Abstract
Optic pathway gliomas (OPGs) occur in 15%–20% of children with neurofibromatosis type 1 (NF1); up to half become symptomatic. There is little information regarding ophthalmologic outcomes after chemotherapy. A retrospective multicenter study was undertaken to evaluate visual outcomes following chemotherapy for NF1-associated OPG, to identify risks for visual loss, and to ascertain indications for treatment. Subjects included children undergoing initial treatment for OPGs with chemotherapy between January 1997 and December 2007. Of 115 subjects, visual acuity (VA) decline and tumor progression were the primary reasons to initiate treatment, although there were significant differences in the pattern of indications cited among the institutions. Eighty-eight subjects and 168 eyes were evaluable for VA outcome. At completion of chemotherapy, VA improved (32% of subjects), remained stable (40%), or declined (28%). Tumor location was the most consistent prognostic factor for poor VA outcome. There was poor correlation between radiographic and VA outcomes. Although visual outcomes for NF1-associated OPG are not optimal, approximately one-third of children regain some vision with treatment. Since radiographic outcomes do not predict visual outcomes, their use as the primary measure of treatment success is in question. The lack of consensus regarding the indications for treatment underlines the need for better standardization of care. Future clinical trials for OPG require standardized visual assessment methods and clear definitions of visual outcomes.
Keywords: neurofibromatosis, optic glioma, outcomes, visual acuity
Optic pathway gliomas (OPGs) arise in 15%–20% of children with the common, autosomal-dominant, cancer-predisposition syndrome of neurofibromatosis type 1 (NF1).1 The majority of these tumors are diagnosed prior to the age of 6 years, although symptomatic tumors have been reported in older children.2 No more than half of NF1-associated OPG patients will develop vision loss. Therefore, initial management typically involves close observation. When therapy is indicated, chemotherapy is usually the treatment of choice.
Some clinicians advocate treatment when there is radiographic progression or visual deterioration.1,3,4 By contrast, others reserve treatment for only patients with documented visual deterioration. In addition, often factored into the decision to treat are tumor size/extent, tumor enhancement, tumor location, progressive proptosis, visual field loss, optic pallor, hydrocephalus, endocrine dysfunction, and the presence of diencephalic syndrome.1,5–7
A paucity of data exist regarding the clinical outcome of children with OPG who receive chemotherapy. Much of the published literature on the chemotherapeutic response of NF1-OPG has focused on radiographic outcomes and not on changes in vision.8,9 Those studies that include visual outcomes in their analyses are hampered by small sample sizes, an admixture of patients with NF1-associated and sporadic OPG, inclusion of subjects previously treated with radiation, a lack of within-subjects comparison, inconsistent endpoints for follow-up, or reporting of outcomes at the end of all treatments (which often include 2 or more different therapies), making it difficult to draw clear conclusions regarding the efficacy of chemotherapy for NF1-OPG.4,10–13 Moreover, in many of these studies, the criteria for a change in vision are often not well defined.14–18 In addition, there are multiple case reports/series in which the radiographic and ophthalmologic outcomes do not match.5,11,13
For these reasons, we launched a large retrospective multicenter study to evaluate visual outcomes following frontline chemotherapy for NF1-associated OPG, to identify risks for visual loss, and to ascertain the indications for treatment.
Patients and Methods
This retrospective multicenter review was approved by the ethics committees of all 10 participating sites (Children's Hospital Boston, MA; Children's Hospital of Philadelphia, PA; Children's Memorial Hospital, Chicago, IL; Children's National Medical Center, Washington DC; Cincinnati [OH] Children's Hospital Medical Center; Guy's and St Thomas’ Hospital, Royal Marsden Hospital, London; St Louis [MO] Children's Hospital; The Children's Hospital at Westmead, Sydney, Australia; The Hospital for Sick Children, Toronto, ON; University of Utah, Salt Lake City); consent was waived. The study population consisted of children younger than 18 years of age with a diagnosis of NF1 who had undergone initial treatment with chemotherapy for an OPG. Subjects must have started chemotherapy no earlier than January 1997 and completed or failed treatment before December 31, 2007. Of note, subjects whose initial chemotherapy regimen changed because of carboplatin allergy or other toxicity were not considered to have failed or completed initial therapy; completion of therapy for these patients was defined as the end of treatment with the new regimen. Subjects who had been treated previously with radiotherapy or chemotherapy were excluded.
