Abstract
BRAF rearrangements and BRAF V600E point mutations are recurring events in pediatric low-grade gliomas. However, their clinical significance, including possible interactions between these markers and other glioma biomarkers, is unclear. In this study a retrospective cohort of 198 pediatric low-grade gliomas (including 40 treated with adjuvant therapy) was analyzed for BRAF rearrangements, BRAF V600E, p16/CDKN2A deletion, p53 expression, and MIB1 proliferation index. In tumors with BRAF rearrangement, homozygous p16 deletion correlated with shorter progression-free survival (P = .04). A high MIB1 proliferation index trended toward worse response to adjuvant radiotherapy compared to BRAF-rearranged, p16-intact tumors (P = .08). On multivariate analysis, the 2 most consistently powerful independent adverse prognostic markers were midline location (P = .0001) and p16 deletion (P = .03). Tumors with BRAF V600E had a strong trend toward an increased risk for progression (hazard ratio = 2.48, P = .07), whereas those with BRAF rearrangement had a milder trend toward reduced risk (hazard ratio = .54, P = .15). These data suggest that p16 deletion adversely impacts the outcomes of BRAF-driven gliomas, that high proliferation index may be a better marker of progression risk than BRAF, that BRAF rearrangement and BRAF V600E might not necessarily produce comparable outcomes, and that none of these markers is stronger than tumor location in determining prognosis in pediatric low-grade gliomas.
Keywords: BRAF, MIB1, p16, p53, V600E
The discovery of BRAF alterations in pediatric low-grade gliomas has shed some light on the etiology of these tumors. Initial studies identified a biologically active gain of the BRAF gene on 7q34 in pilocytic astrocytomas (PAs)1,2 due to a tandem duplication producing a KIAA1549:BRAF fusion protein,3,4 although a minority of cases involve BRAF fusion with other genes.4–6 The end result of all these rearrangements is loss of the Ras-binding domain on BRAF, producing constitutive BRAF activity in the tumorigenic MAPK/ERK pathway.
BRAF rearrangements are present in about 70% of PAs versus 15% of all other low-grade gliomas in the PA differential1–4,7–10 and are less common in high-grade gliomas.3,10 Rearrangement frequency differs as a function of region and age, being present in 75%–80% of cerebellar PAs versus 55% of noncerebellar PAs1–4,6–8,10,11 and is far less common in adults.12 Roughly half of all pilomyxoid astrocytomas contain BRAF rearrangements,4,7 underscoring the link between these tumors and PAs but making a distinction based on BRAF rearrangement impossible.
Recent work showed that BRAF rearrangement is an independent favorable prognostic factor in supratentorial low-grade pediatric gliomas7; a similar trend toward reduced risk of recurrence was seen in cerebellar PAs.8 Another group showed that posterior fossa tumors diagnosed as grade II gliomas but with BRAF rearrangement were quite indolent despite their histologic appearance.3 Thus, BRAF rearrangement appears to be a relatively favorable prognostic marker.
In addition to rearrangement, the constitutively active BRAF V600E point mutation, previously described in melanomas, colonic adenocarcinomas, and papillary thyroid carcinomas, has been identified in up to 15% of grade II–IV pediatric astrocytomas.13,14 Up to 10% of pediatric PAs contain the mutation, which appears to be more frequent in noncerebellar tumors.2–4,6,7,10,14 V600E is common in other low-grade pediatric gliomas, including 25% of gangliogliomas and 80% of pleomorphic xanthoastrocytomas.7,10,14,15 At times, the mutation can occur in conjunction with a BRAF rearrangement in the same tumor.7
The biological consequences of BRAF activation are currently under investigation. Constitutive BRAF activity induces MAPK activity and gliomagenesis in xenografted mice but eventually leads to tumor senescence.7,16–18 Higher-grade gliomas also have MAPK activation but are usually accompanied by impairment of the p53/Rb cell cycle pathway, which could explain why such tumors undergo progression rather than senescence. In fact, loss of p16 expression in PAs appears to correlate with reduced senescence and with higher risk of aggressive behavior.13,16,19 High p53 expression and an elevated MIB1 proliferation index have already been identified as adverse markers in high-grade pediatric gliomas,20–24 as has high MIB1 in unresectable PAs.25 Yet whether p16, p53, and/or MIB1 can specifically modify the prognostic value of BRAF abnormalities is not completely understood. Furthermore, it is not clear whether BRAF rearrangement and BRAF V600E have the same kind and degree of prognostic impact in pediatric low-grade gliomas or whether any of these biomarkers can predict adjuvant therapeutic response. Here we describe the results of outcome comparisons between BRAF, p16, p53, and MIB1 in a large cohort of pediatric low-grade gliomas.
