Abstract
Vertebrate gap junctions are composed of proteins from the connexin family. Co-immunoprecipitation, in vitro binding and far western experiments demonstrate that mammalian CASK (also known as LIN2) directly interacts with Cx43. Immunoprecipitation studies indicate that the CASK mainly interacts with the hypophosphorylated form of Cx43. Functional co-regulation of these proteins was found in MDCK cells migrating into a scratch wound, where expression of either protein individually inhibits migration but their coexpression abrogates this inhibitory effect. Immunofluorescence shows colocalization of Cx43 and CASK in mouse brain astrocytes and in response to wounding in human foreskin. During wounding, CASK is mobilized to the plasma membrane where it colocalizes with Cx43 and CADM1 1 hour after skin explant wounding. Together, these studies indicate that CASK interaction with Cx43 occurs relatively early in the connexin life cycle and imply a plasma membrane targeting role for the interaction that apparently affects cellular processes including cellular migration and wound healing.
Key words: Gap junctions, Connexin, CASK (Lin-2)
Introduction
In vertebrates, connexin proteins assemble into tightly packed gap junctional channels to connect the cytoplasms of adjacent cells via an aqueous-filled channel. Gap junctions allow the passive diffusion of molecules lower than 1000 Da in size such as ions, amino acids, nutrients and secondary messengers (Laird, 2010; Scemes et al., 2007). Gap junctional intercellular communication has been shown to play important roles in tissue differentiation, transport, development, synchronous muscle contraction, homeostasis and conduction in excitable tissues (Goodenough and Paul, 2009). Studies utilizing transgenic mice with altered connexin genes and linkage of connexin gene alterations to human disease support their role in regulation of cell growth control and embryonic development (Dobrowolski and Willecke, 2009; Mese et al., 2007; Naus and Laird, 2010). Cx43, the most ubiquitous connexin, oligomerizes into a hexamer or “connexon” in the trans-Golgi network (TGN) (Musil and Goodenough, 1993) and, after transport to the plasma membrane and association with other connexons, can form a gap junction.
Zona Occudens-1 (ZO-1), a member of the membrane-associated guanylate kinase (MAGUK) family of proteins, interacts with Cx43 through one of its PSD95–Dlg–ZO-1 (PDZ) domain proteins (Giepmans and Moolenaar, 1998; Toyofuku et al., 1998). MAGUK proteins are highly conserved and characterized by the presence of multiple protein–protein interaction domains that have been shown to be important in localizing target membrane proteins to specialized membrane domains, maintaining cell polarity and acting as scaffolds. Another MAGUK, calcium/calmodulin-dependent serine kinase, CASK (also known as LIN2 and PALS) is a causative gene in X-linked mental retardation (Najm et al., 2008), and its deletion in mice is lethal (Atasoy et al., 2007). In renal epithelial cells, CASK exists as a complex with LIN7 (also known as VELI and MALS) (Straight et al., 2000), whereas in the brain CASK and LIN7 are complexed with LIN10 (also known as X11α and MINT1) (Borg et al., 1998; Butz et al., 1998). In Caenorhabditis elegans, Lin-2 is responsible for the basolateral targeting of the Let-23 growth factor receptor (homologous to the mammalian EGF receptor) in vulval precursor cells (Hoskins et al., 1996; Kaech et al., 1998). In mammalian epithelial and neuronal cells, the CASK protein scaffolding complex is involved in the assembly of junctional components (Borg et al., 1998; Butz et al., 1998; Craven and Bredt, 1998; Dimitratos et al., 1999; Kaech et al., 1998; Kamberov et al., 2000; Perego et al., 2000).
Here, we demonstrate that the C-terminus of Cx43 associates directly with the mammalian CASK protein in a non-PDZ-dependent interaction. Furthermore, the CASK protein is strongly immunoprecipitated by an antibody that preferentially interacts with the hypophosphorylated form of Cx43 compared to other phosphospecific and general Cx43 antibodies. Immunofluorescence studies in skin tissues show specific colocalization between Cx43, CASK and CADM1 [a protein that regulates epidermal adhesion and wound repair (Giangreco et al., 2009)] that varies in a temporally and spatially regulated manner during wound healing.
Results
Cx43 interacts directly with CASK and not with LIN7 protein
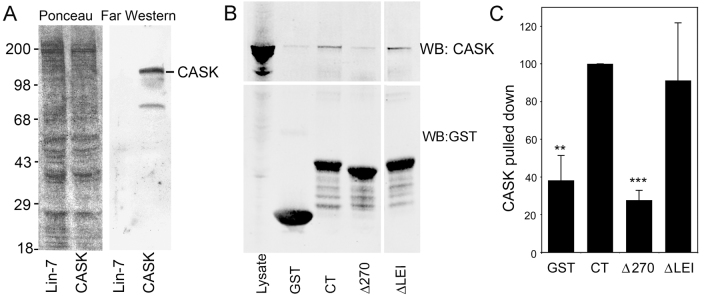
Multiple protein interaction domains and phosphorylation sites in the C terminal tail of Cx43 have been implicated in regulating Cx43 trafficking, assembly, gating and turnover. Previously, we applied an approach combining liquid chromatography with tandem mass spectrometry (LC-MS/MS) to identify Cx43 interacting proteins (Singh and Lampe, 2003). Briefly, the C-terminal tail of Cx43 (amino acid residues 246–382) fused to GST was incubated with whole cell lysates from normal rat kidney (NRK) cells. After washing, elution and trypsinization, peptides were analyzed by LC-MS/MS. Based on the MS data, we reported multiple possible Cx43 interacting partners including LIN7 (Singh and Lampe, 2003). Given that LIN7 exists as a hetero-trimeric complex with CASK, we performed a far western overlay assay to investigate whether Cx43 interacted directly with either protein. Cell lysates from 293T cells transfected with either pRK5-Myc-CASK (amino acids 1–897) or pRK5-Myc-LIN7 (amino acids 1–197) were run on a SDS-PAGE and then transferred to a nitrocellulose membrane. The membrane was blocked and then incubated with a 32P-labeled GST–Cx43CT (amino acids 236–382) probe. After washing, a band corresponding to CASK (approximately 120 kDa) but no LIN7 band was observed (Fig. 1A), suggesting that the direct interaction between GST–Cx43CT and CASK might have led to the LIN7 pulldown by linkage via CASK.
Fig. 1.