Potential cases were identified from existing clinical databases (oncology, ophthalmology, neurology, and/or NF clinic) at each site. Charts of eligible subjects were abstracted for demographic and clinical information, including date of birth and diagnosis of OPG, familial inheritance or sporadic occurrence of NF1, reason for initial imaging, tumor location at the start of therapy, indications for starting treatment (both the primary [ie, main reasons] and secondary indications), treatment regimen, and treatment dates. Recorded were visual acuity (VA) and testing method (Teller, Lea, HOTV, Snellen) within ∼3 months of the start and end of treatment and other pertinent ophthalmologic outcomes (visual fields, optic disc pallor or swelling, proptosis, nystagmus, and ocular alignment). VA response was determined by the coordinating study neuro-ophthalmologist (G.T.L.) after review of the data from each site, taking into account the testing method and acuity norms for age. A 2-line (eg, Snellen chart) decrease in VA compared with the prechemotherapy examination was defined as worsening. Similarly, improvement was defined as a 2-line increase in acuity. For per-subject outcomes, if one eye worsened, the outcome was defined as worsening, no matter the outcome of the other eye. If one eye improved and the other remained stable, the per-subject outcome was scored as improved.
In addition, to evaluate radiographic response, a neuroradiologist at 8 of the sites compared the immediate postchemotherapy MRI scans with the prechemotherapy scans; central review of the scans was not performed. The size of the tumor was assessed using the product of the largest 2 perpendicular diameters on T2-weighted or fluid attenuation inversion recovery imaging. Changes in the intensity of post–gadolinium contrast enhancement were recorded but not used as a parameter to judge response. Radiographic response was defined as follows: complete response (CR) = complete disappearance of tumor; partial response (PR) = reduction in size of the solid parts of the tumor by more than 50%; minor response (MR) = reduction in size of the solid parts of the tumor between 50% and 25%; stable disease (SD) = reduction of the size of the solid parts of the tumor of less than 25%, no change in tumor size, or tumor progression of no more than 25% and no appearance of new tumor lesions; progressive disease (PD) = enlargement of the primary tumor by greater than 25% or the appearance of new lesions. Tumor location was characterized by the most posteriorly involved structure of the visual pathway.
Statistical Analysis
Analyses and calculations were performed using Stata 11 (StataCorp). Univariate analyses to determine the relation of tumor location, age at treatment, gender, NF1 type, chemotherapy regimen, optic pallor or edema at diagnosis, or radiographic outcome to visual outcome (as a categorical variable) were performed using Fisher's exact tests (analyses of proportions for 2 × 2, 3 × 2, 3 × 3, and 4 × 2 tables). Age was divided for analysis into 3 groups (<2 y, 2–5 y, >5 y), as most prior studies evaluating age as a risk factor for progression following treatment used 2 or 5 years as the cutoff.8,19–21 Logistic regression models, with age categories as indicator variables, were used to examine the association between visual outcome and potential predictors, including tumor location and optic disc pallor, accounting simultaneously for age. Analyses were performed on a per subject or per eye basis as appropriate. Type I error for significance was set at P< .05.
Results
Demographics
One hundred fifteen subjects who met eligibility criteria were identified from 10 institutions with large, established, NF clinical programs. The clinical characteristics of the study population are detailed in Table 1. Approximately half of the subjects had tumor involvement of the optic tracts or radiations. Of note, all of these subjects also had involvement of the optic chiasm, and all but 3 had optic nerve involvement. When the chiasm was the most posteriorly involved structure, the nerve was always affected.
Table 1.
Characteristics of study population
| Characteristic | n (%) |
|---|---|
| Gender | |
| Female | 71 (62) |
| Male | 44 (38) |
| NF1 type | |
| Familial | 55 (48) (30 F, 25 M) |
| Sporadic | 56 (49) (38 F, 18 M) |
| Unknown | 4 (3) |
| Tumor locationa | |
| Nerve | 17 (15) |
| Chiasm | 27 (23) |
| Hypothalamus | 16 (14) |
| Tracts/radiations | 55 (48) |
| Chemotherapy regimen | |
| Vcr/carbo (weekly) | 91 (79) |
| Vcr/carbo (monthly) | 14 (12) |
| Carbo (monthly) | 8 (7) |
| Carbo (every other week) | 1 (1) |
| Vinblastine | 1 (1) |
Abbreviations: NF1, neurofibromatosis type 1; vcr, vincristine; carbo, carboplatin; F, female; M, male.
aLocation is the most posteriorly involved part of the visual pathway.