Materials and Methods
Cohort
The cohort in this study used formalin-fixed paraffin embedded tissue (FFPE) from an institutional neuropathology bank, as previously described.8,26 Institutional review board approval was obtained before initiating the study. Tumors in neurofibromatosis type 1 (NF1) patients were excluded from the cohort. All cases were originally diagnosed as low-grade gliomas; for this study, all were rechecked by 2 neuropathologists (C.H. and R.L.H.). While precise information regarding the degree of resection was not available in every case, over 75% of cerebellar and cerebral cases and less than 10% of midline tumors were judged to have had gross total resection via neurosurgical review of postoperative radiographs (by I.F.P.). Similarly, tumors that were treated with adjuvant therapy were evaluated radiologically and placed into 1 of 3 broad categories: (1) good response, with manageable residual disease; (2) partial response with eventual regrowth; and (3) poor response, usually requiring additional surgical and adjuvant interventions. All radiologic, immunohistochemical, and molecular analyses were performed while blinded to outcome and, when possible, location.
Analysis of P53, MIB1, P16, BRAF, and Morphology
P53 and MIB1 immunohistochemistry and p16 and BRAF fluorescence in situ hybridization (FISH) were performed and quantified as described previously.8,20,24,26–28 A p53 score of 0–1 was considered low, with scores of 2–3 being high. In each case, the area showing highest proliferation on MIB1 immunostaining was targeted for quantification. MIB1 proliferation index was considered high if greater than 10%; this was empirically determined to be the best cutoff using a series of Kaplan–Meier analyses of progression-free survival (PFS; data not shown).
BRAF FISH has been shown to correlate well with real-time (RT) PCR breakpoint analysis and comparative genomic hybridization,7 and its use in the PA subset of this cohort has been previously reported.8 Briefly, the BRAF FISH probe consisted of 3 clones of P1-derived artificial chromosomes: RP4-726N20, RP5-839B19, and RP4-813F11, with a centromeric enumeration probe for chromosome 7 (CEP7) serving as a ploidy control. At least 50 cells were scored per case. The BRAF rearrangement pattern was scored if BRAF:CEP7 was greater or equal to 1.15 and over 20% of tumor cells showed relative BRAF gain. P16 FISH was performed using a probe targeted against 9p21 (Abbott Molecular), with CEP9 serving as the ploidy control. Deletion for 9p21 was scored if both signals were lost (homozygous deletion) in at least 20% of tumor nuclei.
Specific key morphologic variables were semiquantified as previously described.26 Briefly, overall nuclear atypia were scored in each case on a scale of 0 = mild, 1 = moderate, and 2 = severe. Mitoses were counted per 10 cm2. Rosenthal fibers, eosinophilic granular bodies, and necrosis were scored from 0 = none, 1= sparse, 2 = moderate, and 3 = frequent.
BRAF V600E
Tumor targets were manually microdissected from the 5-micron unstained histologic sections. DNA was isolated from each target using the DNeasy Blood and Tissue kit (Qiagen) according to the manufacturer's instructions. The quantity of isolated DNA was assessed using a NanoDrop 1000 spectrophotometer (Thermo Scientific). Detection of BRAF V600E mutation was performed using RT-PCR and post-PCR fluorescence melting curve analysis (FMCA) on a LightCycler (Roche Applied Science) as previously described.29 Briefly, RT-PCR amplification was performed using 5–50 ng of DNA, 40 pmol of each primer, and 2 pmol of each hybridization probe on a LightCycler FastStart DNA Master HybProbe Kit (Roche). The reaction mixture was subjected to 40 cycles of PCR amplification consisting of denaturation at 95°C for 5 s, annealing at 54°C for 20 s, and extension at 72°C for 12 s. Post-amplification FMCA was performed by gradual heating of samples at a rate of 0.1°C/s from 45°C to 95°C. Samples that were positive for mutations on PCR/FMCA were confirmed by Sanger sequencing.