Interaction of Cx43 with LIN7 and CASK. (A) Lysates from 293T cells transfected with pRK5-MycCASK (CASK; amino acids 1-897) or pRK5-MycLin7 (LIN7; amino acids 1–197) were separated by SDS-PAGE and transferred to a nitrocellulose membrane (Ponceau S staining of the membrane is shown in the left panel). The membrane was then probed with a 32P-labeled GST–Cx43CT probe and exposed to an X-ray film (autoradiographic image is shown in the right panel). (B) GST–Cx43CT (CT), GST–Cx43CT missing residues 260–280 (Δ270) and GST–Cx43 missing residues 380–382 (ΔLEI) were incubated with NRK cell lysates. Pulled down proteins were analyzed via immunoblot with anti-GST and CASK antibodies. (C) The mean ratio of CASK signal to recombinant Cx43 for five experiments is shown in the bar graph (χ + s.d.). **P<0.01, ***P<0.001 compared with CT values.
Because PDZ domains recognize a three-residue peptide motif in the C-termini of their binding partners (Songyang et al., 1997) and the terminal Leu-Glu-Ile (LEI) residues of Cx43 are crucial for interaction with the PDZ-domain-containing proteins ZO-1 and ZO-2, we suspected that the interaction with CASK involves the C-terminal region of Cx43. Therefore, we utilized different GST–Cx43 fusion constructs including the entire cytoplasmic tail (CT) region (residues 236–382) of Cx43 and the same construct lacking residues 260–280 (Δ270) or lacking the terminal three amino acids (ΔLEI) in a GST pull-down assay. As shown in Fig. 1B, both CT and ΔLEI could effectively pull down CASK. Because these constructs eliminate the PDZ binding domain of Cx43, the CASK binding to Cx43 was probably not via the PDZ domain. However, the construct lacking residues 260–280 was significantly (P<0.01) less effective at pulling down CASK and was comparable to the GST-alone control level (Fig. 1C) indicating that this region, known to be important for Cx43 regulation of turnover and gating (Thomas et al., 2003; Warn-Cramer et al., 1996), is crucial for CASK binding.
CASK interaction shows a preference for hypophosphorylated Cx43
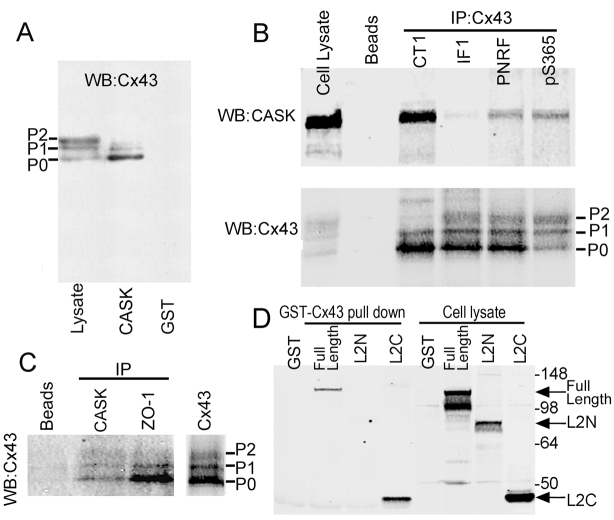
Further examination of the Cx43–CASK interaction involved incubation of GST–CASK with cell lysates from NRK cells in a pull-down assay. In SDS-PAGE, Cx43 separates into multiple bands typically indicated as P0, P1 and P2. GST–CASK indeed interacted with endogenous Cx43 in the cell lysates with a preference for hypophosphorylated Cx43, i.e. P0 (Fig. 2A). This result was confirmed in co-immunoprecipitation assays using several Cx43 antibodies with affinities for different phosphorylated isoforms of Cx43 (Fig. 2B). We observed that the CT1 antibody, which shows a strong preference for the hypophosphorylated isoform (Sosinsky et al., 2007), immunoprecipitated the most CASK, whereas IF1, an antibody that shows more preference for phosphorylated isoforms, precipitated 84% less (n=5, a representative example is shown). Similar results (albeit with fewer repetitions) were obtained with MDCK cells expressing Cx43 (see supplementary material Fig. S1). A polyclonal pan-Cx43 antibody (PNRF) and an antibody specific for Cx43 phosphorylated at Ser365 also immunoprecipitated less CASK than the CT1 antibody. We then performed the reverse immunoprecipitation experiments with CASK antibodies and probed for the presence of Cx43 in an immunoblot. The anti-CASK antibody and an antibody to ZO-1 (a known Cx43-interacting protein) could co-immunoprecipitate Cx43, with both proteins showing some preference for the P0 isoform (Fig. 2C). A control precipitation of lysate performed with protein A beads did not co-precipitate Cx43. To map the portion of CASK that interacts with Cx43, we utilized N- and C-terminal constructs of CASK representing residues 1–612 (L2N, containing intact kinase, L27 and PDZ domains), 578–897 (L2C, containing SH3 and GUK domains) and 1–897 (full length). Both the full length and L2C, C-terminal piece of CASK, could bind to Cx43 (Fig. 2D), but not L2N, indicating that the interaction is not through the PDZ domain but could involve the SH3 or GUK domains.
Fig. 2.
Non-phosphorylated Cx43 binds to CASK. (A) Cell lysates from NRK cells were incubated with either GST alone or GST CASK in a pull-down assay. Bound Cx43 was detected by immunoblotting with a Cx43 antibody. One tenth of the lysate used in the pull-down experiment was run as a whole cell control. (B) Cx43 antibodies including mouse anti-Cx43 antibodies CT1 and IF1 and rabbit antibodies to the C-terminal region of Cx43 (PNRF) and specific for Cx43 phosphorylated at Ser365 (pS365) were used for immunoprecipitation studies. The resulting immunoblot was cut and incubated with either a CASK antibody (upper panel) or a Cx43 antibody (lower panel). (C) Co-immunoprecipitation of Cx43 from NRK cells using antibodies to CASK, ZO-1 (positive control), Cx43 (positive control) or beads alone (negative control). (D) Different portions of CASK including full-length (residues 1–897), N-terminal (L2N; residues 1–612) and C-terminal (L2C; residues 578–897) were expressed in 293-T cell. Lysates were pulled down with GST–Cx43 or GST alone (negative control).
Immunolocalization of Cx43 and CASK in tissues
To investigate whether CASK could interact with Cx43 prior to cellular lysis in tissues, we examined co-immunolabeling in brain and skin using confocal microscopy. In Fig. 3B, CASK (green) and Cx43 (red) show specific colocalization in sections of mouse brain. DAPI and glial fibrillary acidic protein (GFAP) staining of the same field indicate higher Cx43 expression in areas of higher astrocyte cell density (Fig. 3A). CASK and Cx43 were found to colocalize in what appear to be cell bodies (arrowheads) and along astrocytic processes (emphasized in Fig. 3C).
Fig. 3.