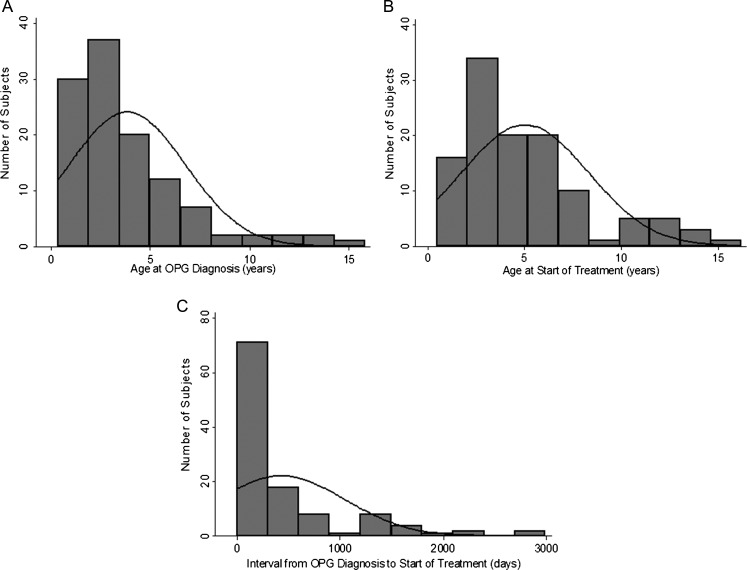
The median age at OPG diagnosis was 2.66 years (range, 0.36–15.8) (Fig. 1A). Indications for diagnostic MRI included abnormal eye symptoms or examination (44%), screening neuroimaging in asymptomatic patients (43%), headache with or without other symptoms (4%), evaluation of an orbital or head plexiform neurofibroma (3%), and various other indications (each <2%). No subject was diagnosed because of precocious puberty. The median interval from diagnosis to initiation of treatment was 133 days (range, 1–2990) (Fig. 1C). As expected, almost all of the subjects received a carboplatin-based chemotherapy regimen, 79% on a weekly schedule and 19% on a monthly schedule.
Fig. 1.
Age at OPG diagnosis, age at start of treatment, and interval between OPG diagnosis and treatment. (A) Age at OPG diagnosis: median 2.66 y (range, 0.36–15.8 y). (B) Age at first chemotherapy dose: median, 4.04 y (range, 0.48–16.2 y). (C) Interval from OPG diagnosis to initiation of treatment: median, 133 days (range, 1–2990 days).
Indications for Treatment
Indications for treatment are listed in Table 2. VA loss and tumor growth were the most frequently listed reasons to initiate therapy, and these were cited together as primary indications in 23 cases. For most subjects, more than 1 primary factor drove the decision to treat (median, 2; mean, 2.1; range, 1–6). Only 29 subjects commenced therapy for a single indication. There appear to be differences in the pattern of indications cited among the institutions (Table 3), with some sites treating more often for VA decline (sites 1–4), and others more often for radiographic tumor progression (sites 5–7). Other sites (8–10) had more of an equal balance between these 2 most commonly cited treatment indications. The differences among institutions also extended to other frequently mentioned indications. Of note, only 2 subjects were treated for visual field loss without VA loss, and only 3 of 28 subjects with abnormal visual fields at the start of treatment had normal VA.
Table 2.
Indications for treatment (n = 115 subjects)
| Total | Primary | Single | |
|---|---|---|---|
| No. indications per subject | |||
| Range | 1–7 | 1–6 | |
| Median | 2 | 2 | |
| Mean | 2.5 | 2.1 | |
| Indication | |||
| Visual acuity loss | 70 | 68 | 18 |
| Tumor growth | 64 | 62 | 10 |
| Tumor size/extent | 24 | 18 | |
| Tumor enhancement | 23 | 16 | |
| Visual field loss | 17 | 16 | |
| Tumor location | 21 | 13 | |
| Progressive proptosis | 10 | 10 | 1 |
| Optic disc pallor | 19 | 8 | |
| Decrease acuity in 1 eye with risk to other eye | 10 | 8 | |
| Unreliability of visual exam in young child | 13 | 7 | |
| Optic disc swelling | 3 | 3 | |
| Change in VEP latency | 2 | 2 | |
| Decrease in color vision | 2 | 2 | |
| Growth of non-OPG tumor | 2 | 1 | |
| Hydrocephalus | 2 | 1 | |
| Pain | 1 | 1 | |
| Patient age | 1 | 1 | |
Abbreviations: VEP, visual evoked potential; OPG, optic pathway glioma.
Total = primary (ie, main reasons; there may be >1 per subject) + secondary indications.
Single = only 1 indication for treatment reported.
Table 3.
Primary indications per site
| Indication | Site |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Visual acuity loss | 4 | 7 | 12 | 14 | 3 | 3 | 2 | 15 | 4 | 4 |
| Tumor growth | 1 | 4 | 3 | 7 | 10 | 6 | 5 | 17 | 5 | 4 |
| Tumor size/extent | • | • | • | • | ||||||
| Tumor enhancement | • | • | • | • | • | |||||
| Visual field loss | • | • | • | • | • | |||||
| Tumor location | • | • | • | |||||||
| # of subjects | 5 | 10 | 13 | 17 | 12 | 7 | 5 | 32 | 8 | 6 |
• = Indication in >15% subjects at that site. Sites are listed in random order.
