Abstract
A defining feature of malignant tumor progression is cellular penetration through the basement membrane and interstitial matrices that separate various cellular compartments. Accumulating evidence supports the notion that invasive cells employ specialized structures termed invadopodia to breach these structural barriers. Invadopodia are actin-based, lipid-raft-enriched membrane protrusions containing membrane-type-1 matrix metalloproteinase (MT1-MMP; also known as matrix metalloproteinase 14; MMP14) and several signaling proteins. CD147 (emmprin, basigin), an immunoglobulin superfamily protein that is associated with tumor invasion and metastasis, induces the synthesis of various matrix metalloproteinases in many systems. In this study we show that upregulation of CD147 is sufficient to induce MT1-MMP expression, invasiveness and formation of invadopodia-like structures in non-transformed, non-invasive, breast epithelial cells. We also demonstrate that CD147 and MT1-MMP are in close proximity within these invadopodia-like structures and co-fractionate in membrane compartments with the properties of lipid rafts. Moreover, manipulation of CD147 levels in invasive breast carcinoma cells causes corresponding changes in MT1-MMP expression, invasiveness and invadopodia formation and activity. These findings indicate that CD147 regulates invadopodia formation and activity, probably through assembly of MT1-MMP-containing complexes within lipid-raft domains of the invadopodia.
Key words: CD147, Invadopodia, MT1-MMP (MMP14), Invasion, Breast cancer
Introduction
Focal penetration through the basement membrane is a defining event in the transition from a dysplastic carcinoma in situ to an invasive and malignant cancer. Invasive cancers probably utilize various mechanisms to breach the surrounding extracellular matrix (ECM) scaffolds that segregate different tissue compartments (Friedl and Wolf, 2003). Cellular protrusions directly abutting and penetrating the ECM have been observed in many cancers as invasion progresses (Rowe and Weiss, 2008) and very similar, ventrally located, membrane superstructures called invadopodia are seen when invasive cells are cultured on two-dimensional substrata (Artym et al., 2006). A recent study demonstrated a direct role for invadopodia in promotion of metastasis (Eckert et al., 2011). Invadopodia are composed of actin filaments and modulators of actin polymerization, including cortactin (Artym et al., 2006; Clark et al., 2007), and are enriched in kinases, cell-adhesion molecules and matrix metalloproteinases (MMPs), notably membrane-type 1 MMP (MT1-MMP; also known as matrix metalloproteinase 14; MMP14), a crucial enzyme for basement membrane and interstitial matrix degradation (Frittoli et al., 2010; Poincloux et al., 2009). A coordinated step-wise model for invadopodia formation has been described, in which cortactin–actin puncta form initially at sites of matrix attachment, followed by delivery and accumulation of MT1-MMP at the invadopodia–ECM interface (Artym et al., 2006; Oser et al., 2009; Schoumacher et al., 2010). Importantly, many studies have demonstrated that cancer cells primarily employ MT1-MMP targeted to invadopodia to degrade underlying ECM, which is orchestrated by a number of diverse proteins that integrate various signals to induce focal MT1-MMP localization and matrix degradation (Artym et al., 2006; Clark et al., 2007; Frittoli et al., 2010; Liu et al., 2009; Murphy and Courtneidge, 2011). Interestingly, a recent study found that MT1-MMP and other major invadopodia constituents localize in detergent-resistant membrane fractions characteristic of lipid raft domains (Yamaguchi et al., 2009). Thus, an emerging model describes invadopodia as dynamic cellular depots where diverse cell signaling inputs, vesicular trafficking and protease activity converge in spatially defined membrane compartments at the leading edge of an invasive cancer cell.
CD147 (also known as emmprin and basigin) is a single pass integral membrane protein, belonging to the Ig superfamily, that is variably glycosylated across different tissue types. It is usually expressed at low levels in most normal tissues, but is highly upregulated during dynamic cellular events, such as tissue remodeling and cancer progression (Gabison et al., 2009; Nabeshima et al., 2006; Yan et al., 2005). Emmprin was originally identified as a cell surface factor that induces MMP production in stromal cells (Ellis et al., 1989; Kataoka et al., 1993), but later studies revealed that emmprin is identical to basigin-2 (Biswas et al., 1995; Liao et al., 2011), and is multifunctional; it is now widely known as CD147. CD147 induces several MMPs at both the mRNA and protein level, including MMP1, MMP2, MMP3, MMP9, MT1-MMP and MT2-MMP, although there is variation in the particular MMPs induced in different systems (Nabeshima et al., 2006; Yan et al., 2005). Furthermore, CD147 has been shown to induce MMPs in tumor cells themselves as well as in stromal cells (Caudroy et al., 2002; Sun and Hemler, 2001). CD147 is a predominant marker in breast cancer micrometastases (Klein et al., 2002; Reimers et al., 2004) and is enriched in malignant pleural effusions and circulating tumor cells (Davidson et al., 2004; Pituch-Noworolska et al., 2007), suggesting a prominent role for CD147 in tumor progression. The role of CD147 in invasion has been demonstrated in several model systems, including a study in vivo in which CD147-transfected breast cancer cells injected into mammary fat pads of nude mice were found to be more locally invasive than control-transfected cells and in several animals metastasized to the liver, spleen, mediastinum, lymph nodes and mesentery (Zucker et al., 2001).
In this study we show that upregulation of CD147 is sufficient to induce many features of invadopodia in non-transformed breast epithelial cells and that manipulation of CD147 levels in invasive breast carcinoma cells causes corresponding changes in the formation and activity of invadopodia.
Results
Upregulation of CD147 is sufficient for induction of invasiveness in non-transformed breast epithelial cells
We first evaluated endogenous CD147 protein levels in non-transformed MCF-10A cells, a spontaneously immortalized, phenotypically normal, human breast epithelial cell line, and the resulting expression of CD147 after treatment of these cells with recombinant CD147 adenovirus or β-galactosidase (β-gal) adenovirus (as control). We found that a multiplicity of infection (MOI) of 2 was effective in upregulating CD147 protein expression to a moderate degree (Fig. 1A), within the range observed previously in invasive cancer cells; an MOI of 2 was used for the following experiments. It should be noted, however, that the ~38 kDa form of CD147 was increased to a greater extent than the ~52 kDa form, as observed previously when using recombinant CD147 adenovirus (Li et al., 2001). The 38 kDa and 52 kDa forms of CD147 have been shown previously to differ in their degrees of glycosylation of the same ~27 kDa protein core (Li et al., 2001; Tang et al., 2004a).
Fig. 1.
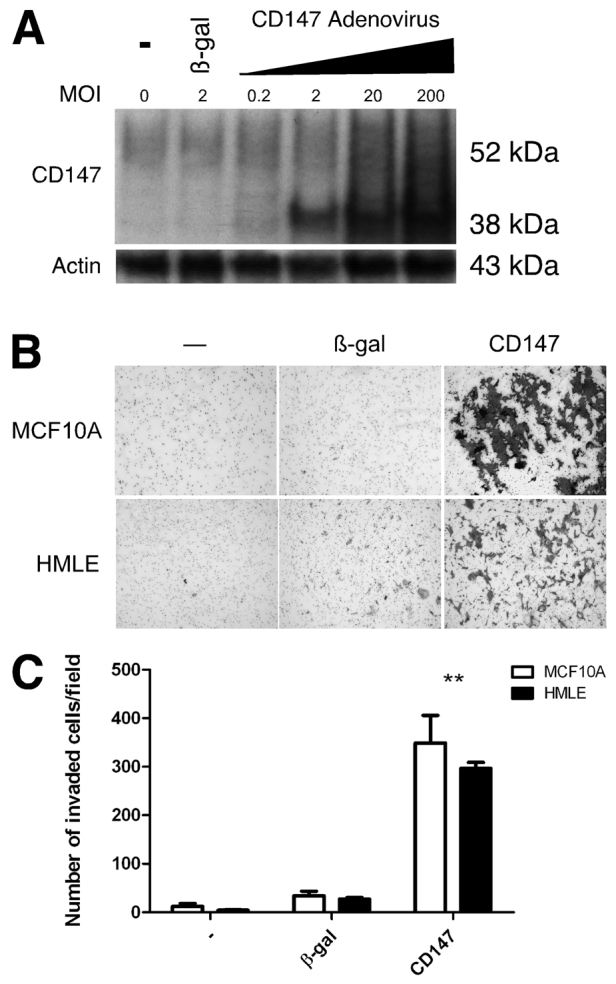
Upregulation of CD147 in non-transformed breast epithelial cells increases invasion. (A) Western blot showing endogenous CD147 protein expression in MCF-10A cells and increased CD147 expression after upregulation with increasing MOI of CD147 adenovirus. An MOI of 2 was used in all subsequent experiments. (B) MCF-10A or HMLE cells that were untreated or infected with β-gal or CD147 adenovirus were plated on Matrigel-coated invasion chambers and analyzed for invasion. (C) Quantification of invasion; the mean number of cells (± s.e.m.) invaded per field in three independent experiments. **P≤0.01.