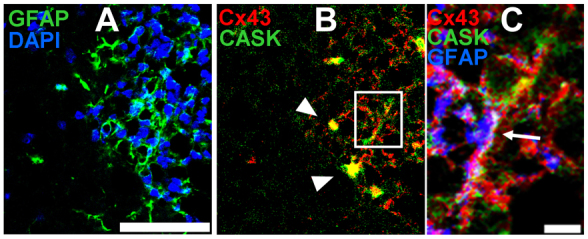
Confocal immunofluorescence staining of CASK and Cx43 in mouse brain tissue. (A) Single tissue section was immunostained with a GFAP (green) antibody and DAPI (blue). (B) Single tissue section was stained with CASK (green) and Cx43 antibodies (red), with colocalization indicated by arrowheads. (C) Higher magnification of the boxed region of B. Colocalization of Cx43, GFAP and CASK is shown in white (indicated by arrow). Scale bars: 50 μm (A,B) and 5 μm (C).
We next examined Cx43 and CASK localization during wounding in human foreskin explant tissue. Samples were examined in unwounded tissue and at 1 hour after epidermal wounding. The dashed line in Fig. 4 marks the basement membrane, indicating the boundary between dermis (below) and epidermis (above). In Fig. 4B, the arrowhead indicates the edge of the wound. The colored panels show overlay of Cx43 (red) and CASK (green) staining with nuclei indicated in blue. Note that in some cases (especially in Fig. 4A) the CASK antibody was bound nonspecifically to the upper, dead cornified layer of cells, which is commonly observed with some antibodies (Aho et al., 2004; Harper et al., 2005; Lampe et al., 1998). Extensive colocalization was observed 1 hour after wounding (Fig. 4B). Projections (positions indicated by white lines) of the x–z and y–z panels confirmed colocalization in all three dimensions. The grayscale images show immunostaining for the individual antibodies. In unwounded skin (Fig. 4A), both Cx43 and CASK are concentrated in the upper differentiating layers of the epidermis, though CASK appears to be localized to the cytoplasm whereas Cx43 is found around the boundaries of cells, in apparent gap junction plaques. This subcellular localization is most apparent in the grayscale images. Consistent with expression in these different compartments, we see very little colocalization of these proteins in unwounded skin but extensive Cx43–CASK colocalization at 1 hour (Fig. 4B). This indicates that CASK is mobilized from the cytoplasm to the plasma membrane at this time point. Previous reports have shown that Cx43 (Goliger and Paul, 1995; Lampe et al., 1998; Saitoh et al., 1997) and CASK (Ojeh et al., 2008) expression are lost near the wound edge as wound healing proceeds.
Fig. 4.
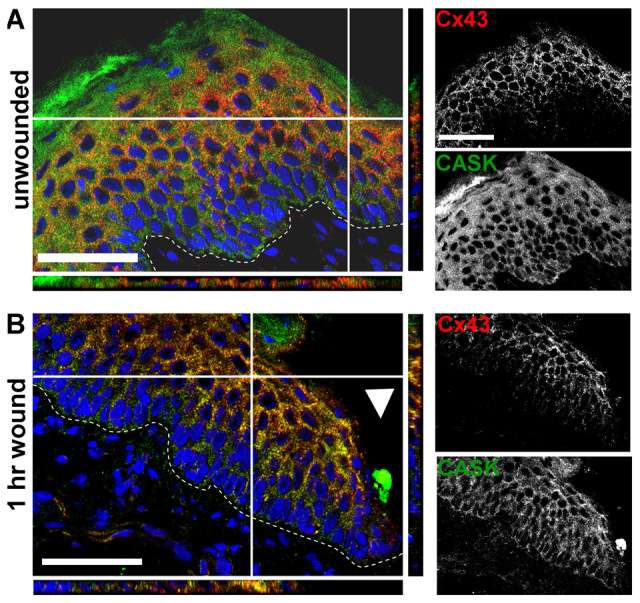
Human foreskin analyzed by immunostaining with antibodies to Cx43 and CASK. The x–y, x–z and y–z plane projections are shown in color and individual antibody staining is shown in grayscale. Cx43 staining is red and CASK staining is green. Nuclei were counterstained with DAPI (blue). Dashed line indicates basement membrane. Foreskins were wounded via punch biopsy and wound margin is indicated by white arrowhead. (A) Unwounded foreskin. (B) Skin at wound margin after 1 hour. Note that the CASK antibody, like many other antibodies, sticks nonspecifically to dead cells within the upper cornified layer, leading to the diffuse green staining. Scale bars: 50 μm.
Cx43 and CASK expression affect cell migration
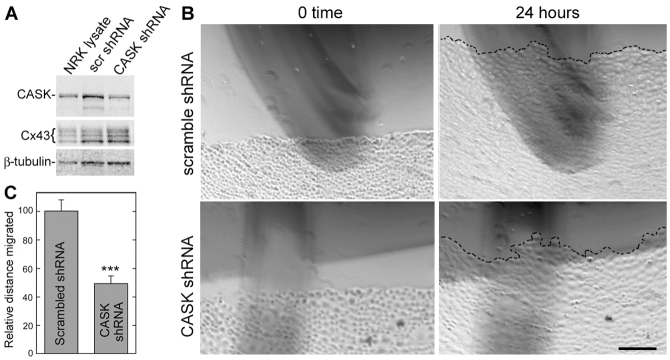
In order to further investigate the role that Cx43 and CASK expression play in migration, we exogenously expressed these proteins in MDCK cells that normally do not express Cx43 and only express low levels of CASK (Fig. 5A). We then examined their migration in a scratch wound assay (Fig. 5B). When CASK or Cx43 were expressed alone, the migration distance travelled in 24 hours was 50–60% less than in parental cells (Fig. 5C). However, coexpression of Cx43 and CASK resulted in a significant rescue of this inhibition (coexpression of Cx43 and CASK led to significantly more migration than either CASK or Cx43 expression alone; P<0.001). These results indicate that CASK and Cx43 functionally cooperate during migration, whereas expression of either protein alone actually inhibits migration.
Fig. 5.