Visual Acuity Outcome
Eighty-eight subjects and 168 eyes were evaluable for VA outcome. The disparity between the number of subjects and eyes reflects patients who had enucleation of 1 eye and cases in which only binocular acuity or quantitative data on one eye were provided. At the completion of chemotherapy, VA had improved in 32% of subjects and 22% of eyes, remained stable in 40% of subjects and 57% of eyes, and declined in 28% of subjects and 21% of eyes (Table 4; Supplementary material, Table S1). Of 74 subjects with outcome data on both eyes, 44 (59%) had concordant acuity outcomes, and only 2 had one eye improve and the other deteriorate.
Table 4.
Visual acuity outcome by tumor location (per subject)
| Tumor Locationa (n) | Visual Acuity Outcome (%, n) |
||
|---|---|---|---|
| Improved | Stable | Worse | |
| Nerve (14) | 36 (5) | 43 (6) | 21 (3) |
| Chiasm (20) | 40 (8) | 45 (9) | 15 (3) |
| Hypothalamus (12) | 42 (5) | 42 (5) | 17 (2) |
| Tracts/radiations (42) | 24 (10) | 36 (15) | 40 (17) |
| Total (88) | 32 (28) | 40 (35) | 28 (25) |
aLocation is the most posteriorly involved part of visual pathway.
On univariate analysis, tumor involvement of the optic tracts/radiations (P = .02 per subject, P = .01 per eye) and optic pallor at the start of treatment (P = .005 per eye) were associated with worse VA outcome. Age was also a prognostic factor for poor acuity outcome (P = .04 per subject, P = .02 per eye); children 2 to 5 years of age were less likely to have a meaningful decline in acuity compared with subjects younger than2 or older than 5 years (Table 5). Not only were subjects younger than2 years at highest risk for visual decline, they also were least likely to demonstrate improvement in VA with treatment (P = .07 per subject, P = .08 per eye). In fact, no subject in this age group had improvement in VA. Gender, NF1 type (familial versus sporadic), chemotherapy regimen (weekly versus monthly carboplatin), optic edema at the start of treatment, and radiographic outcome were not risk factors for visual deterioration in either per subject or per eye analyses.
Table 5.
Visual acuity outcome by age at time of treatment (per subject)
| Age (n) | Visual Acuity Outcome (%, n) |
||
|---|---|---|---|
| Improved | Stable | Worse | |
| <2 y (8) | 0 (0) | 50 (4) | 50 (4) |
| 2–5 y (38) | 37 (14) | 47 (18) | 16 (6) |
| >5 y (42) | 33 (14) | 31 (13) | 36 (15) |
Multivariate analysis (per subject), including age and tumor location, revealed tumor involvement of the optic tracts/radiations (odds ratio [OR], 3.0; 95% confidence interval [CI], 1.1–8.3; P = .032) as the only significant prognostic factor for worsening of VA; however, there was a trend toward an association with age younger than2 years (OR, 0.19; 95% CI, 0.03–1.0; P = .055) or older than5 years (OR, 0.38; 95% CI, 0.12–1.1; P = .085). In the per-eye analysis, tumor location (OR, 2.7; 95% CI, 1.1–6.7; P = .035), optic pallor (OR, 2.8; 95% CI, 1.1–7.5; P = .034), and age >5 years (OR, 0.36; 95% CI, 0.14–0.94; P = .038) were prognostic factors, while age <2 years was not significant (OR, 0.33; 95% CI, 0.07–1.7; P = .186).
Correlation of Visual Acuity and Radiographic Outcomes
Data were available on the radiographic outcome at the end of treatment in 96 subjects (CR 0, PR 21, MR 10, SD 57, PD 8). Seventy-one subjects were evaluable for both VA and radiographic outcomes. Of these, there were 16 PR (22.5%), 8 MR (11.3%), 43 SD (60.6%), and 4 PD (5.6%).
There was a poor correlation between radiographic and VA outcomes (Table 6). Using strict oncologic response criteria (CR + PR only), 25% of subjects with a response by MRI and 29% of those with stable disease (SD + MR) had worsening of VA (P = .44). If response is defined more loosely (CR + PR + MR), the correlation is no better (29% with radiographic response and 28% with SD have worsening of vision, P = .28). In addition, 33% (SD + MR) and 37% (SD only) of those with stable disease had improvement in vision, and 1 of 4 subjects had improvement in acuity despite radiographic PD.