We then evaluated the effect of increased CD147 on invasion of Matrigel, a reconstituted basement membrane. We found that the β-gal-treated MCF-10A cells were non-invasive whereas increased CD147 expression induced their ability to invade (Fig. 1B,C). To ensure that this effect was not restricted to MCF-10A cells, we examined the effect of increased CD147 on invasiveness in HMLE cells, another immortalized, non-transformed, human breast epithelial cell line; these cells also express low endogenous levels of CD147 (not shown). In similar fashion to the MCF-10A cells, upregulation of CD147 induced invasion by these cells as compared with β-gal-adenovirus-treated cells (Fig. 1B,C). These results suggest that upregulating CD147 is sufficient to induce an invasive cellular phenotype in non-transformed breast epithelia.
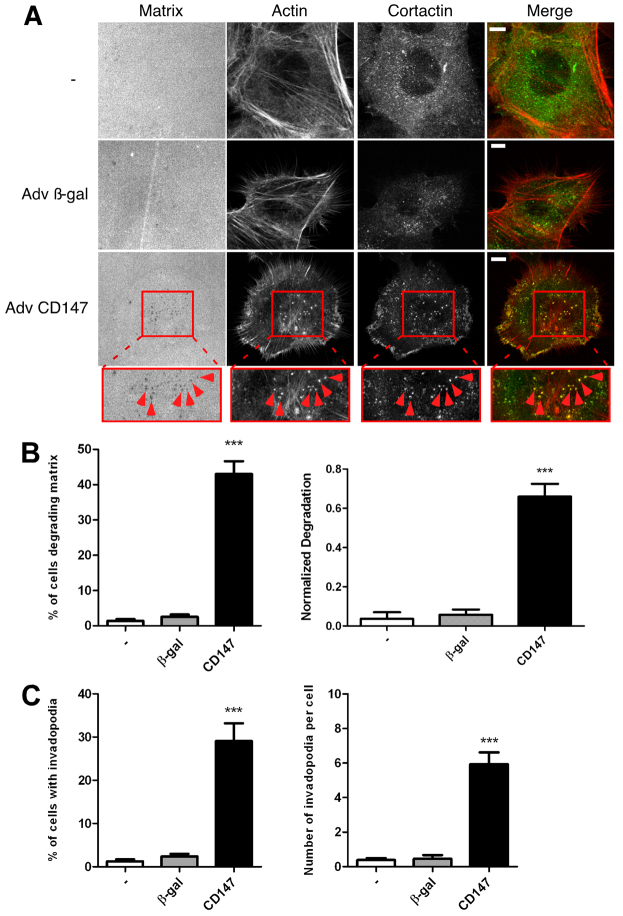
Upregulation of CD147 is sufficient for induction of matrix-degrading invadopodia-like structures in non-transformed breast epithelial cells
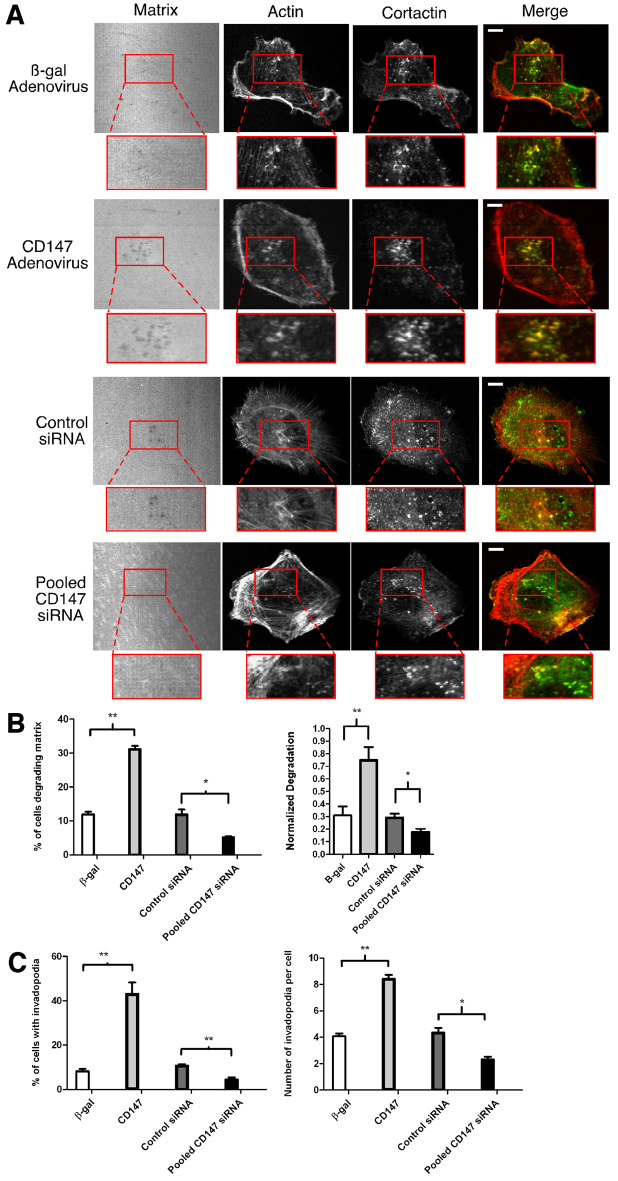
Parental MCF-10A cells normally do not form invadopodia (Sung et al., 2009). Thus we next investigated whether the CD147-induced increase in invasion is associated with invadopodia formation and activity. Initially, we found that untreated and control β-gal-virus-treated cells cultured on thin fluorescent matrices were unable to degrade the underlying matrix, whereas CD147-adenovirus-treated cells degraded the matrix significantly; moreover, the areas of degradation appeared as discrete spots in the matrix (Fig. 2A, left panels). To evaluate whether this matrix degradation was due to invadopodia activity, we probed the cells for cortactin and actin; we observed that only in cells with increased CD147 was there substantial colocalization of cortactin and actin in a punctate manner (Fig. 2A), which is indicative of invadopodia formation. CD147 upregulation increased the percentage of cells degrading matrix to approximately 40%, compared with less than 5% in the control groups (Fig. 2B, left panel); the areas of matrix degradation corresponded to areas of actin–cortactin colocalization in ~30% of cells examined (Fig. 2C, left panel), indicating induction of active invadopodia by CD147 in these cells. We further evaluated the degradation areas and number of invadopodia present in each of the cell groups and found that CD147 upregulation resulted in a greater area of matrix degradation per total cell area compared with parental and β-gal-treated cells (Fig. 2B, right panel). Moreover, whereas the parental and β-gal-treated cells had essentially no invadopodia, cells with increased CD147 expression had an average of approximately six invadopodia per cell (Fig. 2C, right panel).
Fig. 2.
CD147 induces the formation of matrix-degrading invadopodia-like structures in non-transformed breast epithelial cells. MCF-10A cells, non-infected or infected with β-gal or CD147 adenovirus, were cultured on a fluorescent gelatin matrix for 15 hours. (A) Representative micrographs of non-infected, β-gal- and CD147-adenovirus-infected cells cultured on fluorescent matrix. After fixation, cells were immunolabeled for cortactin (primary 4F11 antibody followed by secondary Alexa-Fluor-488 antibody) and Alexa-Fluor-647–phalloidin. Actin and the gelatin matrix were pseudo-colored red and blue, respectively allowing easier visualization of colocalization (yellow) of actin (red) and cortactin (green). Invadopodia-mediated matrix degradation appears as dark black foci in the bright fluorescent matrix field. The boxed regions are shown at higher magnification below. These are examples of invadopodia (arrowheads), identified by colocalization of cortactin and actin punctae over areas of degraded matrix. Scale bars: 10 μm. (B,C) Quantification of invadopodia characteristics. (B) Left panel: percentage of cells degrading the matrix. Right panel: normalized degradation, calculated as area of degradation divided by the total cell area defined by the actin channel. (C) Left panel: percentage of cells with active invadopodia, defined as cortactin–actin aggregates over degraded matrix. Right panel: number of invadopodia per cell. Each parameter was calculated by evaluating 10 random fields containing at least 15 cells per field over three independent experiments. Values are means ± s.e.m. ***P≤0.001.
In order to visualize the induction of invadopodia more clearly, we compiled a volumetric three-dimensional (3D) reconstruction of serial confocal images of MCF-10A cells treated with CD147 adenovirus, using Amira 3D image software. Fig. 3A depicts the 3D environment of a single cell, where the fluorescent matrix appears red, the actin cytoskeleton is blue and cortactin is labeled in green. Approximately six invadopodia can be seen penetrating through to the bottom of the matrix (Fig. 3B) and a cross section through the cell–matrix interface demonstrates colocalization of actin and cortactin in these protrusions (Fig. 3C); videos showing protrusion of several of these invadopodia-like structures through the underlying matrix can be seen in supplementary material Movies 1 and 2.
Fig. 3.