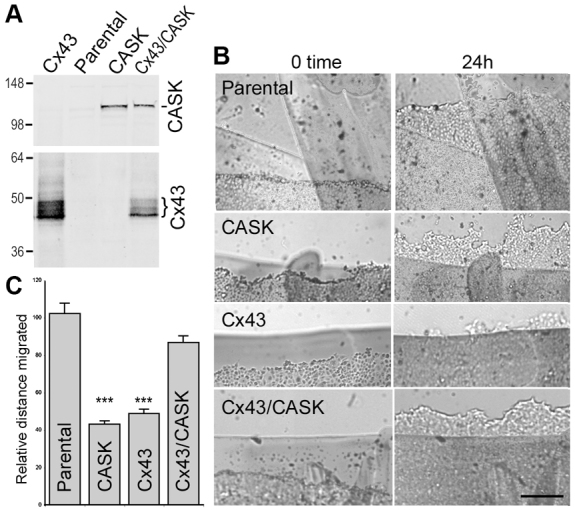
Migration of MDCK cells transfected with CASK and/or Cx43 in a scratch wound assay. (A) Overexpression of Cx43 and CASK in MDCK cells. (B) Examples of migrating cells (note that image is heavily contrasted and manipulated to emphasize the cell front and deemphasize the marks used for orientation and migration measurement). Scale bar: 100 μm. (C) The distance migrated in 24 hours was determined, quantified and the means and standard deviations determined. There was a significant difference between the migration of cells expressing CASK or Cx43 alone and the migration of parental cells or cells coexpressing Cx43 and CASK; ***P<0.001.
We next wanted to test whether CASK knockdown in cells that endogenously express significant levels affected migration after scratch wounding. NRK cells express readily detectable levels of CASK, and expression of short hairpin RNA (shRNA) to CASK reduced the level of the protein by 60–80% in different transduction experiments. Because the cells showing 80% knockdown did not retain this reduced level, we used the cells that maintained stable 60% knockdown in the experiments shown (Fig. 6A). Examples of NRK cells at 0 time and 24 hours after scratch wounding are shown in Fig. 6B. Measurement of the distance migrated in 24 hours showed approximately a 50% reduction in the presence of shRNA against CASK compared with the control (Fig. 6C). We also noticed that the migratory front of the shRNA-expressing cells appeared to be more irregular than in the control. We quantified this difference by measuring the length of a line drawn immediately after scratching and compared it with a similar line drawn after 24 hours of migration and found almost a twofold difference (control, 5.6±2.5; shRNA, 11.8±6.3, 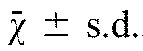 , P=0.015) potentially indicating a loss of migrational control when CASK expression was reduced.
, P=0.015) potentially indicating a loss of migrational control when CASK expression was reduced.
Fig. 6.
Knockdown of CASK reduces the extent and regularity of migration of scratch-wounded NRK cells. (A) shRNA knockdown of CASK was performed and the levels of CASK were reduced as shown in the western immunoblot. Cells treated with scrambled (scr) shRNA were used as control. (B) Examples of scratch-wounded cells immediately after scratch (0 time) and after 24 hours. The front of cells is marked with a dotted line. Scale bar: 100 μm. (C) Quantification of the difference in distance migrated. ***P<0.001.
Next, we were interested in determining how Cx43 and CASK levels and their coordinated function might be regulated. Because CADM1 has been shown to regulate CASK expression levels (Giangreco et al., 2009), we decided to investigate its colocalization in the Cx43- and CASK-expressing MDCK cells and found extensive colocalization in specific regions (apparent as white staining in the overlay in Fig. 7) at 6 hours after scratching (Fig. 7A) and at 1 hour after foreskin wounding (Fig. 7B) with less colocalization at the other time points studied (data not shown). Specifically, the MDCK cells at 6 hours after scratching showed colocalization of all three, or of two of the three proteins, at different sites in the plasma membrane, primarily 2–3 cell diameters from the edge of the scratch. Similarly, suprabasal cells relatively near the wound margin in foreskin showed extensive CADM1 and Cx43 overlap and frequent colocalization of all three proteins (Fig. 7). Increased magnification and line scan analysis of regions of apparent colocalization showed frequent co-incident increases and decreases in these proteins indicating ‘real’ overlay (Fig. 7C). Immunoprecipitation of Cx43 from MDCK cells expressing Cx43 and CASK showed both CASK and a band in a region reported for CADM1 when probed with their respective antibodies (see supplementary material Fig. S1).
Fig. 7.
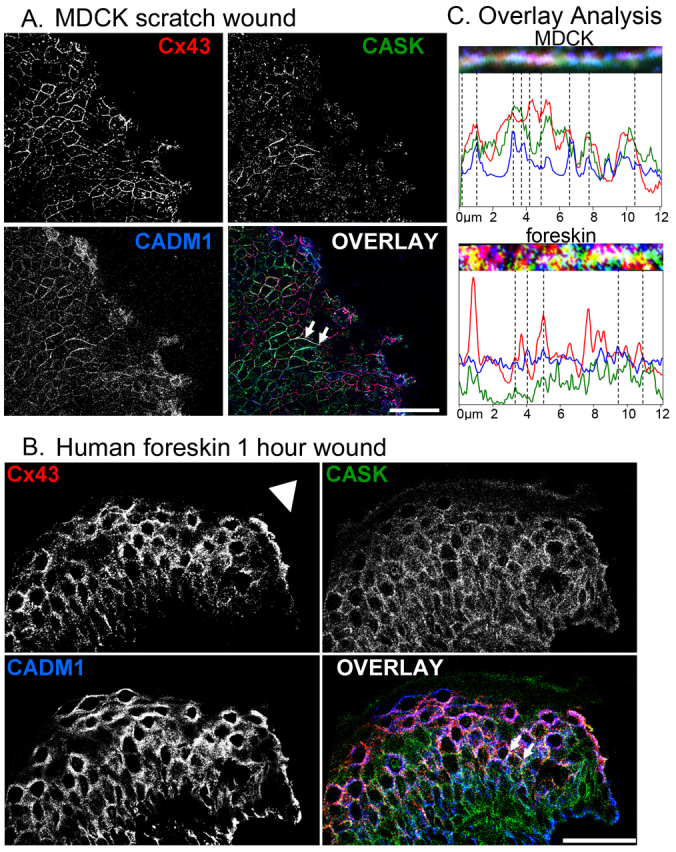
Cx43, CASK and CADM1 colocalize at specific times after wounding. (A) MDCK cells stained 6 hours after scratch wounding show different amounts of interaction between each of the three proteins, with the most interaction between the three approximately 50 μm from the wound edge. (B) Foreskin after 1 hour of wounding shows extensive co-labeling in the suprabasal region within 50–150 μm of the wound edge (indicated by arrowhead). (C) Regions of apparent colocalization (taken from A and B in the regions between the two arrows) and line scan analysis of these regions (2 pixel width, peaks indicating colocalization are shown by dashed lines) are presented with size in μm indicated on the x-axis. Scale bars: 50 μm.
Discussion
We have described five findings: (1) Cx43 and CASK interact directly in vitro and the colocalization can be detected in vivo (skin and brain). (2) CASK interacts with the C-terminal region of Cx43 independently of its PDZ domain. (3) Cx43 phosphorylation events that reduce the migration of Cx43 in SDS-PAGE appear to negatively regulate the interaction, i.e. the most extensive interaction occurs between the hypophosphorylated isoform of Cx43 (P0) and CASK. (4) Cx43 and CASK colocalize in brain and skin and the interaction is dynamic and coordinated during epidermal wounding. (5) Cx43, CADM1 and CASK appear to mechanistically cooperate in regulating migration in MDCK cells.