Table 6.
Correlation between MRI and visual acuity outcome
| MRI Outcome (n) | Visual Acuity Outcome (%, n) |
||
|---|---|---|---|
| Improved | Stable | Worse | |
| Improved (CR + PR) (16) | 31 (5) | 44 (7) | 25 (4) |
| Stable (MR + SD) (51) | 33 (17) | 37 (19) | 29 (15) |
| Worse (PD) (4) | 25 (1) | 0 (0) | 75 (3) |
| Total (71) | 32 (23) | 37 (26) | 31 (22) |
Abbreviations: CR, complete response; PR, partial response; MR, minor response; SD, stable disease; PD, progressive disease.
Other Visual Outcomes
Information on other visual parameters (Supplementary material, Table S2) was not obtained or recorded in 11% (optic pallor) to 48% (visual fields) of the subjects at the start of treatment. Of note, by the end of therapy, the degree of proptosis had improved in 55% of subjects with proptosis prior to treatment. Visual fields worsened (eg, progressed from a quadrantanopia to a hemianopia) in 42%. The visual field outcome mostly mirrored that of VA (data not shown).
Discussion
Although most subjects with NF1-OPG had improvement or stabilization of vision after treatment with chemotherapy, VA worsened in 28% of subjects and 21% of eyes despite treatment. It is unknown whether some of the visual worsening during therapy reflected damage that had already occurred prior to treatment as opposed to continued tumor progression. In this regard, there are few studies in the literature specifically designed to assess the utility of chemotherapy for vision preservation. A recent meta-analysis identified only 8 “high-quality” publications in the literature that examined the visual outcome of OPG treated with chemotherapy.11 Only 1 of these studies was multi-institutional, and the largest included 57 subjects with visual outcomes. Of the 174 subjects in the meta-analysis, 14.4% had improvement, 47.1% stability, and 38.5% worsening of vision following chemotherapy. The applicability of the conclusions to clinical practice suffers from the limitations of the studies reviewed, including lack of a clear definition of visual response, lack of quantitative assessment of vision, or lack of within-subject evaluation. In addition, the wide range of time points chosen as endpoints for visual assessment, and the grouping of both NF1-associated and sporadic OPGs together, make it difficult to identify risk factors for visual outcome. In prior studies, NF1-OPGs were less likely than sporadic OPGs to have associated visual impairment at diagnosis and to exhibit radiographic progression over time.22–24
While several studies suggest that the visual loss prior to OPG treatment is irreversible,3,11 we clearly document VA improvement in 32% of subjects and 22% of eyes. This is important information for families and has significant implications for treating physicians when making decisions. Given that half of our subjects initiated treatment within 4½ months of OPG diagnosis, our study population may differ from those of prior studies in the duration of visual loss prior to therapy, although this was not directly assessed. Thus, treating patients with recent visual loss prior to irreversible damage might result in better functional outcomes. In addition, we do not have a rate of VA improvement for untreated NF1-OPG patients for comparison. It has been reported in the literature that some patients with NF1-OPG and VA loss improve spontaneously, and this has been suggested as a reason to hesitate initiating chemotherapy in patients with vision loss. Given the rarity of this phenomenon, its potential impact on the rate of improvement seen in our study is likely to be minimal.
We identified several factors associated with poor visual outcomes despite treatment. These include age (<2 y or >5 y) and optic pallor at the time of treatment. The former finding is consistent with previous studies that reveal that young age is a poor prognostic factor for tumor progression; however, the age of highest risk is variably reported as less than 1, 2, or 5 years.10,19,25 In contrast, age >5 years was associated with worse progression-free survival in the seminal publication on the efficacy of vincristine and carboplatin for the treatment of newly diagnosed, progressive low-grade glioma.8 While the prognostic significance of age disappears in the per-subject multivariate analysis, there is a trend toward an association. In addition, in the per-eye evaluation, age >5 years remains prognostic. This discrepancy may be a reflection of sample size and points to the need for a larger prospective study to evaluate the contribution of age to VA outcome.
The predictive value of optic pallor is difficult to determine, given that pallor often occurs in patients with OPG and no visual deficits. It is possible that pallor is an indicator of preexisting damage and heralds subsequent vision loss. It is also conceivable that the degree of optic pallor is the important marker of visual outcome5; however, we did not capture quantitative data on optic pallor.
Although prognostic factors for OPG progression have been assessed in numerous studies, the focus has been on radiographic tumor progression rather than visual outcomes. Tumor involvement in the most posterior portion (postchiasmatic) of the visual pathway has been associated with a higher likelihood of VA loss,26,27 although not all studies support this conclusion.28,29 In our univariate and multivariate analyses, tumor involvement of the optic tracts/radiations was significantly associated with progressive visual loss despite chemotherapy. Hypothalamic involvement did not confer a poor visual prognosis, consistent with its anatomic location outside of the visual pathway.
