [Google Scholar]
- 30.Baker E. J., Gleeson T. T. 1999. The effects of intensity on the energetics of brief locomotor activity. J. Exp. Biol. 202, 3081–3087 [DOI] [PubMed] [Google Scholar]
- 31.De Zwaan A., Thompson R. J., Livingstone D. R. 1980. Physiological and biochemical aspects of the valve snap and valve closure responses in the giant scallop Placopecten magellanicus. II. Biochemistry. J. Comp. Physiol. B: Biochem., Syst., Environ. Physiol. 137, 105–114 10.1007/BF00689208 (doi:10.1007/BF00689208) [DOI] [Google Scholar]
- 32.Ansell A. D., Cattaneo-Vietti R., Chiantore M. 1998. Swimming in the Antarctic scallop Adamussium colbecki: analysis of in situ video recordings. Antarct. Sci. 10, 369–375 10.1017/S0954102098000455 (doi:10.1017/S0954102098000455) [DOI] [Google Scholar]
- 33.Livingstone D. R., De Zwaan A., Thompson R. J. 1981. Aerobic metabolism, octopine production and phosphoarginine as sources of energy in the phasic and catch adductor muscles of the giant scallop Placopecten magellanicus during swimming and the subsequent recovery period. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 70, 35–44 10.1016/0305-0491(81)90120-6 (doi:10.1016/0305-0491(81)90120-6) [DOI] [Google Scholar]
- 34.Guderley H. E., Rojas F. M., Nusetti O. A. 1995. Metabolic specialization of mitochondria from scallop phasic muscles. Mar. Biol. 122, 409–416 10.1007/BF00350873 (doi:10.1007/BF00350873) [DOI] [Google Scholar]
- 35.Gaesser G. A., Brooks G. A. 1984. Metabolic bases of excess post-exercise oxygen consumption: a review. Med. Sci. Sports Exerc. 16, 29–43 [PubMed] [Google Scholar]
- 36.Robson A. A., Thomas G. R., Garcia de Leaniz C., Wilson R. P. 2009. Valve gape and exhalant pumping in bivalves: optimization of measurement. Aquat. Biol. 6, 191–200 10.3354/ab00128 (doi:10.3354/ab00128) [DOI] [Google Scholar]
- 37.Halsey L. G., Green J. A., Wilson R. P., Frappell P. B. 2009. Accelerometry to estimate energy expenditure during activity: best practice with data loggers. Physiol. Biochem. Zool. 82, 396–404 10.1086/589815 (doi:10.1086/589815) [DOI] [PubMed] [Google Scholar]
- 38.Shepard E. L. C., Wilson R. P., Halsey L. G., Quintana F., Laich A. G., Gleiss A. C., Liebsch N., Myers A. E., Norman B. 2008. Derivation of body motion via appropriate smoothing of acceleration data. Aquat. Biol. 4, 235–241 10.3354/ab00104 (doi:10.3354/ab00104) [DOI] [Google Scholar]
- 39.Point D., et al. 2007. Biological control of trace metal and organometal benthic fluxes in a eutrophic lagoon (Thau Lagoon, Mediterranean Sea, France). Estuar. Coast. Shelf Sci. 72, 457–471 10.1016/j.ecss.2006.11.013 (doi:10.1016/j.ecss.2006.11.013) [DOI] [Google Scholar]
- 40.Grenz C., Denis L., Boucher G., Chauvaud L., Clavier J., Fichez R., Pringault O. 2003. Spatial variability in sediment oxygen consumption under winter conditions in a lagoonal system in New Caledonia (South Pacific). J. Exp. Mar. Biol. Ecol. 285, 33–47 10.1016/S0022-0981(02)00518-X (doi:10.1016/S0022-0981(02)00518-X) [DOI] [Google Scholar]
- 41.Bricelj V. M., Shumway S. 1991. Physiology: energy acquisition and utilization. Scallops: biology, ecology and aquaculture, pp. 305–346 New York, NY: Elsevier [Google Scholar]