Both Cx43 and CASK have independently been shown to be important in brain and skin function. We decided to focus on skin because we could examine the effect of well-known Cx43 expression pattern changes in response to wounding (Di et al., 2001; Goliger and Paul, 1995; Lampe et al., 1998; Richard, 2000; Richards et al., 2004; Saitoh et al., 1997; Salomon et al., 1994). Mice with reduced levels of Cx43 heal wounds more rapidly (Kretz et al., 2003; Mori et al., 2006). Cx43 antisense application to wounds accelerated the rate of keratinocyte migration, reduced inflammation and increased the rate of wound repair, resulting in less scarring (Qiu et al., 2003). Ojeh and colleagues have also recently shown that CASK expression changes during wounding (Ojeh et al., 2008) and that the ‘migratory tongue’ of epithelial cells shows reduced CASK expression. Consistent with this observation, Giangreco and colleagues reported that overexpression of CADM1 (Necl2) leads to increased CASK expression and eliminates the reduction of CASK at the migratory tongue, causing a reduction in keratinocyte migration and delayed wound healing (Giangreco et al., 2009). Together these data indicate that CADM1 regulation of CASK levels could regulate cellular migration during wound healing, potentially through their action on Cx43.
Previous data from cultured primary keratinocytes (Richards et al., 2004) indicated that gap junctional communication dropped near the wound edge at 1 hour after wounding but that blockage of intercellular communication by carbenoxolone at the time of wounding inhibited subsequent cell migration. This indicates that gap junction communication is involved in migration initiation. Thus, the combination of these data and the data reported here indicate an important functional interaction between Cx43 and CASK in initiating migration in response to wounding. Given that it appears that reductions in both CASK and Cx43 are necessary for proper wound healing, we speculate that their removal is also coordinated and might involve CADM1.
Although it is not yet clear exactly how these interactions mediate their effects, the known functions of these proteins can give us clues. CASK is a MAGUK protein which, like other family members, exists as a complex with other proteins. It is part of an evolutionarily conserved tripartite complex on the membrane that is composed of modular adaptor proteins (CASK, LIN7, LIN10). Many MAGUK proteins are known to play key roles in the apical–basolateral targeting of proteins in polarized cells, including sorting in the trans-Golgi network and endocytic compartments. For instance, mutations in C. elegans Lin proteins result in the mislocalization of the Let-23 receptor (Budnik et al., 1996; Kaech et al., 1998), whereas mutations in the Drosophila Dlg (Discs large protein), another MAGUK protein, cause overgrowth of Drosophila imaginal discs and abnormal larval neuromuscular junctions (Budnik et al., 1996). We show here that CASK interacts preferentially with the P0 form of Cx43. Notably, the P0 form of Cx43 is found predominantly in cytoplasmic and nonjunctional plasma membranes, presumably en route to gap junction formation. Cx43 becomes increasingly phosphorylated and exhibits retarded migration in SDS-PAGE as it is incorporated into gap junction plaques. Our laboratory has shown that phosphorylation at serine residues 365, 325, 328 and 330 occur as Cx43 assembles into a gap junction, and that these events are responsible for most of the shift in the SDS-PAGE migration of Cx43 (Lampe et al., 2006; Solan et al., 2007). CASK localization is similarly dynamic, shifting from cytoplasmic to membrane localization during differentiation and to the nucleus upon entry into S phase of the cell cycle (Ojeh et al., 2008). The fact that CASK preferentially interacts with P0 Cx43 suggests that CASK is involved in the transport of non-phosphorylated Cx43 to the plasma membrane where phosphorylation and interaction with proteins involved in forming the gap junction plaque could supplant the interaction, resulting in a ‘hand-off’ of Cx43 to interacting proteins involved in gap junction assembly. Recently, CASK has been shown to have Mg2+-independent neurexin kinase activity that could conceivably be involved in Cx43 phosphorylation (Mukherjee et al., 2008). However, our very preliminary experiments did not show Cx43 phosphorylation in immunoprecipitated complexes of the two proteins (data not shown), but the phosphorylation could also be indirect. A model consistent with these results could involve an initial wound and CADM1-induced recruitment of CASK to the plasma membrane promoting Cx43 phosphorylation, loss of interaction with CASK and gap junction assembly. At later time points, a reduction in CADM1 expression or localization might cause a reduction in CASK and Cx43 phosphorylation, thereby reducing gap junction stability and resulting in cells that can more efficiently migrate and close wounds. Because gap junctional communication is necessary soon after wounding to initiate migration (Richards et al., 2004), it could play a role in reducing CADM1 levels and thus in triggering the loss of CASK and Cx43 observed at 24 hours. Alternatively, dissociation of interactions between CASK, CADM1 and/or Cx43 could trigger degradation and/or migration.
Cx43 and CASK also play important roles in mammalian cell development. Disruption of the CASK locus by transgenic insertion in mice results in cleft palate and death within 24 hours of birth (Laverty and Wilson, 1998). Similarly, Cx43 knockout is neonatally lethal due to conotruncal defects in the heart (Reaume et al., 1995). The crucial roles of these apparently co-regulated proteins during development and wound repair, their temporally and spatially regulated colocalization and their roles in the control of migration indicate both a physical and functional interaction between Cx43, CASK and CADM1 that is of significant biological importance.
Materials and Methods
Reagents, antibodies and cDNA constructs
All general chemicals, unless otherwise noted, were purchased from Fisher Scientific. Anti-Myc mouse monoclonal 9E10 (Cell Signaling Technology, Beverly, MA), rabbit anti-Cx43 (C6219; Sigma, St Louis, MO), rabbit anti-CADM1 (SynCAM, S4945, Sigma), rabbit anti-Cx43 [termed PNRF and obtained from A. Boynton (Hossain et al., 1998)], rabbit antibody against Ser365-phosphorylated Cx43 (Solan et al., 2007), mouse anti-Cx43 antibodies Cx43CT1 and Cx43IF1 raised against amino acids 360–382 (Cooper and Lampe, 2002; Sosinsky et al., 2007), Cx43NT1 raised against residues 1–20 of Cx43 (Solan et al., 2007), rabbit polyclonal anti-CASK (UM196) (Straight et al., 2000), anti-GFAP (Zymed/Invitrogen, Carlsbad, CA), anti-CASK (BD Biosciences, San Jose, CA), and rabbit anti-CASK (Invitrogen) antibodies were purchased or prepared as described in the associated reference.