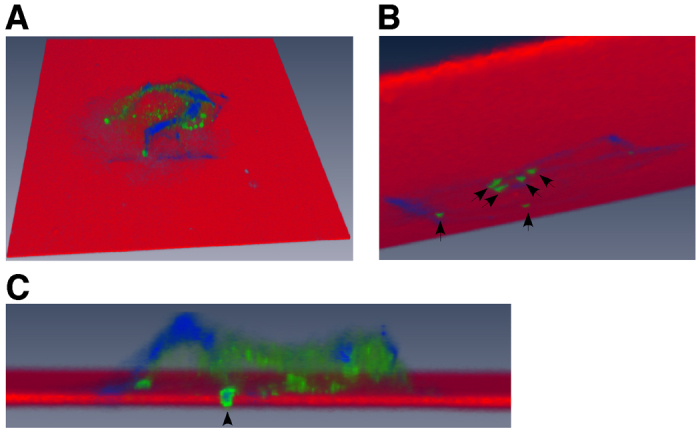
Three-dimensional volumetric reconstruction of CD147-induced invadopodia-like structures penetrating the underlying matrix. CD147-adenovirus-infected MCF-10A cells cultured on a fluorescent gelatin matrix were analyzed using confocal microscopy. Leica SP5 confocal software was employed to obtain an optimal image stack from single cell images and was further processed with Amira software to reconstruct a 3D volumetric image of a cell producing invadopodia-like protrusions that are penetrating the underlying fluorescent matrix. Cells were stained with Alexa-Fluor-647–phalloidin (blue) to image actin filaments and cortactin (green) to identify invadopodia; the fluorescent matrix is depicted in red. (A) Overhead view of a single cell cultured on fluorescent matrix. (B) View underneath the fluorescent matrix showing approximately six invadopodia (arrows) penetrating the matrix. (C) Magnified cross-section through the cell demonstrating one of the invadopodia-like protrusions (actin and cortactin positive; arrowhead) penetrating the fluorescent matrix. See supplementary material Movies 1 and 2.
These observations indicate that CD147 plays an important role in the induction of invadopodia formation and activity.
CD147-induced, invadopodia-mediated, matrix degradation is dependent on membrane-type MMPs
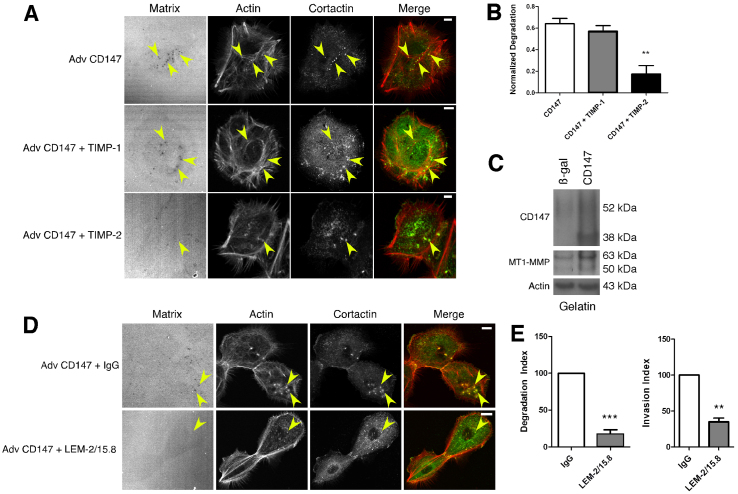
Although many MMPs effectively degrade components of the ECM and mediate invasiveness in vitro, several studies have shown that invadopodia utilize membrane-type MMPs, particularly MT1-MMP, to degrade the ECM (Artym et al., 2006; Frittoli et al., 2010; Liu et al., 2009; Poincloux et al., 2009; Rowe and Weiss, 2008). Because CD147 induces both soluble and membrane-type MMPs in various systems (Nabeshima et al., 2006; Yan et al., 2005), we employed the MMP inhibitors, TIMP-1 and TIMP-2, to distinguish which class of MMP was responsible for CD147-induced, invadopodia-mediated matrix degradation. TIMP-1 selectively inhibits the activity of soluble MMPs, whereas TIMP-2 is an inhibitor of both soluble and membrane-bound MMPs (Hotary et al., 2006). As expected, TIMP-2 inhibited most CD147-induced matrix degradation whereas TIMP-1 did not (Fig. 4A,B). These results show that cells expressing increased levels of CD147 utilize MT-MMPs to facilitate invadopodia-mediated matrix degradation.
Fig. 4.
CD147-induced, invadopodia-mediated, matrix degradation is dependent on membrane-type MMPs. (A,B) MCF-10A cells infected with CD147 adenovirus were pre-treated with the protease inhibitors, TIMP-1 (0.5 μg/ml) and TIMP-2 (0.5 μg/ml) for 30 minutes and then plated on fluorescent matrices for 12 hours in the presence of these inhibitors. (A) Representative micrographs of matrix degradation in control and CD147-upregulated cells in the presence of protease inhibitors. Scale bars: 10 μm. (B) Quantification of normalized degradation area, expressed as means ± s.e.m. **P≤0.01. The experiment was repeated three times. (C) Western blot of CD147 and MT1-MMP in aliquots of lysates obtained from MCF-10A cells that were cultured on gelatin and treated with control (β-gal) or CD147 adenovirus. Actin was used as a loading control; n=3. (D,E) MCF-10A cells infected with CD147 adenovirus were pre-treated with function-blocking antibody against MT1-MMP (LEM-2/15.8; 12 μg/ml) or control IgG and then plated on fluorescent matrices for 12 hours in the presence of the blocking antibody or IgG. (D) Representative micrographs of matrix degradation in CD147-upregulated cells in the presence of blocking antibody or IgG. Scale bars: 10 μm. (E) Left panel: quantification of normalized degradation area. Right panel: quantification of invasion through the Matrigel. Values are means ± s.e.m. **P≤0.01; ***P≤0.001; experiments were repeated three times.
Because the major membrane-type MMP associated with invadopodia formation and activity is MT1-MMP, we next evaluated whether increased CD147 in MCF-10A cells induces MT1-MMP expression. We found that MCF-10A cells expressed low amounts of MT1-MMP, but treatment with CD147 adenovirus increased MT1-MMP as well as CD147 protein levels (Fig. 4C). CD147 induced expression of both the active form (~50 kDa) and pro-form (~63 kDa) of MT1-MMP when the cells were cultured on gelatin (Fig. 4C), the conditions employed for invadopodia formation. However, when the CD147-upregulated cells were grown on polystyrene, only the ~63 kDa MT1-MMP pro-form was detected (data not shown).
To evaluate further whether the invasive phenotype of CD147-upregulated cells was due to MT1-MMP activity, we treated the cells with a function-blocking antibody targeting the catalytic domain of MT1-MMP. We found that the MT1-MMP blocking antibody decreased both CD147-induced matrix degradation and Matrigel invasion compared with a control IgG antibody (Fig. 4D,E).
CD147 associates with MT1-MMP in CD147-induced invadopodia-like structures and in membrane sub-fractions with the properties of lipid rafts
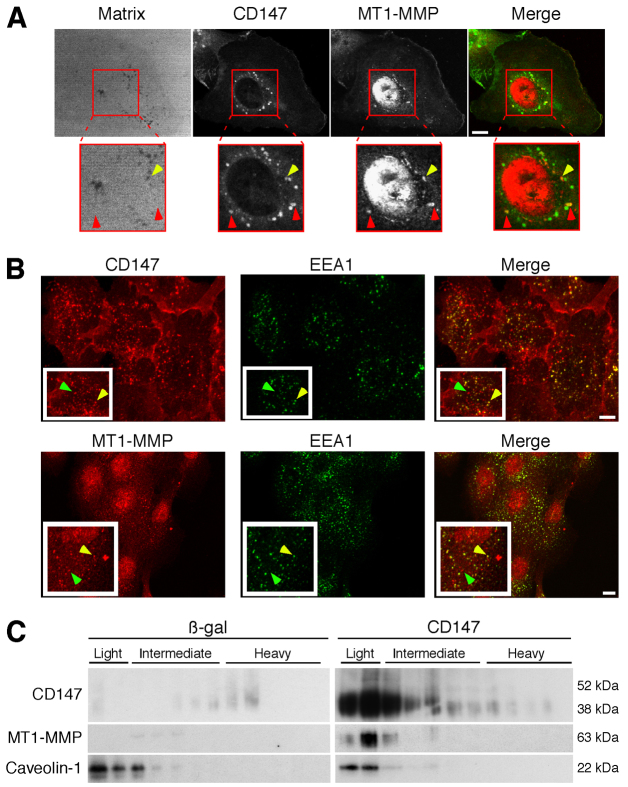
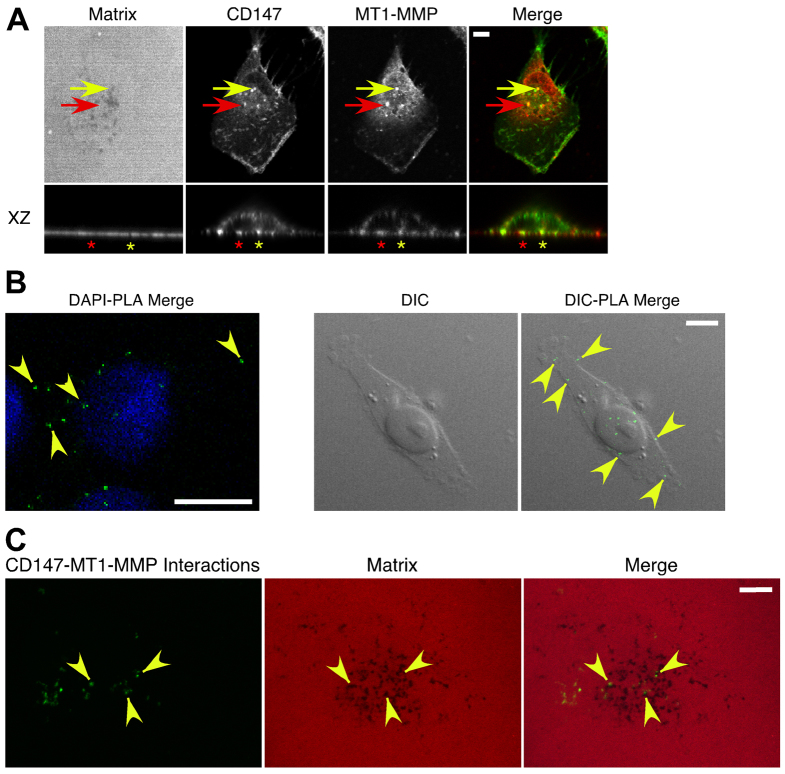
Because the data above support regulation of MT1-MMP expression by CD147, we investigated whether CD147 colocalizes with MT1-MMP and whether colocalization occurs within the induced invadopodia-like structures. We found that the two proteins colocalize over a subset of foci of degraded matrix (Fig. 5A, yellow arrows), but also colocalize in foci that did not coincide with matrix degradation (Fig. 5A, red arrows). In β-gal-treated cells the corresponding staining for MT1-MMP or CD147 was relatively weak (supplementary material Fig. S1). Previous studies have shown that CD147 and MT1-MMP are present in endocytic compartments, as well as in the plasma membrane (Eyster et al., 2009; Jiang et al., 2001). Thus, we evaluated whether sub-populations of CD147 and MT1-MMP were present in a vesicular compartment within MCF-10A cells over-expressing CD147, and found that distinct sub-populations of CD147 and MT1-MMP are colocalized in EEA1-positive vesicles (Fig. 5B). These results, together with those in Fig. 5A, indicate that sub-populations of CD147 and MT1-MMP are present in vesicular compartments that are distinct from the populations that are localized in invadopodia. These findings are consistent with FACS analyses (data not shown), which showed that upregulation of CD147 induced ~30% increase in cell surface MT1-MMP compared with a total increase in MT1-MMP expression of ~80% (from western blots), suggesting that a substantial fraction of induced MT1-MMP remains inside the cells.
