Abstract
We have characterized a cDNA pGPX1211 encoding rat glutathione peroxidase I. The selenocysteine in the protein corresponded to a TGA codon in the coding region of the cDNA, similar to earlier findings in mouse and human genes, and a gene encoding the formate dehydrogenase from E. coli, another selenoenzyme. The rat GSH peroxidase I has a calculated subunit molecular weight of 22,155 daltons and shares 95% and 86% sequence homology with the mouse and human subunits, respectively. The 3'-noncoding sequence (greater than 930 bp) in pGPX1211 is much longer than that of the human sequences. We found that glutathione peroxidase I mRNA, but not the polypeptide, was expressed under nutritional stress of selenium deficiency where no glutathione peroxidase I activity can be detected. The failure of detecting any apoprotein for the glutathione peroxidase I under selenium deficiency and results published from other laboratories supports the proposal that selenium may be incorporated into the glutathione peroxidase I co-translationally.
Full text
PDF






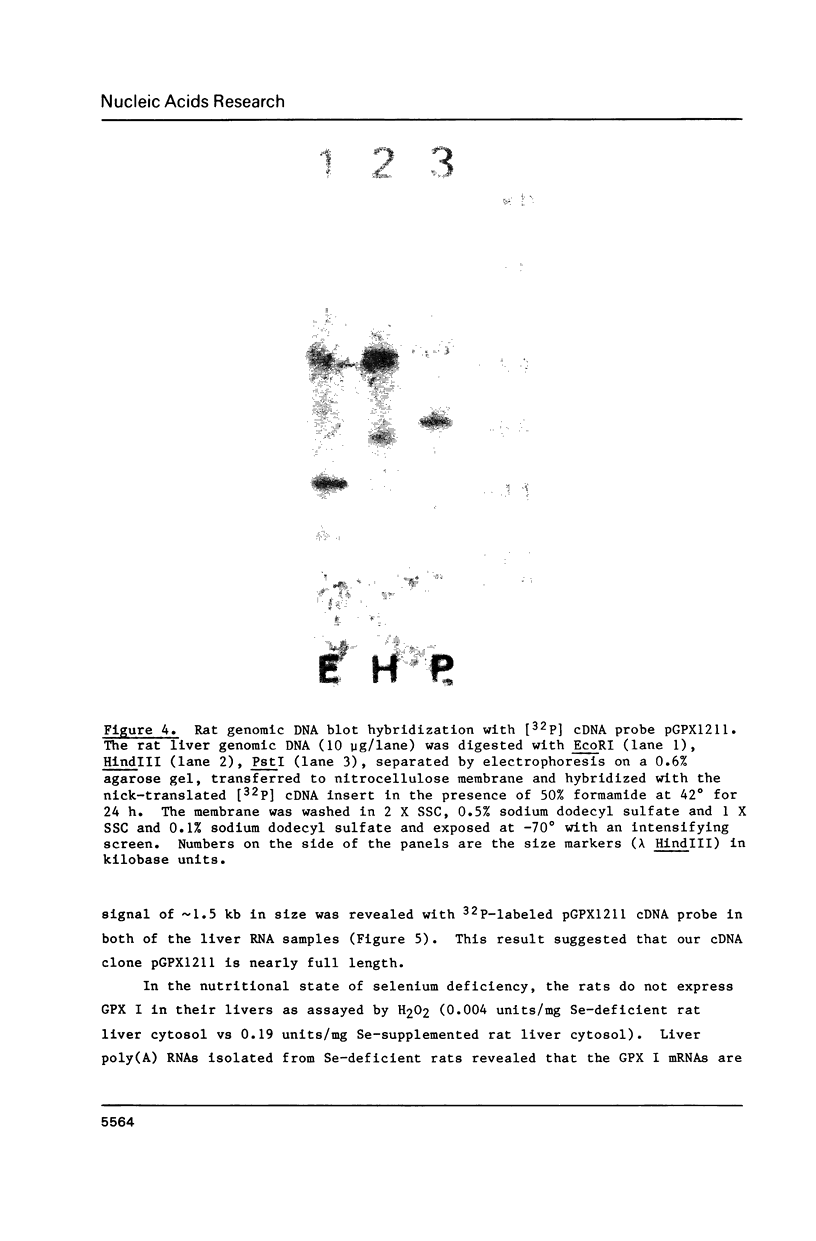




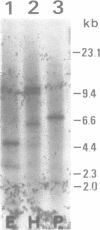
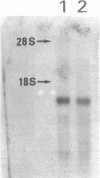
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J., Fridovich I. Inactivation of glutathione peroxidase by superoxide radical. Arch Biochem Biophys. 1985 Aug 1;240(2):500–508. doi: 10.1016/0003-9861(85)90056-6. [DOI] [PubMed] [Google Scholar]
- Bryant R. W., Bailey J. M. Role of selenium-dependent glutathione peroxidase in platelet lipoxygenase metabolism. Prog Lipid Res. 1981;20:189–194. doi: 10.1016/0163-7827(81)90035-7. [DOI] [PubMed] [Google Scholar]
- Chambers I., Frampton J., Goldfarb P., Affara N., McBain W., Harrison P. R. The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the 'termination' codon, TGA. EMBO J. 1986 Jun;5(6):1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Christophersen B. O. Reduction of X-ray-induced DNA and thymine hydroperoxides by rat liver glutathione peroxidase. Biochim Biophys Acta. 1969 Aug 20;186(2):387–389. doi: 10.1016/0005-2787(69)90017-3. [DOI] [PubMed] [Google Scholar]
- Condell R. A., Tappel A. L. Amino acid sequence around the active-site selenocysteine of rat liver glutathione peroxidase. Biochim Biophys Acta. 1982 Dec 20;709(2):304–309. doi: 10.1016/0167-4838(82)90472-1. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Proteins. Sci Am. 1985 Oct;253(4):88–99. doi: 10.1038/scientificamerican1085-88. [DOI] [PubMed] [Google Scholar]
- Epp O., Ladenstein R., Wendel A. The refined structure of the selenoenzyme glutathione peroxidase at 0.2-nm resolution. Eur J Biochem. 1983 Jun 1;133(1):51–69. doi: 10.1111/j.1432-1033.1983.tb07429.x. [DOI] [PubMed] [Google Scholar]
- Günzler W. A., Steffens G. J., Grossmann A., Kim S. M., Otting F., Wendel A., Flohé L. The amino-acid sequence of bovine glutathione peroxidase. Hoppe Seylers Z Physiol Chem. 1984 Feb;365(2):195–212. doi: 10.1515/bchm2.1984.365.1.195. [DOI] [PubMed] [Google Scholar]