Cx43CT (C-terminus of Cx43) encoding amino acids 236–382 of Cx43 was cloned in pGEX-2T or pGEX-2TK vector to generate GST–Cx43CT, Δ270 (i.e. missing amino acids 260–280), and LEI (missing amino acids 380–382). Essentially, all of the CASK constructs were created in the Margolis laboratory and have been previously described (Borg et al., 1999; Borg et al., 1998; Straight et al., 2001; Straight et al., 2000). All GST fusion proteins were expressed and purified as described earlier (Singh et al., 2005). Protein concentrations were determined by running GST fusion proteins on SDS-PAGE and comparing to known concentrations of bovine serum albumin (BSA). Approximately 5 μg of protein was used in each binding study.
Cell culture and transfections
NRK epithelial cells (NRK-E51; American Tissue Culture Collection, Manassas, VA), MDCK and 293-T cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Atlanta, GA), 100 units/ml penicillin G and 100 μg/ml streptomycin in a humidified chamber with 5% CO2 at 37°C. Cell lysates were harvested in cold RIPA lysis buffer [25 mM Tris-HCl, 100 mM NaCl, 10 mM EDTA, 50 mM NaF, 500 μM Na3VO4, 0.25% Triton X-100, 0.25% deoxycholate, 0.02% NaN3, 2 mM phenylmethylsulfonyl fluoride and 1× Complete Protease Inhibitors (Roche Applied Science, Indianapolis, IN) pH 7.2]. The lysate was centrifuged at 15,870 g for 10 minutes and pre-cleared with GST bound to glutathione beads or protein A for use in pull-down or immunoprecipitation experiments, respectively. 293-T and MDCK cells were grown to 80% confluence and transiently transfected using Fugene reagent (Roche Applied Science) according to the manufacturer's instructions. Transfected cells were harvested in RIPA precipitation assay buffer after 48 hours.
shRNA-based CASK knockdown
For CASK depletion, NRK cells were transduced with shRNA-expressing lentiviruses. The following shRNA-encoding pGIPZ vectors were obtained from OpenBiosystems: V2LMM_193314 (5′-TGCTGTTGACAGTGAGCGCGGGAGTATTACCTTCAAGATTTAGTGAAGCCACAGATGTAAATCTTGAAGGTAATACTCCCTTGCCTACTGCCTCGGA-3′), V2LHS_91774 (5′-TGCTGTTGACAGTGAGCGCGGTGACATCATCCAGATTATTTAGTGAAGCCACAGATGTAAATAATCTGGATGATGTCACCATGCCTACTGCCTCGGA-3′), and a scramble shRNA. Lentiviral particles were generated by standard procedures (Kappes et al., 2003). Briefly, 60% confluent 293FT cells were transfected with shRNA-encoding lentiviral pGIPZ vector, psPAX2 and MD2.G (4:2:1 ratio, respectively) using Lipofectamine 2000 accordingly to the manufacturer's specifications (Invitrogen). At 72 hours after transfection, supernatants were harvested and cleared by centrifugation at 1,200 g for 10 minutes. Viral preparations were concentrated by using PEG-it Virus Precipitation Solution according to the manufacturer's specifications (SBI, System Biosciences, Mountain View. CA). NRK cells were transduced with the concentrated viral supernatant (MOI ~1) in the growth media supplemented with 0.5 mg/ml polybrene for 16 hours at 31°C. At 48 hours post-transduction, puromycin (4 mg/ml) was added to the medium and cells incubated for 5 days. shRNA-expressing cells were maintained in growth medium with 1 mg/ml puromycin. Knockdown efficiency was estimated by western blot.
Pull-down, immunoprecipitation and in vitro binding assays
For GST pull-down assays, 5 μg of the individual GST fusion proteins bound to glutathione-agarose beads (Thermo Scientific, Rockford, IL) were incubated at 4°C for 1.5 hours with 400 μl of cell lysate (prepared as described above). For immunoprecipitation experiments, the antibodies were incubated with cell lysate for 1.5 hours followed by immunoprecipitation with protein A-Sepharose beads for 1 hour. The beads were washed extensively in PBS, and bound protein was eluted in Laemmli sample buffer followed by separation on SDS-PAGE (10%) and immunoblotting as previously described (Solan et al., 2007). For immunoblot detection, primary antibodies were visualized with fluorescent dye-labeled secondary antibodies. Alexa-Fluor-680-conjugated goat anti-rabbit (Invitrogen) and IRDye800-conjugated donkey anti-mouse IgG (Rockland Immunochemicals, Gilbertsville, PA) both extensively cross-reacted against other species. The immunoblots were analyzed using the Li-Cor Biosciences Odyssey infrared imaging system and associated software (inverted images are presented).
The far western assay has been described previously (Singh et al., 2005). Briefly, 10–20 μg of GST–Cx43CT fusion protein cloned in the pGEX-2TK vector was suspended in kinase buffer (50 mM potassium phosphate, pH 7.2, 10 mM MgCl2, 5 mM NaF, and 5 mM dithiothreitol) to which the catalytic subunit of protein kinase A (Sigma) and 50 μCi of [γ-32P]ATP (Blu002; PerkinElmer Life Sciences, Waltham, MA) were added for 30 minutes. After washing and elution of the labeled Cx43, it was incubated with a blot containing lysates from cells expressing CASK or LIN7. Ponceau S (Sigma) staining of the membrane was performed according to the manufacturer's directions.
Immunofluorescence and immunostaining
Mouse brain and neonatal human foreskin were frozen in OCT (VWR, West Chester, PA) and cut as 8-μm sections on a cryostat microtome. The sections were fixed in ice-cold acetone and methanol (1:1), washed twice in PBS and then incubated with 5% normal serum and 1% BSA in PBS for 1 hour at room temperature. Slices were then incubated with rabbit anti-Cx43 (1:200) and mouse anti-CASK monoclonal (1:200) in blocking solution for 1 hour. After four washes in PBS, the cells were incubated with Alexa-Fluor-488-conjugated goat anti-mouse and Alexa-Fluor-546-conjugated goat anti-rabbit secondary antibodies (Invitrogen) for 1 hour. For the triple labeling experiments, Cx43 was labeled with anti-mouse IgG-specific Alexa Fluor 546 and CADM1 with anti-rabbit IgG Alexa Fluor 633 (Invitrogen). The slides were washed in PBS and then labeled with anti-mouse IgG1-specific Alexa Fluor 488 (Invitrogen) to detect CASK. After washing, the slides were mounted in ProGold anti-fade medium (Invitrogen). Images were acquired using a Zeiss LSM 510 Confocal Laser Scanning microscope with a 40× oil objective. Z-planes analysis of images including linescan analysis utilized Metamorph 7.0 (Universal Imaging, Westchester, PA).