Fig. 5.
CD147 associates with MT1-MMP in invadopodia-like structures and in membrane sub-fractions with the properties of lipid rafts. (A) Representative micrographs of CD147 and MT1-MMP colocalizing over degraded matrix in CD147-overexpressing MCF-10A cells. Yellow arrows indicate colocalization of CD147 (green) and MT1-MMP (red) over foci of degraded matrix; red arrows indicate colocalization of CD147 and MT1-MMP over areas of matrix that is not degraded. Scale bar: 10 μm. (B) Representative micrographs showing colocalization of CD147 (top panels) or MT1-MMP (bottom panels) with EEA1-positive vesicles. Yellow arrows indicate colocalization of proteins; green arrows indicate lack of colocalization. Scale bars: 10 μm. (C) MCF-10A cells infected with β-gal or CD147 adenovirus were plated on gelatin-coated plates overnight and were subjected to detergent-resistant membrane isolation. Light fractions are from the gradient interface (0–20%) where detergent-resistant membrane domains such as lipid rafts localize; n=2.
Recent studies have shown that invadopodia are highly enriched in lipid raft domains and are dependent on these raft domains for their initiation and degradative activity (Yamaguchi et al., 2009). Independent studies have shown that CD147 (Schwab et al., 2007; Tang and Hemler, 2004) and MT1-MMP (Annabi et al., 2001; Yamaguchi et al., 2009) are at least in part localized in membrane compartments similar to lipid rafts. Thus, we examined whether CD147 influences MT1-MMP localization to lipid rafts. Using a detergent-based lipid raft isolation protocol we showed that upregulation of CD147 in MCF-10A cells results in an enrichment of both CD147 and MT1-MMP in the raft fractions (light fraction) compared with the levels in β-gal-treated cells (Fig. 5C). Notably, most of the CD147 in the raft fractions was the lower molecular mass form of ~38 kDa. These results, in conjunction with earlier findings (Yamaguchi et al., 2009), suggest that upregulation of CD147 leads to enrichment of MT1-MMP within specialized regions of the plasma membrane, most probably within invadopodia. However, some of these lipid raft domains might reside in the membranes of intracellular vesicles, as discussed above.
CD147 regulates invadopodia function in invasive breast cancer cells
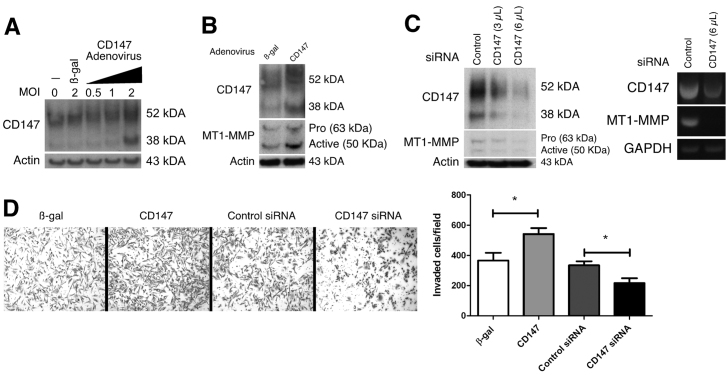
Many cell models have been used to study invadopodia dynamics, but the majority of these studies have utilized the invasive human breast cancer cell line MDA-MB-231 (Artym et al., 2006; Hu et al., 2011; Liu et al., 2009; Yamaguchi et al., 2009). Thus, we also evaluated whether CD147 regulates invadopodia dynamics in MDA-MB-231 cells. Fig. 6A shows the endogenous level of CD147 expression in MDA-MB-231 cells and the resulting expression of CD147 after treatment of these cells with recombinant CD147 adenovirus or β-gal adenovirus as control. We found that an MOI of 2 was effective in upregulating CD147 protein expression to a moderate degree (Fig. 6A), within the range observed in various invasive cells; this MOI was used for the following experiments. As with the MCF-10A cells, the ~38 kDa form of CD147 was increased to a greater extent than the ~52 kDa form. The MDA-MB-231 cells express MT1-MMP and invade Matrigel to a moderate level. However, upregulation of CD147 in these cells was accompanied by increased MT1-MMP expression (Fig. 6B) and increased Matrigel invasiveness (Fig. 6D) as compared with controls. Bands corresponding to the pro-form and active form of MT1-MMP were detected in western blots. We also treated MDA-MB-231 cells with pooled target-specific siRNAs. Dose-dependent downregulation of CD147 was observed and was accompanied by corresponding partial decreases in MT1-MMP mRNA and protein (Fig. 6C), as well as invasiveness in Matrigel (Fig. 6D).
Fig. 6.
CD147 regulates MT1-MMP expression and Matrigel invasion in invasive breast cancer cells. (A) Western blot showing endogenous CD147 protein expression, CD147 expression following treatment with control adenovirus (β-gal), and CD147 expression following treatment with increasing amounts of recombinant CD147 adenovirus in MDA-MB-231 cells; n=2. An MOI of 2 was chosen for upregulating CD147 in subsequent experiments. β-actin was used as the loading control. (B) Representative western blot of increased MT1-MMP levels following CD147 upregulation in MDA-MB-231 cells; n=3. β-gal treatment was used as the control; β-actin demonstrates equal loading. (C) Left panel: western blot demonstrating that knockdown of CD147 with increasing concentrations (3 and 6 μl) of pooled target-specific siRNA results in decreased MT1-MMP protein expression. Non-specific siRNA was used as a control and β-actin was used to demonstrate equal loading; n=3. Right panel: RT-PCR analysis of CD147 and MT1-MMP mRNA after treatment with control or CD147-targeted siRNAs. (D) Left panel: representative images of Matrigel invasion assay with MDA-MB-231 cells treated with β-gal or CD147 adenovirus or with control or CD147-specific siRNA. Right panel: quantification of invaded cells per field by evaluating four separate fields in three independent experiments. Values are means ± s.e.m. *P≤0.05.
We next determined whether changes in CD147 expression in MDA-MB-231 cells led to corresponding changes in invadopodia formation and activity. Cells treated with β-gal adenovirus showed a moderate number of invadopodia and degradation spots in the fluorescent matrix field, in similar numbers to those reported in earlier studies (Artym et al., 2006). However, when cells were treated with CD147 adenovirus there was an increase in the percentage of cells degrading the matrix, concomitant with an increase in the percentage of cells with invadopodia (Fig. 7A–C). Upregulation of CD147 resulted in an increase in invadopodia, from approximately four per cell to approximately eight per cell (Fig. 7C, right panel), coincident with large increases in degradation area per cell (Fig 7B, right panel). Partial downregulation of endogenous CD147, using pooled siRNAs (Fig. 6C), resulted in corresponding partial decreases in the percentage of cells degrading the matrix, cells with invadopodia, number of invadopodia per cell and area of matrix degradation per cell, whereas cells treated with control siRNA were comparable to β-gal-treated cells (Fig. 7A–C).
Fig. 7.