Statistical Analysis
Survival rates were compared via log-rank tests on Kaplan–Meier curves. Cox proportional hazards regression was used to generate multivariate survival models. Means were compared between multiple groups by parametric analysis of variance (ANOVA; Kruskal–Wallis test and Dunn's post hoc) or parametric ANOVA (Tukey's post hoc) where appropriate. Fisher's exact test generated hazard ratios (HRs) between 2 groups. Spearman rank correlation assessed trends between biomarkers and response to adjuvant therapy. All of the aforementioned analyses were done using GraphPad software and StatPages (http://statpages.org/prophaz.html); differences were considered significant at P < .05.
Results
Cohort Characteristics and Survival by Location
One hundred ninety-eight non–NF1-related pediatric low-grade gliomas were retrospectively analyzed (Table 1). Follow-up data were available for 160 cases (80.8%), with a median interval of 6.3 years. Fifty of the 160 cases (31.3%) had disease progression at time of analysis, while 9 (5.6%) had died. The most frequent histologic diagnosis was PA (143 cases, 72.2%), followed by ganglioglioma (27 cases, 13.6%). The most common primary site was the cerebellum, with 96 cases (48.5%, Table 2). Midline tumors, defined as those arising in the diencephalon (thalamus and hypothalamus), midbrain, brainstem, and spinal cord (DMBS), as well as in the basal ganglia, totaled 54 cases (27.3%). Forty-eight tumors arose in nonmidline cerebral sites (24.2%). All PAs had been previously studied8,26 and had updated outcome information for the current analysis.
Table 1.
Cohort characteristics
| General statistics | |
| 198 cases total | 160 cases with follow-up data |
| 111 male, 87 female | Mean follow-up 7.3 ± 0.4 years |
| Mean age 8.2 ± 0.3 years | Median follow-up 6.3 years |
| Median age 7.6 years | Follow-up range 21.3 years |
| Age range 30 days—18.8 years | 9 dead of disease |
| Diagnoses | |
| 143 pilocytic astrocytomas | 4 grade II diffuse astrocytomas |
| 27 gangliogliomas | 2 grade II oligodendrogliomas |
| 7 low-grade gliomas nos | 2 subependymal giant cell astrocytomas |
| 6 pilomyxoid astrocytomas | 1 dysembryoplastic neuroepithelial tumor |
| 6 pleomorphic xanthoastrocytomas | |
| Treatment | |
| 158 surgery only | 40 with adjuvant therapy |
| 13 chemotherapy only | |
| 19 radiation only | |
| 8 radiochemotherapy | |
Abbreviations: nos, not otherwise specified.
A total of 204 low-grade pediatric gliomas made up the cohort, 160 of which had available follow-up information. *Six cases were from NF1 patients and are not included in the rest of these analyses (see Supplementary material, Table S1).
Table 2.
Frequency of non-NF1 pediatric low-grade gliomas by diagnosis, location, and BRAF status
| Diagnosis | Location |
BRAF status |
||||||
|---|---|---|---|---|---|---|---|---|
| Cerebellum | Cerebrum | Diencephalon | Basal ganglia | Midbrain/brainstem | Spinal cord | Rearrangement | Mutation | |
| Pilocytic astrocytoma | 94 | 7 | 21 | 1 | 12 | 8 | 79.6% | 9.1% |
| 90/133 | 10*/110 | |||||||
| (7 failed, 3 NA) | (18 failed, 15 NA) | |||||||
| Ganglioglioma | 0 | 24 | 1 | 1 | 1 | 0 | 40.0% | 22.7% |
| 10/25 | 5/22 | |||||||
| (2 NA) | (3 failed, 2 NA) | |||||||
| Pilomyxoid astrocytoma | 0 | 1 | 5 | 0 | 0 | 0 | 60.0% | 20.0% |
| 3/5 | 1/5 | |||||||
| (1 NA) | (1 NA) | |||||||
| Pleomorphic xanthoastrocytoma | 1 | 5 | 0 | 0 | 0 | 0 | 66.7% | 40.0% |
| 4/6 | 2/5 | |||||||
| (1 failed) | ||||||||
| Other low-grade glioma (diffuse astrocytoma, oligodendroglioma, nos) | 1 | 10 | 2 | 0 | 0 | 0 | 0.0% | 8.3% |
| 0/10 | 1/12 | |||||||
| (3 NA) | (1 failed) | |||||||
| Subependymal giant cell astrocytoma | 0 | 0 | 1 | 1 | 0 | 0 | 1/2 | 0/2 |
| Dysembryoplastic neuroepithelial tumor | 0 | 1 | 0 | 0 | 0 | 0 | 1 NA | 0/1 |
| BRAF rearrangement | 80.0% | 42.9% | 42.3% | 16.7% | 50.0% | |||
| 72/90 | 18/42 | 11/26 | 1/3 | 2/12 | 4/8 | |||
| (4 failed, 2 NA) | (6 NA) | (2 failed, 2 NA) | (1 failed) | |||||
| BRAF mutation | 8.1% | 20.9% | 18.2% | 0/1 | 0.0% | 0.0% | ||
| 6*/74 | 9/43 | 4/22 | (1 failed, 1 NA) | 0/10 | 0/7 | |||
| (13 failed, 9 NA) | (5 failed) | (3 failed, 5 NA) | (1 failed, 2 NA) | (1 failed) | ||||
Abbreviations: NA, insufficient remaining tissue available for analysis; nos, not otherwise specified.