Wounding of human neonatal foreskin
Neonatal foreskin tissue was obtained from a local hospital under an approved Institutional Review Board protocol. Human foreskins were placed in serum-free keratinocyte growth media (KGM, Lonza, Walkersville, MD) for up to 24 hours. Wounds were created via punch biopsy then fixed immediately in 10% neutral buffered formalin or maintained in culture for 1 and 24 hours prior to fixation. The tissue was analyzed via immunofluorescence with antibodies to Cx43 and CASK as indicated and counterstained with DAPI.
In vitro scratch-wounding assay
Cells were cultured to confluence on 10-cm plates, and the medium was replaced with Opti-MEM I (Invitrogen) reduced serum medium 24 hours before scratching with a 200 μl pipette tip. Detached cells were removed, and the remaining cells were incubated for 0 and 24 hours. At least eight scratched areas for each sample were marked and photographed with a Nikon TE-400 inverted microscope immediately and after 24 hours of incubation. Migration was evaluated as previously described (Richards et al., 2004) by measuring the distance migrated at 24 hours compared to the position at 0 time. For measurements of migrating front regularity, the length of a line drawn along the front edge of the leading cells was compared to the length of a line drawn immediately after the scratch was performed, as previously described (Richards et al., 2004).
Supplementary Material
Acknowledgements
We wish to thank Ben Margolis and Samuel Straight, previously in the Margolis laboratory, for their advice and sharing of essentially all of the CASK reagents used in this study. This research was supported by grant GM55632 from the National Institutes of Health.
Footnotes
Funding
This research was supported by the National Institutes of Health [grant number GM55632]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.084400/-/DC1
References
- Aho S., Li K., Ryoo Y., McGee C., Ishida-Yamamoto A., Uitto J., Klement J. F. (2004). Periplakin gene targeting reveals a constituent of the cornified cell envelope dispensable for normal mouse development. Mol. Cell. Biol. 24, 6410-6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D., Schoch S., Ho A., Nadasy K. A., Liu X., Zhang W., Mukherjee K., Nosyreva E. D., Fernandez-Chacon R., Missler M., et al. (2007). Deletion of CASK in mice is lethal and impairs synaptic function. Proc. Natl. Acad. Sci. USA 104, 2525-2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg J. P., Straight S. W., Kaech S. M., de Taddeo-Borg M., Kroon D. E., Karnak D., Turner R. S., Kim S. K., Margolis B. (1998). Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J. Biol. Chem. 273, 31633-31636 [DOI] [PubMed] [Google Scholar]
- Borg J. P., Lopez-Figueroa M. O., de Taddeo-Borg M., Kroon D. E., Turner R. S., Watson S. J., Margolis B. (1999). Molecular analysis of the X11-mLin-2/CASK complex in brain. J. Neurosci. 19, 1307-1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V., Koh Y. H., Guan B., Hartmann B., Hough C., Woods D., Gorczyca M. (1996). Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron 17, 627-640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz S., Okamoto M., Sudhof T. C. (1998). A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell 94, 773-782 [DOI] [PubMed] [Google Scholar]
- Cooper C. D., Lampe P. D. (2002). Casein kinase 1 regulates connexin43 gap junction assembly. J. Biol. Chem. 277, 44962-44968 [DOI] [PubMed] [Google Scholar]
- Craven S. E., Bredt D. S. (1998). PDZ proteins organize synaptic signaling pathways. Cell 93, 495-498 [DOI] [PubMed] [Google Scholar]
- Di W. L, Rugg E. L, Leigh I. M, Kelsell D. P. (2001). Multiple epidermal connexins are expressed in different keratinocyte subpopulations including connexin 31. J. Invest. Dermatol. 117, 958-964 [DOI] [PubMed] [Google Scholar]
- Dimitratos S. D., Woods D. F., Stathakis D. G., Bryant P. J. (1999). Signaling pathways are focused at specialized regions of the plasma membrane by scaffolding proteins of the MAGUK family. Bioessays 21, 912-921 [DOI] [PubMed] [Google Scholar]
- Dobrowolski R., Willecke K. (2009). Connexin-caused genetic diseases and corresponding mouse models. Antioxid. Redox. Signal. 11, 283-295 [DOI] [PubMed] [Google Scholar]
- Giangreco A., Jensen K. B., Takai Y., Miyoshi J., Watt F. M. (2009). Necl2 regulates epidermal adhesion and wound repair. Development 136, 3505-3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giepmans B. N., Moolenaar W. H. (1998). The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr. Biol. 8, 931-934 [DOI] [PubMed] [Google Scholar]
- Goliger J. A., Paul D. L. (1995). Wounding alters epidermal connexin expression and gap junction-mediated intercellular communication. Mol. Biol. Cell 6, 1491-1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A., Paul D. L. (2009). Gap junctions. Cold Spring Harb. Perspect Biol. 1, a002576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper E. G., Alvares S. M., Carter W. G. (2005). Wounding activates p38 map kinase and activation transcription factor 3 in leading keratinocytes. J. Cell Sci. 118, 3471-3485 [DOI] [PubMed] [Google Scholar]
- Hoskins R., Hajnal A. F., Harp S. A., Kim S. K. (1996). The C. elegans vulval induction gene lin-2 encodes a member of the MAGUK family of cell junction proteins. Development 122, 97-111 [DOI] [PubMed] [Google Scholar]
- Hossain M. Z., Ao P., Boynton A. L. (1998). Rapid disruption of gap junctional communication and phosphorylation of connexin43 by platelet-derived growth factor in T51B rat liver epithelial cells expressing platelet-derived growth factor receptor. J. Cell. Physiol. 174, 66-77 [DOI] [PubMed] [Google Scholar]
- Kaech S. M., Whitfield C. W., Kim S. K. (1998). The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell 94, 761-771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamberov E., Makarova O., Roh M., Liu A., Karnak D., Straight S., Margolis B. (2000). Molecular cloning and characterization of Pals, proteins associated with mLin-7. J. Biol. Chem. 275, 11425-11431 [DOI] [PubMed] [Google Scholar]
- Kappes J. C., Wu X., Wakefield J. K. (2003). Production of trans-lentiviral vector with predictable safety. Methods Mol. Med. 76, 449-465 [DOI] [PubMed] [Google Scholar]
- Kretz M., Euwens C., Hombach S., Eckardt D., Teubner B., Traub O., Willecke K., Ott T. (2003). Altered connexin expression and wound healing in the epidermis of connexin-deficient mice. J. Cell Sci. 116, 3443-3452 [DOI] [PubMed] [Google Scholar]