Particularly striking is the poor correlation between visual and radiographic outcomes. Utilizing a clear definition of radiographic response, only 34%–38% of subjects (depending on whether those with MR are considered to be stable or improved) had concordant visual and radiographic outcomes, while 7%–11% of subjects had one outcome improved while the other was worse. Several smaller series have noted similar disparate results,5,13,30 although a clear objective definition of radiographic response was not always reported in these studies.5,13 Our findings call into question the traditional oncology method of defining response simply in terms of changes in tumor size rather than incorporating functional outcomes. It is possible that our results are affected by the inherent difficulty of measuring OPG size reliably in NF1 patients, who often have concurrent non-neoplastic areas of hyperintensity on T2-weighted MRI sequences (formerly referred to as unidentified bright objects). This seems unlikely given our use of standardized oncology criteria to define tumor response and progression (minimum 25% change) and review of the MRI scans by a neuroradiologist at each participating site.
There appears to be little consensus regarding the indications for treatment in our study, despite the involvement of high-volume NF clinical centers with large patient populations. Although VA loss and tumor progression were the main reasons for treatment, a combination of factors drove the responsible physicians to treat in most cases. Whether these differences among centers are due to the weight that individual physicians place on certain factors, differences in the referral patterns, or institutional practice biases, they demonstrate the need for uniform criteria for treatment.
For this cohort of NF1-OPGs that required treatment, the need for treatment was apparent early, as the median time from diagnosis of OPG to the initiation of treatment in our cohort was less than 4½ months. Since more than65% of subjects initiated treatment within 1 year of diagnosis and approximately 85% within 3 years, we advocate that patients with newly diagnosed NF1-OPGs undergo neuro-ophthalmology and neuro-oncology evaluation every 3 months for the first year, every 6 months for the next 2 years, and yearly thereafter. The identification of late presentations of symptomatic OPG suggests that continued yearly evaluations for up to 8 years after OPG diagnosis may be warranted. The optimal frequency of neuro-imaging follow-up has yet to be determined.
The strengths of the present study include its large sample size, involvement of centers with clinical expertise in the treatment of children with NF1, uniform population of NF1-associated OPGs, standardized assessment time points, and quantitative, clearly defined visual outcomes that were applied consistently across all centers. However, given that our outcome assessments were performed at the completion of therapy, no comment can be made on the durability of visual response. Our study had a higher percentage of subjects younger than 2 years old who were inevaluable for VA outcome because of a lack of quantitative data, highlighting the difficulty in obtaining reliable vision examinations in very young children and the need to explore potential surrogate markers for acuity, such as optical coherence tomography.31 In addition, adequate data on most of the ancillary visual outcomes (visual fields, ocular alignment, etc) were lacking. This deficiency underscores the challenge inherent in retrospective evaluations of visual parameters that are not traditionally reported in a quantitative fashion.
In summary, our multicenter study identified several important findings with clear clinical importance. First, although visual outcomes after treatment with chemotherapy for NF1-OPG are not optimal, there are children who regain vision with treatment. Second, tumor involvement of the optic tracts/radiations is the most consistent prognostic factor for poor visual outcome. Third, the lack of correlation between visual and radiographic outcomes argues against the use of MRI response as the gold standard of treatment success for this tumor. Fourth, the lack of agreement on indications for treatment of OPG among the large centers participating in this study highlights the need for better standardization of the care of these patients. Fifth, the short interval from diagnosis to initiation of treatment in the majority of NF1-OPGs has implications for the intensity of follow-up. These observations provide the key questions that can only be adequately addressed with a prospective collaborative study involving neuro-oncologists and neuro-ophthalmologists, which would employ standardized visual assessment methods and clear definitions of visual outcomes and data acquisition time points.
Supplementary Material
Acknowledgments
The authors thank the NF1 Optic Pathway Glioma Working Group (Stewart Goldman, Gary Hedlund, Mira Irons, Karine Lascelles, Jane Leitch, Danny Morrison, Kathryn North, Brian Weiss), Anne Albers, Annie Kuo, Angie Miller, Pamela Nguyen, Elena Tsangaris, Janice Lasky Zeid. Children's Tumor Foundation Optic Pathway Glioma Conference, Utah, June 2007 (David H. Gutmann, Meeting Chair). This study was presented in part in abstract form at the following scientific meetings: Joint Meeting of the Society for Neuro-Oncology and the AANS/CNS Section on Tumors, New Orleans, LA, October 2009; 36th Annual Meeting of the North American Neuro-Ophthalmology Society, Tuscon, AZ, March 2010; Children's Tumor Foundation Neurofibromatosis Conference, Baltimore, MD, June 2010; 14th International Symposium on Pediatric Neuro-Oncology, Vienna, Austria, June 2010.