- Hawkes W. C., Lyons D. E., Tappel A. L. Identification of a selenocysteine-specific aminoacyl transfer RNA from rat liver. Biochim Biophys Acta. 1982 Dec 31;699(3):183–191. doi: 10.1016/0167-4781(82)90106-3. [DOI] [PubMed] [Google Scholar]
- Hawkes W. C., Tappel A. L. In vitro synthesis of glutathione peroxidase from selenite. Translational incorporation of selenocysteine. Biochim Biophys Acta. 1983 Mar 10;739(2):225–234. doi: 10.1016/0167-4781(83)90033-7. [DOI] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: chemiluminescence and peroxidation. Biochemistry. 1975 Dec 2;14(24):5299–5303. doi: 10.1021/bi00695a011. [DOI] [PubMed] [Google Scholar]
- Knight S. A., Sunde R. A. The effect of progressive selenium deficiency on anti-glutathione peroxidase antibody reactive protein in rat liver. J Nutr. 1987 Apr;117(4):732–738. doi: 10.1093/jn/117.4.732. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leinfelder W., Zehelein E., Mandrand-Berthelot M. A., Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988 Feb 25;331(6158):723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mullenbach G. T., Tabrizi A., Irvine B. D., Bell G. I., Hallewell R. A. Sequence of a cDNA coding for human glutathione peroxidase confirms TGA encodes active site selenocysteine. Nucleic Acids Res. 1987 Jul 10;15(13):5484–5484. doi: 10.1093/nar/15.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium-dependent enzymes. Annu Rev Biochem. 1980;49:93–110. doi: 10.1146/annurev.bi.49.070180.000521. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Specific occurrence of selenium in enzymes and amino acid tRNAs. FASEB J. 1987 Nov;1(5):375–379. doi: 10.1096/fasebj.1.5.2445614. [DOI] [PubMed] [Google Scholar]
- Sunde R. A., Evenson J. K. Serine incorporation into the selenocysteine moiety of glutathione peroxidase. J Biol Chem. 1987 Jan 15;262(2):933–937. [PubMed] [Google Scholar]
- Takahashi K., Newburger P. E., Cohen H. J. Glutathione peroxidase protein. Absence in selenium deficiency states and correlation with enzymatic activity. J Clin Invest. 1986 Apr;77(4):1402–1404. doi: 10.1172/JCI112449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappel A. L. Selenium--glutathione peroxidase: properties and synthesis. Curr Top Cell Regul. 1984;24:87–97. [PubMed] [Google Scholar]
- Taylor S. L., Lamden M. P., Tappel A. L. Sensitive fluorometric method for tissue tocopherol analysis. Lipids. 1976 Jul;11(7):530–538. doi: 10.1007/BF02532898. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Qian B. Human liver glutathione S-transferases: complete primary sequence of an Ha subunit cDNA. Biochem Biophys Res Commun. 1986 Nov 26;141(1):229–237. doi: 10.1016/s0006-291x(86)80358-8. [DOI] [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Stadtman T. C., Böck A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4650–4654. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]