CD147 regulates invadopodia dynamics in invasive breast cancer cells. (A) Representative micrographs of MDA-MB-231 cells treated with β-gal or CD147 adenovirus or with control or CD147-targeted siRNA. Cells were cultured on Alexa-Fluor-568-conjugated gelatin for 5 hours, fixed and probed for actin (Alexa-Fluor-647–phalloidin) and cortactin (primary 4F11 antibody followed by secondary Alexa-Fluor-488 antibody). Actin and the gelatin matrix were pseudo-colored red and blue, respectively; this allows for easier visualization of colocalization (yellow) of actin (red) and cortactin (green). The left panels show invadopodia-mediated matrix degradation (dark holes). Higher magnifications of the boxed regions show invadopodia in more detail. Scale bars: 10 μm. (B,C) Quantification of invadopodia characteristics for MDA-MB-231 cells. (B) Left panel: percentage of cells degrading the matrix; calculated as cells with at least one area of degradation underneath the cell or near the cell border. Right panel: normalized degradation, calculated as area of degradation divided by the total cell area defined by the actin channel. (C) Left panel: percentage of cells with invadopodia, defined as actin–cortactin aggregates localized over degraded matrix. Right panel: number of invadopodia per cell in cells exhibiting at least one invadopodium. Each parameter was calculated by evaluating 10 random fields containing at least 20 cells per field over three independent experiments. Values are means ± s.e.m. *P≤0.05, **P≤0.01.
Colocalization of CD147 and MT1-MMP in invasive breast cancer cells
In a similar manner to our experiments with CD147-treated MCF-10A cells (Fig. 5A), in parental MDA-MB-231 cells CD147 and MT1-MMP colocalized over foci of degraded matrix (Fig. 8A, yellow arrow and asterisk) and non-degraded matrix (Fig. 8A, red arrow and asterisk). These results were confirmed with a monoclonal antibody against MT1-MMP (supplementary material Fig. S2).
Fig. 8.
Colocalization of CD147 and MT1-MMP in invasive breast cancer cells. (A) Representative images demonstrating CD147 and MT1-MMP colocalization in MDA-MB-231 cells. Cells were cultured on Alexa-Fluor-568-conjugated gelatin for 5 hours and probed with Alexa-Fluor-488-conjugated CD147 and MT1-MMP followed by an Alexa-Fluor-647 secondary antibody. A polyclonal antibody against MT1-MMP was used for these images; a monoclonal antibody against MT1-MMP gave similar results (supplementary material Fig. S2). MT1-MMP and the gelatin matrix were pseudo-colored red and blue, respectively; this allowed easier visualization of colocalization (yellow) of CD147 (green) and MT1-MMP (red). Yellow arrow indicates colocalization of CD147 and MT1-MMP over foci of degraded matrix; red arrow indicates colocalization of CD147 and MT1-MMP over an area not showing degraded matrix. XZ section: yellow asterisk, CD147–MT1-MMP colocalization over a region of degraded matrix; red asterisk, CD147–MT1-MMP colocalization over an area not showing matrix degradation. n=3. (B,C) A proximity ligation assay (PLA) was employed to detect close protein–protein interactions (≤40 nm) of CD147 and MT1-MMP in MDA-MB-231 cells (see Materials and Methods for details). (B) Left panel: interactions of CD147 and MT1-MMP (small green dots at arrowheads); cells were also stained with DAPI (blue). Right panel: DIC images of a single cell; arrowheads indicate sites of CD147–MT1-MMP interactions, which are restricted to areas over the cell. (C) PLA combined with gelatin degradation assay, showing that CD147–MT1-MMP interactions occurred over areas of degradation (arrowheads), as well as over areas lacking degradation. Scale bars: 10 μm.
To further evaluate the observed colocalization of CD147 and MT1-MMP, we performed a proximity ligation assay (PLA), which can detect endogenous protein–protein interactions that occur within 40 nm (Soderberg et al., 2006). Probing for CD147 and MT1-MMP in MDA-MB-231 cells using the PLA revealed that endogenous CD147 and MT1-MMP were closely associated (Fig. 8B). In a control experiment using only CD147 primary antibody followed by secondary PLA probes and amplification, no interaction was observed; additional field images demonstrated that CD147–MT1-MMP interactions occur throughout the cell population (supplementary material Fig. S3). Furthermore, we confirmed the specificity of the reaction by performing differential interference contrast (DIC) microscopy, which demonstrated that the interactions occurred only in association with cells and did not represent non-specific staining of the culture substratum (Figure 8B, right panels; supplementary material Fig. S3). We found ~12–15 CD147–MT1-MMP interactions per cell and, when the proximity ligation method was combined with the gelatin degradation assay, these CD147–MT1-MMP interactions were found over areas of both degraded and non-degraded matrix (Fig. 8C), similar to the results in Fig. 8A. These data strongly support close interactions of sub-populations of CD147 and MT1-MMP in actively degrading invadopodia, as well as in other areas of the cell such as EEA1-positive vesicles (Fig. 5B).
Discussion
Cancer cells employ various strategies to compromise the ECM scaffolds that inhibit their spread through different tissue compartments. A major mechanism used by many cancer cell types to degrade the surrounding ECM is the formation of specialized actin-enriched membrane protrusions called invadopodia. The proteolytic activity of invadopodia has been mainly attributed to the localization of MT1-MMP at the cell surface in specialized membrane domains with the properties of lipid rafts, a process that is orchestrated by a diverse group of interacting molecules (Frittoli et al., 2010; Murphy and Courtneidge, 2011; Poincloux et al., 2009). Our findings indicate that CD147 has a major mechanistic role in invadopodia formation and activity, and consequently in invasion.
CD147 expression is associated with many malignant phenotypes, including chemo- and radio-therapy resistance, aerobic glycolysis, anchorage-independent growth, angiogenesis, invasion and increased metastatic potential. Among the best characterized functions of CD147 is induction of MMPs, including MT1-MMP (Nabeshima et al., 2006; Yan et al., 2005). The exact mechanisms by which CD147 induces MMPs is not known, but current evidence indicates that homophilic CD147 interactions mediate this induction (Sun and Hemler, 2001; Tang et al., 2004b) and result in various downstream signaling events that promote MMP synthesis and other activities (Ghatak et al., 2005; Lim et al., 1998; Tang et al., 2008; Venkatesan et al., 2010; Yang et al., 2006). In agreement with earlier studies, we demonstrated that upregulation or downregulation of CD147 increases or decreases, respectively, the levels of MT1-MMP and invasiveness. In addition, we have demonstrated that modulation of CD147 expression correspondingly alters invadopodia function.
Most strikingly, we found that upregulating CD147 expression in non-transformed breast epithelia was sufficient to induce an invasion program similar to that seen in already invasive cancer cells, which was characterized by Matrigel invasion and formation of MT-MMP-dependent, actively degradative invadopodia. This suggests that upregulation of CD147 enriches the cell surface with active pools of MT1-MMP that subsequently degrade the matrix, as shown in the gelatin degradation assays (Fig. 2A). Though the mechanisms are not fully understood, earlier reports have demonstrated that CD147 interacts with actin (Schlosshauer et al., 1995) and that manipulation of CD147 expression results in reorganization of the actin cytoskeleton (Qian et al., 2008) and formation of actin-rich lamellipodia in Drosophila cells (Curtin et al., 2005); whether these findings are related to the mechanism of invadopodia formation is not known. In addition to its interactions with the actin cytoskeleton, CD147 interacts with several other invadopodia-enriched molecules, such as integrins (Berditchevski et al., 1997) and CD44 (Slomiany et al., 2009), that mediate attachment of ECM components to the cell surface and influence cytoskeleton remodeling. Recent evidence also suggests that CD147 can upregulate various transcription factors leading to multiple downstream signaling events associated with ECM remodeling and invasion (Venkatesan et al., 2010). Thus, CD147-regulated invadopodia formation could be due in part to interactions with other ancillary invadopodia-associated proteins.
Another established function of CD147 is in lactate transporter trafficking to the cell surface, where CD147 is an essential chaperone needed to display sub-classes of lactate transporters on the cell surface (Halestrap and Meredith, 2004). Although it is currently unknown whether CD147 is also involved in trafficking of MMPs to the cell surface, our data demonstrate that endogenous CD147 and MT1-MMP are in close proximity and that sub-populations of this complex occur in actively degrading invadopodia whereas other sub-populations are present in EEA1-positive endocytic vesicles. These results suggest that CD147–MT1-MMP complexes cycle between these two compartments. Other investigators have shown that CD147 associates with both the pro and active forms of MT1-MMP (Egawa et al., 2006; Niiya et al., 2009). In addition, we found that upregulation of CD147 in non-transformed MCF-10A epithelial cells results in enrichment of both CD147 and MT1-MMP in membrane compartments with characteristics similar to lipid raft domains, supporting previous observations that invadopodia formation and activity are dependent on these domains (Yamaguchi et al., 2009). These results are compatible with the idea that CD147 plays a role in subcellular trafficking or surface presentation of MT1-MMP. However, further work is required to determine whether CD147 is involved at a number of steps during initiation and maturation of invadopodia or whether it aids solely during the degradation process.
Of interest also is our observation that a relatively low-glycosylated form of CD147 was induced by treatment with recombinant CD147 adenovirus and that this form was the major form targeted to the lipid raft domains (Fig. 5C). The results of some previous studies suggest strongly that high levels of glycosylation are necessary for induction of MMP production (Guo et al., 1997; Tang and Hemler, 2004), whereas others have demonstrated robust MMP production after treatment with non-glycosylated CD147 (Belton et al., 2008) or CD147 substituted only with the disaccharide, chitobiose (Kawakami et al., 2011). Clearly, further investigation is required to elucidate the role of glycosylation in CD147 activities in different contexts.