The most common diagnosis and location was pilocytic astrocytoma of the cerebellum. Overall, BRAF rearrangement was more common than BRAF mutation. In the latter cases, all but 2 were V600E mutations; *1 was a frameshift stop codon.
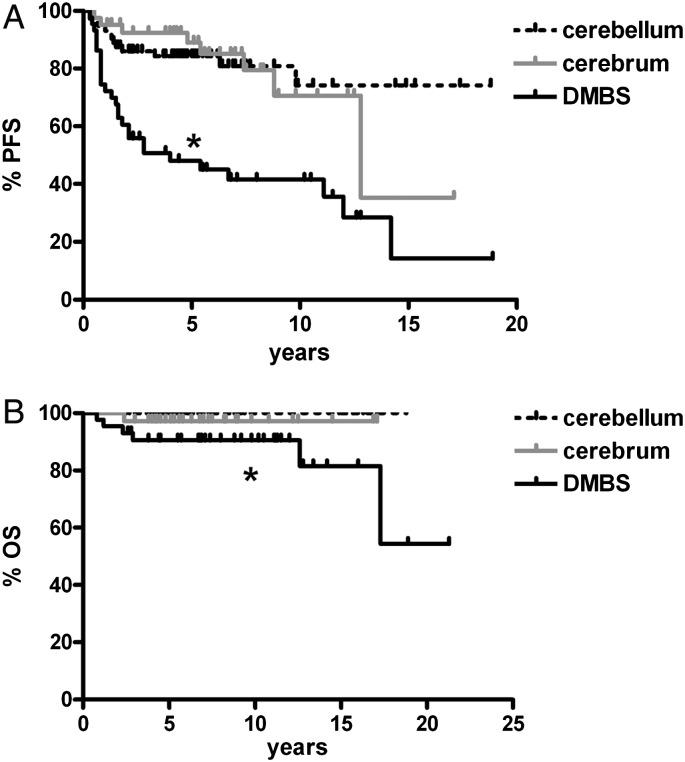
Initial survival analysis by specific locations identified all midline tumor locations as having shorter PFS than cerebral or cerebellar tumors (Supplementary Fig. S1). For all subsequent analyses, the midline tumors were thus combined into the DMBS group, which also included the basal ganglia tumors. As expected, the DMBS subset had shorter PFS and overall survival (OS) compared with cerebral and cerebellar tumors (Fig. 1).
Fig. 1.
Progression-free and overall survival in pediatric low-grade gliomas are worse in midline locations. Tumors arising in the midline (ie, DMBS) had generally worse progression-free survival (A) and overall survival (B) compared with those located in the cerebellum and cerebrum. In (A), *P < .0001 vs cerebellum and = .0003 vs cerebrum; in (B) *P < .001 vs cerebellum and = .17 vs cerebrum.