- 42.Wilkens L. A. 1981. Neurobiology of the scallop. I. Starfish-mediated escape behaviours. Proc. R. Soc. Lond. B 211, 341–372 10.1098/rspb.1981.0011 (doi:10.1098/rspb.1981.0011) [DOI] [Google Scholar]
- 43.Mellon F. 1969. The reflex control of rhythmic motor output during swimming in the scallop. J. Comp. Physiol. A: Neuroethol., Sensory, Neural Behav. Physiol. 62, 318–336 [Google Scholar]
- 44.Zar J. H. 1984. Biostatistical analysis, 2nd edn. Englewood Cliffs, NJ: Prentice Hall, Inc [Google Scholar]
- 45.Robson A. A., Garcia de Leaniz C., Wilson R. P., Halsey L. G. 2010. Effect of anthropogenic feeding regimes on activity rhythms of laboratory mussels exposed to natural light. Hydrobiologia 655, 197–204 10.1007/s10750-010-0449-7 (doi:10.1007/s10750-010-0449-7) [DOI] [Google Scholar]
- 46.Green J. A., Butler P. J., Woakes A. J., Boyd I. L., Holder R. L. 2001. Heart rate and rate of oxygen consumption of exercising macaroni penguins. J. Exp. Biol. 204, 673–684 [DOI] [PubMed] [Google Scholar]
- 47.Brokordt K. B., Himmelman J. H., Nusetti O. A., Guderley H. E. 2000. Reproductive investment reduces recuperation from exhaustive escape activity in the tropical scallop Euvola zizac. Mar. Biol. 137, 857–865 10.1007/s002270000415 (doi:10.1007/s002270000415) [DOI] [Google Scholar]
- 48.Brokordt K. B., Himmelman J. H., Guderley H. E. 2000. Effect of reproduction on escape responses and muscle metabolic capacities in the scallop Chlamys islandica Muller 1776. J. Exp. Mar. Biol. Ecol. 251, 205–225 10.1016/S0022-0981(00)00215-X (doi:10.1016/S0022-0981(00)00215-X) [DOI] [PubMed] [Google Scholar]
- 49.Halsey L. G., Shepard E. L. C., Quintana F., Gomez Laich A., Green J. A., Wilson R. P. 2009. The relationship between oxygen consumption and body acceleration in a range of species. Comp. Biochem. Physiol.-Part A: Mol. Integr. Physiol. 152, 197–202 10.1016/j.cbpa.2008.09.021 (doi:10.1016/j.cbpa.2008.09.021) [DOI] [PubMed] [Google Scholar]
- 50.Baird R. H. 1958. On the swimming behaviour of escallops (Pecten maximus L.). J. Molluscan Stud. 33, 67–71 [Google Scholar]
- 51.Halsey L. G., Jones T. T., Jones D. R., Liebsch N., Booth D. T. 2011. Measuring energy expenditure in sub-adult and hatchling sea turtles via accelerometry. PLoS ONE 6, e22311. 10.1371/journal.pone.0022311 (doi:10.1371/journal.pone.0022311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartnoll R. G. 1967. An investigation of the movement of the scallop, Pecten maximus. Helgol Mar. Res. 15, 523–533 [Google Scholar]
- 53.Winter M. A., Hamilton P. V. 1985. Factors influencing swimming in bay scallops, Argopecten irradians (Lamarck, 1819). J. Exp. Mar. Biol. Ecol. 88, 227–242 10.1016/0022-0981(85)90232-1 (doi:10.1016/0022-0981(85)90232-1) [DOI] [Google Scholar]
- 54.Donovan D. A., Bingham B. L., From M., Fleisch A. F., Loomis E. S. 2003. Effects of barnacle encrustation on the swimming behaviour, energetics, morphometry, and drag coefficient of the scallop Chlamys hastata. J. Mar. Biol. Assoc. UK 83, 813–819 10.1017/S0025315403007847h (doi:10.1017/S0025315403007847h) [DOI] [Google Scholar]