Mechanisms of regulation of endogenous CD147 expression are not well established, although several growth factors and cytokines have been shown to increase CD147 levels in a variety of contexts (Hagemann et al., 2005; Menashi et al., 2003; Reddy et al., 2010; Rucci et al., 2010). Previous studies have shown that Src is a crucial regulator of invadopodia formation (Murphy and Courtneidge, 2011), and a recent study showed that the Src family kinase, Fyn kinase, induces emmprin expression (Ramos and Dang, 2011). We have also found that CD147 is elevated in cells over-expressing wild-type or constitutively active Src (G.D.G., unpublished data), suggesting that Src acts at least in part through induction of CD147.
In the present study, we provide evidence that increased CD147 alone is sufficient to induce the formation of actively degradative invadopodia-like structures in non-transformed epithelial cells and that CD147 could regulate localization of MT1-MMP in lipid raft domains. Furthermore, we have demonstrated that CD147 and MT1-MMP are in close proximity to each other and these interacting pools are present in part within active invadopodia. Because CD147 is enriched on the surface of many malignant cancer cell types, it represents a plausible molecular target for possible future cancer therapies, especially with respect to invasion and metastasis.
Materials and Methods
Cell culture
The human breast adenocarcinoma cell line MDA-MB-231 was obtained from the American Type Culture Collection (ATCC) and was cultured in RPMI 1640 (R-8755) with 2.38 g/l HEPES, 2 g/l sodium bicarbonate and 10% FBS (pH 7.4). The spontaneously immortalized human breast epithelial cell line MCF-10A was obtained from ATCC. The immortalized human mammary epithelial cell line HMLE was a generous gift from Robert Weinberg (MIT, Cambridge, MA). Low passage MCF-10A and HMLE cells were maintained in mammary epithelial cell growth medium (MEGM) with BulletKit supplements (Lonza). All cells were cultured in an incubator in humidified 95% air, 5% CO2 at 37°C.
Antibodies and reagents
The following primary antibodies were obtained for these studies: CD147 (BD Pharmingen); Alexa-Fluor-488-conjugated CD147 (Biolegend); cortactin (clone 4F11) and MT1-MMP (hinge-region) polyclonal (Millipore); MT1-MMP monoclonal (Epitomics); caveolin-1 (BD Biosciences); rabbit and mouse EEA1 (AbCam); and β-actin (Sigma). Alexa-Fluor-488-conjugated goat anti-mouse-IgG, Alexa-Fluor-488-conjugated goat anti-rabbit-IgG, Alexa-Fluor-647-conjugated goat anti-rabbit-IgG, Alexa-Fluor-555-conjugated goat anti-mouse-IgG, Alexa-Fluor-555-conjugated goat anti-rabbit-IgG and Alexa-Fluor-647–phalloidin were purchased from Invitrogen. Goat anti-mouse-HRP and goat anti-rabbit-HRP (Chemicon) were used as secondary antibodies for immunoblotting. Western blotting detection reagent (enhanced chemiluminescence) was purchased from Pierce. Recombinant TIMP-1 and TIMP-2 were purchased from Chemicon and were used at the concentrations indicated in the figure legends. A function-blocking antibody targeting the catalytic domain of MT1-MMP (LEM-2/15.8) and the IgG control antibody were obtained from Millipore.
Recombinant adenovirus infections
Recombinant human CD147 adenovirus and control β-galactosidase (β-gal) adenovirus were constructed and used as described previously (Li et al., 2001).
RT-PCR
Total RNA was isolated using the RNeasy Mini kit (Qiagen) and quality control and quantification was performed with an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.) and Agilent RNA 6000 Pico LabChip kits. cDNA was synthesized from equal total RNA using iScript cDNA synthesis kit (Bio-Rad) according to the procedures of the manufacturer. The primers designed for targeting the genes included the following: CD147 sense, 5′-GGAATAGGAATCATGGCGGCTGCG-3′; CD147 antisense, 5′-GAGCCTCAGGAAGAGTTCCTCTGGC-3′; MMP14 (MT1-MMP) sense, 5′-CCATTGGGCATCCAGAAGAGAGC-3′; MMP14 antisense, 5′-GGATACCCAATGCCCATTGGCCA-3′; GAPDH sense, 5′-ATGTTCGTCATGGGTGTGAA-3′; GAPDH antisense, 5′-GGTGCTAAGCAGTTGGTGGT-3′. The PCR was performed in a DNA thermal cycler (PCR Express Thermo Cycler, Thermo Hybaid) under conditions of 95°C for 2 minutes; 30 cycles each at 95°C for 30 seconds, 59°C for 30 seconds and 72°C for 60 seconds; followed by 72°C for 5 minutes.
RNA interference
Control non-silencing siRNA and target-specific pooled siRNAs for CD147 (#35298) were purchased from Santa Cruz Biotechnology and used according to manufacturer's protocol. For all experiments, cells were transfected with siRNA 72 hours before each experiment.
Matrigel invasion assay
Matrigel invasion inserts (8 μm pores) for 24-well tissue culture plates were purchased from BD Biosciences. Briefly, the Matrigel inserts (upper and lower chambers) were rehydrated for 2 hours at 37°C with serum culture medium (MDA-MB-231) or non-supplemented MEGM (MCF-10A/HMLE). MDA-MB-231 cells (4.0×105) treated with siRNA (72 hours) or adenoviruses (24 hours) were detached and seeded in the upper chamber of the inserts and cultured for 24 hours at 37°C. MCF-10A or HMLE cells (8.0×104), non-treated or treated with adenoviruses, were placed in the upper chamber in non-supplemented MEGM, and medium in the lower chamber was replaced with complete growth medium containing 20 nM EGF, and cultured for 36 hours at 37°C. After incubation, the cells were fixed in 3.7% paraformaldehyde for 15 minutes at room temperature, the chambers were rinsed with PBS and stained with 0.2% Crystal Violet for 10 minutes, followed by extensive washing. After scraping off the cells at the top of the Matrigel membrane with a Q-tip, the cells that passed through the 8 μm pores in the membrane were imaged using a phase-contrast microscope (Leica DFC320). Cells were counted in four separate fields in three independent experiments.
Gelatin degradation assay for invadopodia
This assay was performed as described previously with minor modifications (Artym et al., 2009). Briefly, gelatin was conjugated with Alexa-Fluor-568 dye (Molecular Probes) using the manufacturer's protocol. Glass coverslips (18 mm) were acid washed with 1 M HCl for 12–16 hours and washed extensively with water. Coverslips were sterilized with 70% ethanol and then coated with 50 μg/ml poly-L-lysine for 20 minutes at room temperature, washed with PBS, and fixed with ice-cold 0.5% glutaraldehyde for 15 minutes followed by extensive washing. The coverslips were then inverted on an 80 μl drop of fluorescent gelatin matrix (0.2% gelatin and Alexa-Fluor–gelatin at an 8:1 ratio) and incubated for 15 minutes at room temperature. The coverslips were washed with PBS and the residual reactive groups in the gelatin matrix were quenched with 5 mg/ml sodium borohydride in PBS for 10 minutes followed by further washing in PBS. The coated coverslips were placed in 12-well plates, sterilized in 70% ethanol for 10 minutes, washed in PBS and equilibrated in serum-free medium and switched to serum medium 30 minutes before the addition of cells. For invadopodia assays, 5.0×104 cells were plated on the coated coverslips and incubated at 37°C for 5–6 hours (MDA-MB-231) or 15 hours (MCF-10A). Cells were fixed and permeabilized with 3.7% paraformaldehyde and 0.1% Triton X-100 for 10 minutes, washed with PBS, blocked in 3% BSA in PBS for 1 hour, and incubated with appropriate primary and secondary antibodies in 3% BSA in PBS. Actin filaments were visualized with Alexa-Fluor-647–phalloidin.
In situ proximity ligation assay
Interaction of CD147 and MT1-MMP was detected in situ using Duolink II secondary antibodies and detection kit (Olink Bioscience, Uppsala, Sweden) according to manufacturer's instructions. Briefly, primary antibodies targeting CD147 (BD Pharmingen) and MT1-MMP (Millipore) were applied using standard procedures. Oligonucleotide-conjugated Duolink secondary antibodies were then added, followed by Duolink ligation solution. The oligonucleotides ligate together in a closed circle only when the secondary antibodies are in close proximity (≤40 nm) (Soderberg et al., 2006). Finally, polymerase was added, which amplified any existing closed circles, and detection was achieved with complementary, fluorescently labeled oligonucleotides. Specificity of the reaction was determined by treating cells with a single primary antibody followed by Duolink secondary antibodies, which results in no signal amplification because of the absence of a second oligonucleotide needed for ligation and subsequent closed circle amplification.
Microscopy and image analysis
Images were captured with a Leica Total Confocal System SP5 acoustic optical beam splitter confocal (TCS SP5 AOBS) microscope using a 63×, 1.4 NA oil objective at the Josh Spruill Molecular Morphology & Imaging Center in the Department of Regenerative Medicine and Cell Biology (MUSC, Charleston, SC). Images were acquired at high confocality (pinhole =1 Airy unit) to achieve the thinnest possible optical slices at the matrix–cell interface. Potential overlaps in emission spectra were eliminated by sequential scanning and tuning of the AOBS. Differential interference contrast (DIC) microscopy was performed using the Leica SP5 Confocal System. Three-dimensional volumetric reconstruction of invadopodia-producing cells was carried out using the 3D reconstruction software Amira 5.2 (Visage Imaging, San Diego, CA). Image processing and compilation were performed using Canvas Software (Deneba Systems Inc.) and Adobe Photoshop.