- Laird D. W. (2010). The gap junction proteome and its relationship to disease. Trends Cell Biol. 20, 92-101 [DOI] [PubMed] [Google Scholar]
- Lampe P. D., Cooper C. D., King T. J., Burt J. M. (2006). Analysis of Connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. J. Cell Sci. 119, 3435-3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe P. D., Nguyen B. P., Gil S., Usui M., Olerud J., Takada Y., Carter W. G. (1998). Cellular interaction of integrin α3β1 with laminin 5 promotes gap junctional communication. J. Cell Biol. 143, 1735-1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty H. G., Wilson J. B. (1998). Murine CASK is disrupted in a sex-linked cleft palate mouse mutant. Genomics 53, 29-41 [DOI] [PubMed] [Google Scholar]
- Mese G., Richard G., White T. W. (2007). Gap junctions: basic structure and function. J. Invest. Dermatol. 127, 2516-2524 [DOI] [PubMed] [Google Scholar]
- Mori R., Power K. T., Wang C. M., Martin P., Becker D. L. (2006). Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J. Cell Sci. 119, 5193-5203 [DOI] [PubMed] [Google Scholar]
- Mukherjee K., Sharma M., Urlaub H., Bourenkov G. P., Jahn R., Sudhof T. C., Wahl M. C. (2008). CASK Functions as a Mg2+-independent neurexin kinase. Cell 133, 328-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil L. S., Goodenough D. A. (1993). Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell 74, 1065-1077 [DOI] [PubMed] [Google Scholar]
- Najm J., Horn D., Wimplinger I., Golden J. A., Chizhikov V. V., Sudi J., Christian S. L., Ullmann R., Kuechler A., Haas C. A., et al. (2008). Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat. Genet. 40, 1065-1067 [DOI] [PubMed] [Google Scholar]
- Naus C. C., Laird D. W. (2010). Implications and challenges of connexin connections to cancer. Nat. Rev. Cancer 10, 435-441 [DOI] [PubMed] [Google Scholar]
- Ojeh N., Pekovic V., Jahoda C., Maatta A. (2008). The MAGUK-family protein CASK is targeted to nuclei of the basal epidermis and controls keratinocyte proliferation. J. Cell Sci. 121, 2705-2717 [DOI] [PubMed] [Google Scholar]
- Perego C., Vanoni C., Massari S., Longhi R., Pietrini G. (2000). Mammalian LIN-7 PDZ proteins associate with beta-catenin at the cell-cell junctions of epithelia and neurons. EMBO J. 19, 3978-3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C., Coutinho P., Frank S., Franke S., Law L. Y., Martin P., Green C. R., Becker D. L. (2003). Targeting connexin43 expression accelerates the rate of wound repair. Curr. Biol. 13, 1697-1703 [DOI] [PubMed] [Google Scholar]
- Reaume A. G., de Sousa P. A., Kulkarni S., Langille B. L., Zhu D., Davies T. C., Juneja S. C., Kidder G. M., Rossant J. (1995). Cardiac malformation in neonatal mice lacking connexin43. Science 267, 1831-1834 [DOI] [PubMed] [Google Scholar]
- Richard G. (2000). Connexins: a connection with the skin. Exp. Dermatol. 9, 77-96 [DOI] [PubMed] [Google Scholar]
- Richards T. S., Dunn C. A., Carter W. G., Usui M. L., Olerud J. E., Lampe P. D. (2004). Protein kinase C spatially and temporally regulates gap junctional communication during human wound repair via phosphorylation of connexin43 on serine368. J. Cell Biol. 167, 555-562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M., Oyamada M., Oyamada Y., Kaku T., Mori M. (1997). Changes in the expression of gap junction proteins (connexins) in hamster tongue epithelium during would healing and carcinogenesis. Carcinogenesis 18, 1319-1328 [DOI] [PubMed] [Google Scholar]
- Salomon D., Masgrau E., Vischer S., Ullrich S., Dupont E., Sappino P., Saurat J.-H., Meda P. (1994). Topography of mammalian connexins in human skin. J. Invest. Dermatol. 103, 240-247 [DOI] [PubMed] [Google Scholar]
- Scemes E., Suadicani S. O., Dahl G., Spray D. C. (2007). Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 3, 199-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Lampe P. D. (2003). Identification of connexin-43 interacting proteins. Cell Commun. Adhes. 10, 215-220 [DOI] [PubMed] [Google Scholar]
- Singh D., Solan J. L., Taffet S. M., Javier R., Lampe P. D. (2005). Connexin 43 interacts with zona occludens-1 and -2 proteins in a cell cycle stage-specific manner. J. Biol. Chem. 280, 30416-30421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan J. L., Marquez-Rosado L., Sorgen P. L., Thornton P. J., Gafken P. R., Lampe P. D. (2007). Phosphorylation of Cx43 at S365 is a gatekeeper event that changes the structure of Cx43 and prevents downregulation by PKC. J. Cell Biol. 179, 1301-1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z., Fanning A. S., Fu C., Xu J., Marfatia S. M., Chishti A. H., Crompton A., Chan A. C., Anderson J. M., Cantley L. C. (1997). Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science 275, 73-77 [DOI] [PubMed] [Google Scholar]
- Sosinsky G. E., Solan J. L., Gaietta G. M., Ngan L., Lee G. J., Mackey M. R., Lampe P. D. (2007). The C-terminus of Connexin43 adopts different conformations in the golgi and gap junction as detected with structure specific antibodies. Biochem. J. 408, 375-385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight S. W., Karnak D., Borg J. P., Kamberov E., Dare H., Margolis B., Wade J. B. (2000). mLin-7 is localized to the basolateral surface of renal epithelia via its NH(2) terminus. Am. J. Physiol. Renal Physiol. 278, F464-F475 [DOI] [PubMed] [Google Scholar]
- Straight S. W., Chen L., Karnak D., Margolis B. (2001). Interaction with mLin-7 alters the targeting of endocytosed transmembrane proteins in mammalian epithelial cells. Mol. Biol. Cell 12, 1329-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. A., Zosso N., Scerri I., Demaurex N., Chanson M., Staub O. (2003). A tyrosine-based sorting signal is involved in connexin43 stability and gap junction turnover. J. Cell Sci. 116, 2213-2222 [DOI] [PubMed] [Google Scholar]
- Toyofuku T., Yabuki M., Otsu K., Kuzuya T., Hori M., Tada M. (1998). Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J. Biol. Chem. 273, 12725-12731 [DOI] [PubMed] [Google Scholar]
- Warn-Cramer B. J., Lampe P. D., Kurata W. E., Kanemitsu M. Y., Loo L. W. M., Eckhart W., Lau A. F. (1996). Characterization of the MAP kinase phosphorylation sites on the connexin43 gap junction protein. J. Biol. Chem. 271, 3779-3786 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.