BRAF Rearrangement and V600E in Pediatric Low-Grade Gliomas
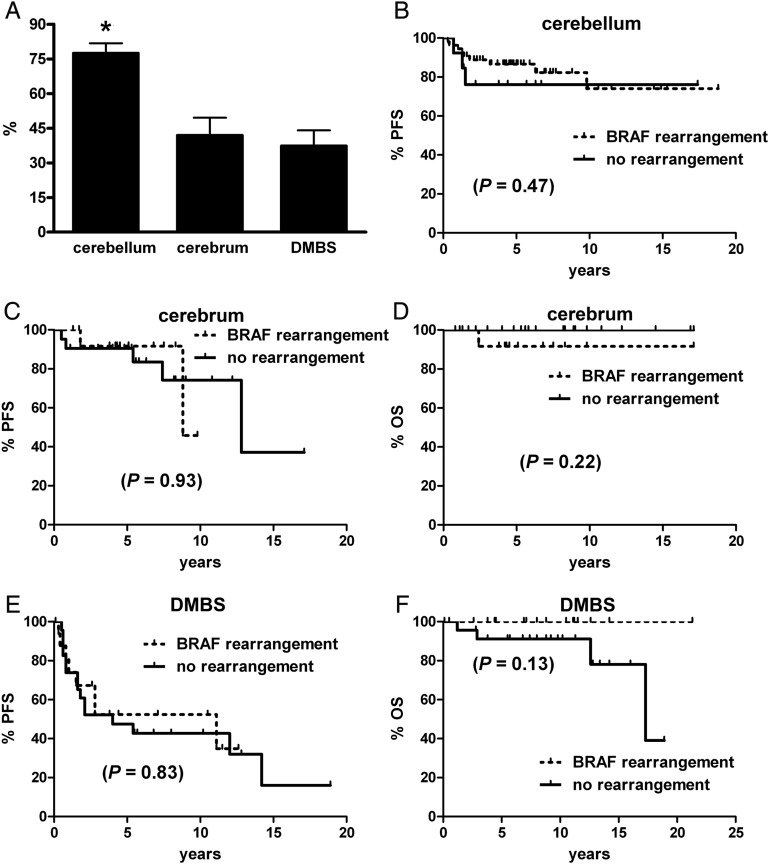
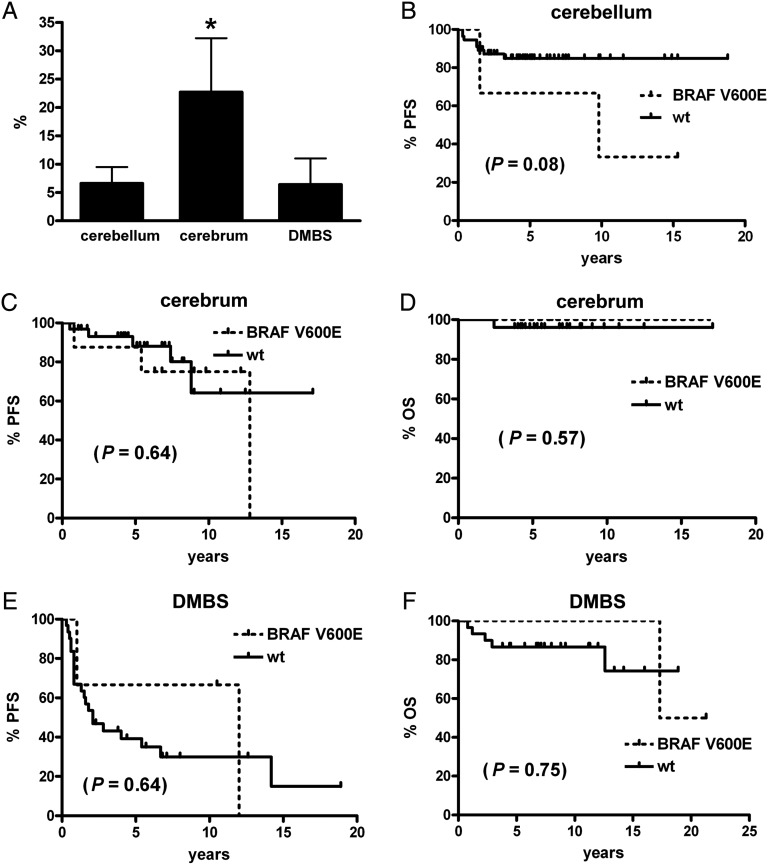
BRAF rearrangement status was able to be determined in 181 cases, while 157 were successfully analyzed for BRAF V600E mutations (Table 2). Rearrangement was far more common overall, with 108 cases showing such a pattern by FISH, whereas only 19 had the V600E mutation. Cerebellar tumors were more likely to have BRAF rearrangement than were either cerebral or DMBS tumors (Fig. 2A), whereas cerebral tumors had a higher rate of V600E mutation than did either cerebellar or DMBS sites (Fig. 3A). In the DMBS subset, only diencephalic tumors had a V600E mutation, meaning that 68.4% of all V600E tumors were located in the supratentorium (P = .02). In contrast, 72.2% of tumors with BRAF rearrangement were infratentorial (P = .0002).