To assess the ability of cells to form invadopodia and degrade matrix, at least 10 randomly chosen fields were imaged per trial and evaluated for degraded matrix foci, which appear as dark ‘holes’ in the bright fluorescent matrix field. A cell degrading at least one hole underneath or near the cell edge was counted as a cell able to degrade matrix. Invadopodia, defined as actin puncta that colocalize with cortactin, were manually counted and analyzed for underlying matrix degradation, and the results presented as the number of invadopodia per cell or the percentage of cells with invadopodia over degraded matrix. Quantification of matrix degradation area per cell was analyzed as described previously (Liu et al., 2009) and was reported as normalized degradation, which is total area of degraded matrix relative to the area of the whole cell. Briefly, the area of degraded matrix in the cell field was measured using ImageJ 1.43s software. Areas of degraded matrix in the bright fluorescent field were converted to 8 bit grayscale files, automatically thresholded into black dots on a white background, followed by automatic outlining of the dots. The area of each individual cell was measured by manually outlining the cell boundary using the β-actin channel. Data were collected in an Excel spreadsheet and used to calculate degradation area per cell area.
Detergent-resistant membrane fractionation
Detergent-resistant membrane domains (e.g. lipid rafts) were isolated from MCF-10A cells as described previously (Thankamony and Knudson, 2006) with minor modifications. Briefly, cells cultured on gelatin-coated D150 plates were washed with ice-cold PBS three times. Cells were lysed in 500 μl lysis buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM dithiothreitol, 10% sucrose, 1% Triton X-100 and protease inhibitors) on ice for 30 minutes. The cells were mechanically disrupted by passaging 20 times through a 3-inch 22-guage needle. The lysates were mixed directly with iodixanol stock solution (60% solution of Optiprep iodixanol) to yield a 40% (v/v) iodixanol–lysate solution, which was placed at the bottom of an ultracentrifuge tube. Equal volumes of 35%, 30%, 25%, 20% and 0% Optiprep in lysis buffer without Triton X-100 were carefully overlaid above the iodixanol–lysate solution. The samples were centrifuged at 160,000 g for 8 hours at 4°C in a SW41-Ti rotor (Beckman Coulter). Equal fractions were carefully collected from the top of the tube and equivalent aliquots of each fraction were subjected to immunoblotting using antibodies recognizing proteins of interest.
Immunoblot analysis
Whole cell lysates were prepared for immunoblotting using a RIPA buffer modified to contain 1 mM PMSF, 10 μg/ml aprotinin and leupeptin, 2 mM sodium orthovanadate and 10 mM sodium fluoride. Protein content was determined using a BCA assay (Pierce), and aliquots were solubilized in SDS sample buffer, resolved on Pierce 4–20% reducing polyacrylamide gels, transferred to nitrocellulose (Osmonics, Westborough, MA) with a Pierce apparatus, and stained with antibody.
Supplementary Material
Acknowledgements
We thank Thomas Trusk for assistance with the Amira reconstructions and Mark Slomiany for helpful discussions.
Footnotes
Funding
This work was supported by Department of Defense predoctoral fellowship [grant number W81XWH-10-1-0083 to G.D.G.]; by National Institutes of Health [grant numbers R01 CA073839 to B.P.T., R01 CA082867 to B.P.T.]; Department of Defense [grant number OC050368 to B.P.T.]; and by the South Carolina Clinical & Translational Research Institute (National Center for Research Resources, National Institutes of Health) [grant number UL1RR029882]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.097956/-/DC1
References
- Annabi B., Lachambre M., Bousquet-Gagnon N., Page M., Gingras D., Beliveau R. (2001). Localization of membrane-type 1 matrix metalloproteinase in caveolae membrane domains. Biochem. J. 353, 547-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artym V. V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K. M., Mueller S. C. (2006). Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 66, 3034-3043 [DOI] [PubMed] [Google Scholar]
- Artym V. V., Yamada K. M., Mueller S. C. (2009). ECM degradation assays for analyzing local cell invasion. Methods Mol. Biol. 522, 211-219 [DOI] [PubMed] [Google Scholar]
- Belton R. J., Jr, Chen L., Mesquita F. S., Nowak R. A. (2008). Basigin-2 is a cell surface receptor for soluble basigin ligand. J. Biol. Chem. 283, 17805-17814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F., Chang S., Bodorova J., Hemler M. E. (1997). Generation of monoclonal antibodies to integrin-associated proteins. Evidence that alpha3beta1 complexes with EMMPRIN/basigin/OX47/M6. J. Biol. Chem. 272, 29174-29180 [DOI] [PubMed] [Google Scholar]
- Biswas C., Zhang Y., DeCastro R., Guo H., Nakamura T., Kataoka H., Nabeshima K. (1995). The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 55, 434-439 [PubMed] [Google Scholar]
- Caudroy S., Polette M., Nawrocki-Raby B., Cao J., Toole B. P., Zucker S., Birembaut P. (2002). Emmprin-mediated MMP regulation in tumor and endothelial cells. Clin. Exp. Metastasis 19, 697-702 [DOI] [PubMed] [Google Scholar]
- Clark E. S., Whigham A. S., Yarbrough W. G., Weaver A. M. (2007). Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 67, 4227-4235 [DOI] [PubMed] [Google Scholar]
- Curtin K. D., Meinertzhagen I. A., Wyman R. J. (2005). Basigin (EMMPRIN/CD147) interacts with integrin to affect cellular architecture. J. Cell Sci. 118, 2649-2660 [DOI] [PubMed] [Google Scholar]
- Davidson B., Konstantinovsky S., Nielsen S., Dong H. P., Berner A., Vyberg M., Reich R. (2004). Altered expression of metastasis-associated and regulatory molecules in effusions from breast cancer patients: a novel model for tumor progression. Clin. Cancer Res. 10, 7335-7346 [DOI] [PubMed] [Google Scholar]
- Eckert M. A., Lwin T. M., Chang A. T., Kim J., Danis E., Ohno-Machado L., Yang J. (2011). Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell 19, 372-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa N., Koshikawa N., Tomari T., Nabeshima K., Isobe T., Seiki M. (2006). Membrane type 1 matrix metalloproteinase (MT1-MMP/MMP-14) cleaves and releases a 22-kDa extracellular matrix metalloproteinase inducer (EMMPRIN) fragment from tumor cells. J. Biol. Chem. 281, 37576-37585 [DOI] [PubMed] [Google Scholar]
- Ellis S. M., Nabeshima K., Biswas C. (1989). Monoclonal antibody preparation and purification of a tumor cell collagenase-stimulatory factor. Cancer Res. 49, 3385-3391 [PubMed] [Google Scholar]
- Eyster C. A., Higginson J. D., Huebner R., Porat-Shliom N., Weigert R., Wu W. W., Shen R. F., Donaldson J. G. (2009). Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic 10, 590-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Wolf K. (2003). Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer 3, 362-374 [DOI] [PubMed] [Google Scholar]
- Frittoli E., Palamidessi A., Disanza A., Scita G. (2010). Secretory and endo/exocytic trafficking in invadopodia formation: The MT1-MMP paradigm. Eur. J. Cell Biol. 90, 108-114 [DOI] [PubMed] [Google Scholar]
- Gabison E. E., Huet E., Baudouin C., Menashi S. (2009). Direct epithelial-stromal interaction in corneal wound healing: Role of EMMPRIN/CD147 in MMPs induction and beyond. Prog. Retin. Eye Res. 28, 19-33 [DOI] [PubMed] [Google Scholar]
- Ghatak S., Misra S., Toole B. P. (2005). Hyaluronan regulates constitutive ErbB2 phosphorylation and signal complex formation in carcinoma cells. J. Biol. Chem. 280, 8875-8883 [DOI] [PubMed] [Google Scholar]
- Guo H., Zucker S., Gordon M. K., Toole B. P., Biswas C. (1997). Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J. Biol. Chem. 272, 24-27 [PubMed] [Google Scholar]
- Hagemann T., Wilson J., Kulbe H., Li N. F., Leinster D. A., Charles K., Klemm F., Pukrop T., Binder C., Balkwill F. R. (2005). Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J. Immunol. 175, 1197-1205 [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Meredith D. (2004). The SLC16 gene family - from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 447, 619-628 [DOI] [PubMed] [Google Scholar]
- Hotary K., Li X. Y., Allen E., Stevens S. L., Weiss S. J. (2006). A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev. 20, 2673-2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Mukhopadhyay A., Truesdell P., Chander H., Mukhopadhyay U. K., Mak A. S., Craig A. W. (2011). Cdc42-interacting protein 4 is a Src substrate that regulates invadopodia and invasiveness of breast tumors by promoting MT1-MMP endocytosis. J. Cell Sci. 124, 1739-1751 [DOI] [PubMed] [Google Scholar]
- Jiang A., Lehti K., Wang X., Weiss S. J., Keski-Oja J., Pei D. (2001). Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc. Natl. Acad. Sci. USA 98, 13693-13698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka H., DeCastro R., Zucker S., Biswas C. (1993). Tumor cell-derived collagenase-stimulatory factor increases expression of interstitial collagenase, stromelysin, and 72-kDa gelatinase. Cancer Res. 53, 3154-3158 [PubMed] [Google Scholar]