Conflict of interest statement. None declared.
References
- 1.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61:189–198. doi: 10.1002/ana.21107. doi:10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Listernick R, Ferner RE, Piersall L, Sharif S, Gutmann DH, Charrow J. Late-onset optic pathway tumors in children with neurofibromatosis 1. Neurology. 2004;63:1944–1946. doi: 10.1212/01.wnl.0000144341.16830.01. [DOI] [PubMed] [Google Scholar]
- 3.Dalla Via P, Opocher E, Pinello ML, et al. Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuro-oncology program. Neuro Oncol. 2007;9:430–437. doi: 10.1215/15228517-2007-031. doi:10.1215/15228517-2007-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shuper A, Horev G, Kornreich L, et al. Visual pathway glioma: an erratic tumour with therapeutic dilemmas. Arch Dis Child. 1997;76:259–263. doi: 10.1136/adc.76.3.259. doi:10.1136/adc.76.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campagna M, Opocher E, Viscardi E, et al. Optic pathway glioma: long-term visual outcome in children without neurofibromatosis type-1. Pediatr Blood Cancer. 2010;55:1083–1088. doi: 10.1002/pbc.22748. doi:10.1002/pbc.22748. [DOI] [PubMed] [Google Scholar]
- 6.Gayre GS, Scott IU, Feuer W, Saunders TG, Siatkowski RM. Long-term visual outcome in patients with anterior visual pathway gliomas. J Neuroophthalmol. 2001;21:1–7. doi: 10.1097/00041327-200103000-00001. doi:10.1097/00041327-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Grill J, Laithier V, Rodriguez D, Raquin MA, Pierre-Kahn A, Kalifa C. When do children with optic pathway tumours need treatment? An oncological perspective in 106 patients treated in a single centre. Eur J Pediatr. 2000;159:692–696. doi: 10.1007/s004310000531. doi:10.1007/s004310000531. [DOI] [PubMed] [Google Scholar]
- 8.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–754. doi: 10.3171/jns.1997.86.5.0747. doi:10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 9.Prados MD, Edwards MS, Rabbitt J, Lamborn K, Davis RL, Levin VA. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32:235–241. doi: 10.1023/a:1005736104205. doi:10.1023/A:1005736104205. [DOI] [PubMed] [Google Scholar]
- 10.Laithier V, Grill J, Le Deley MC, et al. Progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy—results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol. 2003;21:4572–4578. doi: 10.1200/JCO.2003.03.043. doi:10.1200/JCO.2003.03.043. [DOI] [PubMed] [Google Scholar]
- 11.Moreno L, Bautista F, Ashley S, Duncan C, Zacharoulis S. Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer. 2010;46:2253–2259. doi: 10.1016/j.ejca.2010.03.028. doi:10.1016/j.ejca.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Nicolin G, Parkin P, Mabbott D, et al. Natural history and outcome of optic pathway gliomas in children. Pediatr Blood Cancer. 2009;53:1231–1237. doi: 10.1002/pbc.22198. doi:10.1002/pbc.22198. [DOI] [PubMed] [Google Scholar]
- 13.Shofty B, Ben-Sira L, Freedman S, et al. Visual outcome following chemotherapy for progressive optic pathway gliomas. Pediatr Blood Cancer. 2011:481–485.. doi: 10.1002/pbc.22967. doi:10.1002/pbc.22967. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain MC, Grafe MR. Recurrent chiasmatic-hypothalamic glioma treated with oral etoposide. J Clin Oncol. 1995;13:2072–2076. doi: 10.1200/JCO.1995.13.8.2072. [DOI] [PubMed] [Google Scholar]
- 15.Janss AJ, Grundy R, Cnaan A, et al. Optic pathway and hypothalamic/chiasmatic gliomas in children younger than age 5 years with a 6-year follow-up. Cancer. 1995;75:1051–1059. doi: 10.1002/1097-0142(19950215)75:4<1051::aid-cncr2820750423>3.0.co;2-s. doi:10.1002/1097-0142(19950215)75:4<1051::AID-CNCR2820750423>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Massimino M, Spreafico F, Cefalo G, et al. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20:4209–4216. doi: 10.1200/JCO.2002.08.087. doi:10.1200/JCO.2002.08.087. [DOI] [PubMed] [Google Scholar]