Fig. 2.
PFS and OS in pediatric low-grade gliomas by location and BRAF rearrangement status. (A) Tumors in the cerebellum had a higher rate of BRAF rearrangement than did those in the cerebrum or midline, aka DMBS. *P < .001 via Kruskal–Wallis and Dunn's post hoc. (B–F) BRAF rearrangement did not significantly stratify PFS or OS in any of the 3 tumor subgroups. No deaths occurred in any cerebellar tumor.
Fig. 3.
PFS and OS in pediatric low-grade gliomas by location and BRAF V600E status. (A) Tumors in the cerebrum had a higher rate of BRAF V600E mutation than those in the cerebellum (*P < .05) or midline, aka DMBS. While there was a trend toward worse PFS in cerebellar tumors with BRAF V600E (B), BRAF V600E did not significantly stratify PFS or OS in any of the other tumor subgroups (C–F). No deaths occurred in any cerebellar tumor.
None of the histologic subtypes were statistically more or less likely to have a V600E mutation (overall P = .13); even when combining all non-PA tumors, the difference versus PAs was not significant (19.1% vs 7.3%, P = .23). Multiple-group analysis showed that WHO grade I PAs were more likely than grade II diffusely infiltrative gliomas to have BRAF rearrangement (67.7% vs 0%, P < .01), though no other intergroup comparisons were significant. However, PAs had a higher rate of BRAF rearrangement compared with all non-PAs pooled together (67.7% vs 36.7%, P = .001).
Pooling all low-grade gliomas together, the presence of BRAF rearrangement strongly trended toward improved PFS (P = .06, not shown). Only 1 patient with a rearrangement died, and there was a strong trend toward reduced risk of death in such tumors versus those without the rearrangement (HR = 0.30, 95% confidence interval [CI] = 0.05–1.7, P = .05). In contrast, there was no significant change in PFS or the risk of death in V600E tumors, although the HR for death was above 1.0 (HR = 1.6, 95% CI = 0.23–10.3, P = .52).
Adjusting for location, the presence of BRAF rearrangement failed to significantly stratify low-grade gliomas by survival in the cerebellum (Fig. 2B), cerebrum (Fig. 2C and D), or DMBS (Fig. 2E and F), yet it is noteworthy that the only deaths that occurred in the DMBS region were with tumors lacking BRAF rearrangement (Fig. 2F). Likewise, V600E mutation did not produce significant survival differences by subregions (Fig. 3B–F), although there was a trend toward worse PFS in cerebellar tumors (Fig. 3B).
P16 and BRAF in Pediatric Low-Grade Gliomas
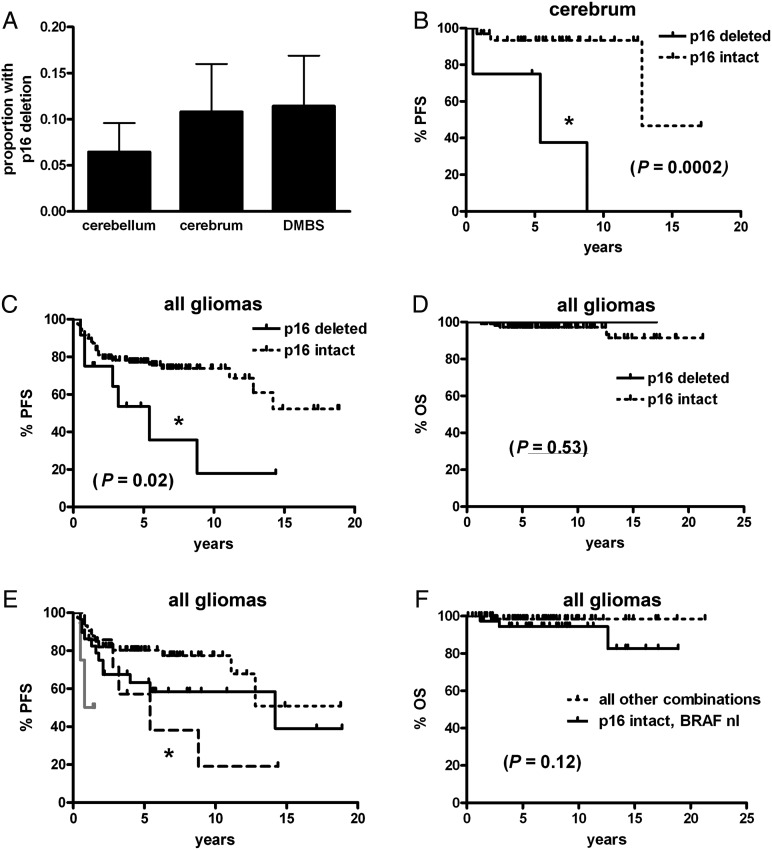