- Kawakami T., Sameshima T., Hojo H., Koga K., Nakahara Y., Toole B. P., Suzumiya J., Okada Y., Iwasaki A., Nabeshima K. (2011). Synthetic emmprin peptides with chitobiose substitution stimulate MMP-2 production by fibroblasts. BMC Cancer 11, 300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C. A., Seidl S., Petat-Dutter K., Offner S., Geigl J. B., Schmidt-Kittler O., Wendler N., Passlick B., Huber R. M., Schlimok G., et al. (2002). Combined transcriptome and genome analysis of single micrometastatic cells. Nat. Biotechnol. 20, 387-392 [DOI] [PubMed] [Google Scholar]
- Li R., Huang L., Guo H., Toole B. P. (2001). Basigin (murine EMMPRIN) stimulates matrix metalloproteinase production by fibroblasts. J. Cell. Physiol. 186, 371-379 [DOI] [PubMed] [Google Scholar]
- Liao C. G., Kong L. M., Song F., Xing J. L., Wang L. X., Sun Z. J., Tang H., Yao H., Zhang Y., Wang L., et al. (2011). Characterization of basigin isoforms and the inhibitory function of basigin-3 in human hepatocellular carcinoma proliferation and invasion. Mol. Cell. Biol. 31, 2591-2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M., Martinez T., Jablons D., Cameron R., Guo H., Toole B., Li J. D., Basbaum C. (1998). Tumor-derived EMMPRIN (extracellular matrix metalloproteinase inducer) stimulates collagenase transcription through MAPK p38. FEBS Lett. 441, 88-92 [DOI] [PubMed] [Google Scholar]
- Liu J., Yue P., Artym V. V., Mueller S. C., Guo W. (2009). The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Mol. Biol. Cell 20, 3763-3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menashi S., Serova M., Ma L., Vignot S., Mourah S., Calvo F. (2003). Regulation of extracellular matrix metalloproteinase inducer and matrix metalloproteinase expression by amphiregulin in transformed human breast epithelial cells. Cancer Res. 63, 7575-7580 [PubMed] [Google Scholar]
- Murphy D. A., Courtneidge S. A. (2011). The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat. Rev. Mol. Cell Biol. 12, 413-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima K., Iwasaki H., Koga K., Hojo H., Suzumiya J., Kikuchi M. (2006). Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol. Int. 56, 359-367 [DOI] [PubMed] [Google Scholar]
- Niiya D., Egawa N., Sakamoto T., Kikkawa Y., Shinkawa T., Isobe T., Koshikawa N., Seiki M. (2009). Identification and characterization of Lutheran blood group glycoprotein as a new substrate of membrane-type 1 matrix metalloproteinase 1 (MT1-MMP): a systemic whole cell analysis of MT1-MMP-associating proteins in A431 cells. J. Biol. Chem. 284, 27360-27369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser M., Yamaguchi H., Mader C. C., Bravo-Cordero J. J., Arias M., Chen X., Desmarais V., van Rheenen J., Koleske A. J., Condeelis J. (2009). Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 186, 571-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pituch-Noworolska A., Drabik G., Szatanek R., Bialas M., Kolodziejczyk P., Szczepanik A., Stachura J., Zembala M. (2007). Immunophenotype of isolated tumour cells in the blood, bone marrow and lymph nodes of patients with gastric cancer. Pol. J. Pathol. 58, 93-97 [PubMed] [Google Scholar]
- Poincloux R., Lizarraga F., Chavrier P. (2009). Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J. Cell Sci. 122, 3015-3024 [DOI] [PubMed] [Google Scholar]
- Qian A. R., Zhang W., Cao J. P., Yang P. F., Gao X., Wang Z., Xu H. Y., Weng Y. Y., Shang P. (2008). Downregulation of CD147 expression alters cytoskeleton architecture and inhibits gelatinase production and SAPK pathway in human hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 27, 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos D. M., Dang D. (2011). EMMPRIN expression in oral SCC is regulated by FYN kinase. Anticancer Res. 31, 1205-1209 [PubMed] [Google Scholar]
- Reddy V. S., Prabhu S. D., Mummidi S., Valente A. J., Venkatesan B., Shanmugam P., Delafontaine P., Chandrasekar B. (2010). Interleukin-18 induces EMMPRIN expression in primary cardiomyocytes via JNK/Sp1 signaling and MMP-9 in part via EMMPRIN and through AP-1 and NF-kappaB activation. Am. J. Physiol. Heart Circ. Physiol. 299, H1242-H1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers N., Zafrakas K., Assmann V., Egen C., Riethdorf L., Riethdorf S., Berger J., Ebel S., Janicke F., Sauter G., et al. (2004). Expression of extracellular matrix metalloprotease inducer on micrometastatic and primary mammary carcinoma cells. Clin. Cancer Res. 10, 3422-3428 [DOI] [PubMed] [Google Scholar]
- Rowe R. G., Weiss S. J. (2008). Breaching the basement membrane: who, when and how? Trends Cell Biol. 18, 560-574 [DOI] [PubMed] [Google Scholar]
- Rucci N., Millimaggi D., Mari M., Del Fattore A., Bologna M., Teti A., Angelucci A., Dolo V. (2010). Receptor activator of NF-kappaB ligand enhances breast cancer-induced osteolytic lesions through upregulation of extra-cellular matrix metalloproteinase inducer/CD147. Cancer Res. 70, 6150-6160 [DOI] [PubMed] [Google Scholar]
- Schlosshauer B., Bauch H., Frank R. (1995). Neurothelin: amino acid sequence, cell surface dynamics and actin colocalization. Eur. J. Cell Biol. 68, 159-166 [PubMed] [Google Scholar]
- Schoumacher M., Goldman R. D., Louvard D., Vignjevic D. M. (2010). Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J. Cell Biol. 189, 541-556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab W., Harada H., Goetz W., Nowicki M., Witt M., Kasper M., Barth K. (2007). Immunocytochemical and biochemical detection of EMMPRIN in the rat tooth germ: differentiation-dependent co-expression with MMPs and co-localization with caveolin-1 in membrane rafts of dental epithelial cells. Histochem. Cell Biol. 128, 195-203 [DOI] [PubMed] [Google Scholar]
- Slomiany M. G., Grass G. D., Robertson A. D., Yang X. Y., Maria B. L., Beeson B., Toole B. P. (2009). Hyaluronan, CD44 and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Res. 69, 1293-1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg O., Gullberg M., Jarvius M., Ridderstrale K., Leuchowius K. J., Jarvius J., Wester K., Hydbring P., Bahram F., Larsson L. G., et al. (2006). Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995-1000 [DOI] [PubMed] [Google Scholar]
- Sun J., Hemler M. E. (2001). Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 61, 2276-2281 [PubMed] [Google Scholar]
- Sung Y. M., Xu X., Sun J., Mueller D., Sentissi K., Johnson P., Urbach E., Seillier-Moiseiwitsch F., Johnson M. D., Mueller S. C. (2009). Tumor suppressor function of Syk in human MCF10A in vitro and normal mouse mammary epithelium in vivo. PLoS One 4, e7445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Wu Y. M., Zhao P., Yang X. M., Jiang J. L., Chen Z. N. (2008). Overexpression of HAb18G/CD147 promotes invasion and metastasis via alpha3-beta1 integrin mediated FAK-paxillin and FAK-PI3K-Ca2+ pathways. Cell. Mol. Life Sci. 65, 2933-2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Hemler M. E. (2004). Caveolin-1 regulates matrix metalloproteinases-1 induction and CD147/EMMPRIN cell surface clustering. J. Biol. Chem. 279, 11112-11118 [DOI] [PubMed] [Google Scholar]
- Tang W., Chang S. B., Hemler M. E. (2004a). Links between CD147 function, glycosylation, and caveolin-1. Mol. Biol. Cell 15, 4043-4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Kesavan P., Nakada M. T., Yan L. (2004b). Tumor-stroma interaction: positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol. Cancer Res. 2, 73-80 [PubMed] [Google Scholar]
- Thankamony S. P., Knudson W. (2006). Acylation of CD44 and its association with lipid rafts are required for receptor and hyaluronan endocytosis. J. Biol. Chem. 281, 34601-34609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan B., Valente A. J., Prabhu S. D., Shanmugam P., Delafontaine P., Chandrasekar B. (2010). EMMPRIN activates multiple transcription factors in cardiomyocytes, and induces interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB and MKK7/JNK/AP-1 signaling. J. Mol. Cell. Cardiol. 49, 655-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Takeo Y., Yoshida S., Kouchi Z., Nakamura Y., Fukami K. (2009). Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer Res. 69, 8594-8602 [DOI] [PubMed] [Google Scholar]
- Yan L., Zucker S., Toole B. P. (2005). Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb. Haemost. 93, 199-204 [DOI] [PubMed] [Google Scholar]
- Yang J. M., O'Neill P., Jin W., Foty R., Medina D. J., Xu Z., Lomas M., Arndt G. M., Tang Y., Nakada M., et al. (2006). Extracellular matrix metalloproteinase inducer (CD147) confers resistance of breast cancer cells to anoikis through inhibition of Bim. J. Biol. Chem. 281, 9719-9727 [DOI] [PubMed] [Google Scholar]
- Zucker S., Hymowitz M., Rollo E. E., Mann R., Conner C. E., Cao J., Foda H. D., Tompkins D. C., Toole B. P. (2001). Tumorigenic potential of extracellular matrix metalloproteinase inducer (EMMPRIN). Am. J. Pathol. 158, 1921-1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.