- 17.Packer RJ, Sutton LN, Bilaniuk LT, et al. Treatment of chiasmatic/hypothalamic gliomas of childhood with chemotherapy: an update. Ann Neurol. 1988;23:79–85. doi: 10.1002/ana.410230113. doi:10.1002/ana.410230113. [DOI] [PubMed] [Google Scholar]
- 18.Petronio J, Edwards MS, Prados M, et al. Management of chiasmal and hypothalamic gliomas of infancy and childhood with chemotherapy. J Neurosurg. 1991;74:701–708. doi: 10.3171/jns.1991.74.5.0701. doi:10.3171/jns.1991.74.5.0701. [DOI] [PubMed] [Google Scholar]
- 19.Ahn Y, Cho BK, Kim SK, et al. Optic pathway glioma: outcome and prognostic factors in a surgical series. Childs Nerv Syst. 2006;22:1136–1142. doi: 10.1007/s00381-006-0086-7. doi:10.1007/s00381-006-0086-7. [DOI] [PubMed] [Google Scholar]
- 20.Fouladi M, Hunt DL, Pollack IF, et al. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children's Cancer Group high-grade glioma study CCG-945. Cancer. 2003;98:1243–1252. doi: 10.1002/cncr.11637. doi:10.1002/cncr.11637. [DOI] [PubMed] [Google Scholar]
- 21.Gururangan S, Cavazos CM, Ashley D, et al. Phase II study of carboplatin in children with progressive low-grade gliomas. J Clin Oncol. 2002;20:2951–2958. doi: 10.1200/JCO.2002.12.008. doi:10.1200/JCO.2002.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Astrup J. Natural history and clinical management of optic pathway glioma. Br J Neurosurg. 2003;17:327–335. doi: 10.1080/02688690310001601216. doi:10.1080/02688690310001601216. [DOI] [PubMed] [Google Scholar]
- 23.Chateil JF, Soussotte C, Pedespan JM, Brun M, Le Manh C, Diard F. MRI and clinical differences between optic pathway tumours in children with and without neurofibromatosis. Br J Radiol. 2001;74:24–31. doi: 10.1259/bjr.74.877.740024. [DOI] [PubMed] [Google Scholar]
- 24.Kornreich L, Blaser S, Schwarz M, et al. Optic pathway glioma: correlation of imaging findings with the presence of neurofibromatosis. AJNR Am J Neuroradiol. 2001;22:1963–1969. [PMC free article] [PubMed] [Google Scholar]
- 25.Nishio S, Takeshita I, Fujiwara S, Fukui M. Optico-hypothalamic glioma: an analysis of 16 cases. Childs Nerv Syst. 1993;9:334–338. doi: 10.1007/BF00302036. doi:10.1007/BF00302036. [DOI] [PubMed] [Google Scholar]
- 26.Balcer LJ, Liu GT, Heller G, et al. Visual loss in children with neurofibromatosis type 1 and optic pathway gliomas: relation to tumor location by magnetic resonance imaging. Am J Ophthalmol. 2001;131:442–445. doi: 10.1016/s0002-9394(00)00852-7. doi:10.1016/S0002-9394(00)00852-7. [DOI] [PubMed] [Google Scholar]
- 27.Tow SL, Chandela S, Miller NR, Avellino AM. Long-term outcome in children with gliomas of the anterior visual pathway. Pediatr Neurol. 2003;28:262–270. doi: 10.1016/s0887-8994(02)00628-8. doi:10.1016/S0887-8994(02)00628-8. [DOI] [PubMed] [Google Scholar]
- 28.Opocher E, Kremer LC, Da Dalt L, et al. Prognostic factors for progression of childhood optic pathway glioma: a systematic review. Eur J Cancer. 2006;42:1807–1816. doi: 10.1016/j.ejca.2006.02.022. doi:10.1016/j.ejca.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Segal L, Darvish-Zargar M, Dilenge ME, Ortenberg J, Polomeno RC. Optic pathway gliomas in patients with neurofibromatosis type 1: follow-up of 44 patients. J AAPOS. 2010;14:155–158. doi: 10.1016/j.jaapos.2009.11.020. doi:10.1016/j.jaapos.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell AE, Elder JE, Mackey DA, Waters KD, Ashley DM. Visual improvement despite radiologically stable disease after treatment with carboplatin in children with progressive low-grade optic/thalamic gliomas. J Pediatr Hematol Oncol. 2001;23:572–577. doi: 10.1097/00043426-200112000-00004. doi:10.1097/00043426-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Avery RA, Liu GT, Fisher MJ, et al. Retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2011;151:542–549. doi: 10.1016/j.ajo.2010.08.046. e542 doi:10.1016/j.ajo.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.