The presence of a p16 deletion was able to be determined via FISH in 167 tumors (see Methods). While the frequency of homozygous p16 deletion did not vary among the 3 tumor regions (Fig. 4A), PFS, but not OS, was markedly shorter in p16-deleted low-grade gliomas (Fig. 4C and D); this was attributable specifically to cerebral tumors (Fig. 4B), as PFS was not significantly different by p16 status in the cerebellar or DMBS locations (P = .67 and P = .45, respectively, data not shown). However, the presence of p16 deletion lowered PFS in gliomas with any BRAF abnormalities (P = .04, Fig. 4E). A similar pattern seemed to be present in BRAF-normal tumors, though there were too few BRAF-normal, p16-deleted cases to generate statistical significance. OS, on the other hand, was not significantly different (Fig. 4F).
Fig. 4.
PFS and OS in pediatric low-grade gliomas by p16 and BRAF status. (A) There was no difference in the frequency of homozygous p16 deletion by tumor site (P = .64). While PFS was shorter in cerebral tumors (B) and all pooled gliomas (C) with p16 deletion, cerebellar and DMBS subsets did not achieve significance (see Results), nor did OS in all gliomas (D). (E) Stratifying all gliomas by p16 deletion and any BRAF abnormality (rearrangement or V600E mutation) showed that when BRAF was abnormal, the concomitant presence of p16 deletion correlated with shorter PFS (short dotted black line = p16 intact, BRAF abnormal; long dotted black line = p16 deleted, BRAF abnormal; solid grey line = p16 deleted, BRAF normal; solid black line = p16 intact, BRAF normal; *P = .04 vs p16 intact, BRAF abnormal). (F) There was, however, no significant stratification of OS by p16 and BRAF in this cohort. DMBS, diencephalon/midbrain/brainstem/spinal cord; nl, normal.
Of note, morphologic semiquantification of nuclear atypia (see Methods) showed a higher degree of atypia in p16-deleted gliomas versus p16-intact gliomas (0.78 ± 0.2 vs 0.30 ± 0.05, P = .037). Neither Ki67 proliferation index, mitotic count, Rosenthal fibers, nor necrosis differed by p16 status (data not shown).
P53 and BRAF in Pediatric Low-Grade Gliomas
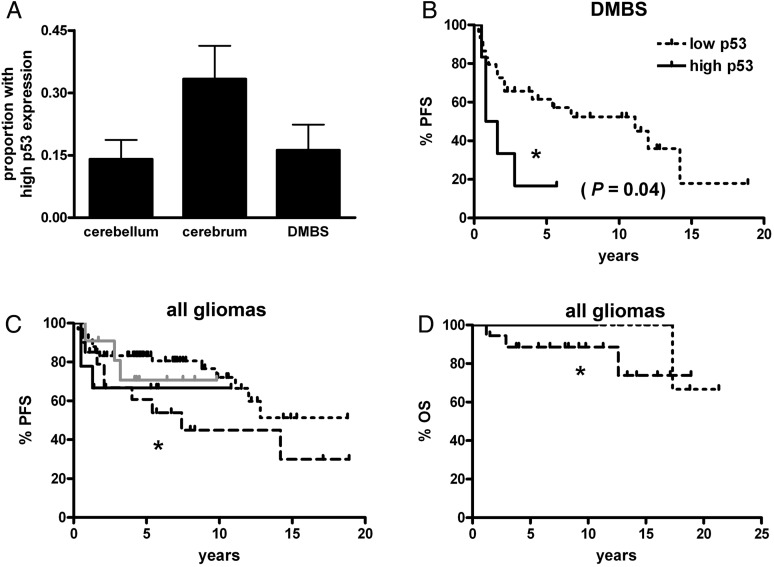
Nuclear p53 expression was semiquantified via immunohistochemical analysis in 166 gliomas (see Methods). Tumors expressing high levels of p53 trended toward being more common in the cerebrum versus the other two regions (Fig. 5A). Yet while p53 did not stratify outcome in all pooled gliomas or in the cerebral or cerebellar subsets (data not shown), high p53 significantly correlated with shorter PFS in DMBS tumors (Fig. 5B). The role of high p53 in BRAF-abnormal gliomas was not clear (Fig. 5C), perhaps because too few tumors had high p53 expression. Interestingly, there was a slight but significantly decreased OS in gliomas with weak p53 expression and normal BRAF versus all other subtypes (Fig. 5D).
Fig. 5.
PFS and OS in pediatric low-grade gliomas by p53 and BRAF status. (A) There was a strong trend toward the cerebrum having more high-expressing p53 tumors than either cerebellar or DMBS subregions (P = .06), although it was only in the DMBS subregion that high-expressing p53 tumors had shorter PFS (B). (C and D) In all pooled gliomas, those with weak p53 expression and normal BRAF had shorter PFS (C, *P = .05) and OS (D, *P = .02) than weak p53-expressing tumors with any BRAF abnormality (either rearrangement or V600E mutation). However, the degree of p53 expression did not modify PFS or OS when adjusting for BRAF status. Short dotted black line = p53 weak, BRAF abnormal; long dotted black line = p53 weak, BRAF normal; solid grey line = p53 strong, BRAF abnormal; solid black line = p53 strong, BRAF normal. DMBS, diencephalon/midbrain/brainstem/spinal cord.
MIB1 and BRAF in Pediatric Low-Grade Gliomas
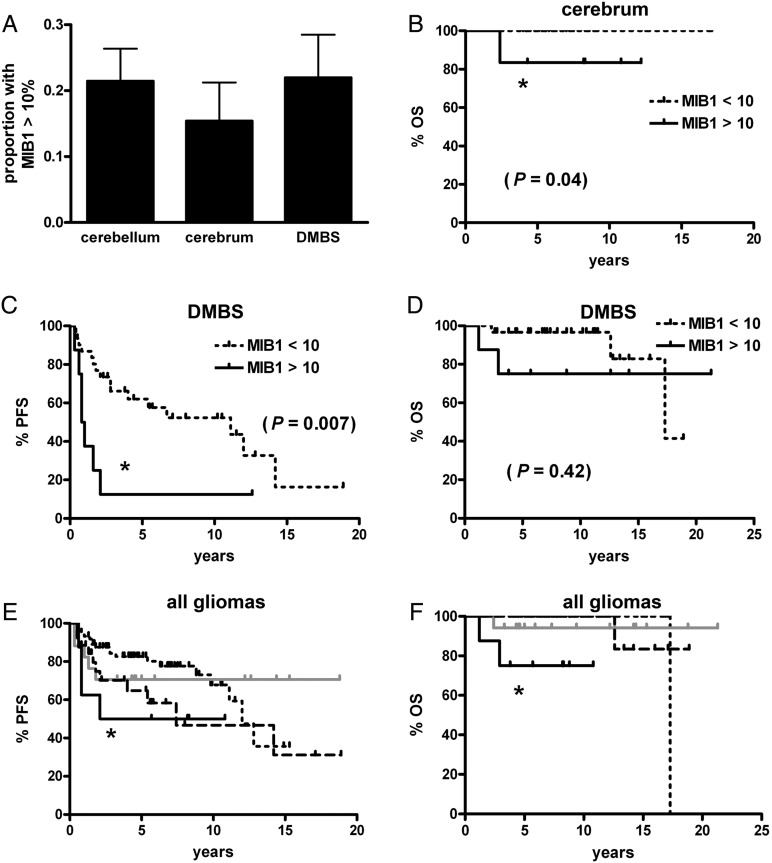
An MIB1 proliferation index was scored in 190 cases (see Methods). The proportion of cases with very high indices, defined as greater than 10%, did not differ among the 3 main tumor regions (Fig. 6A). Likewise, mean indices did not significantly differ among the 3 groups (data not shown). In all gliomas, high proliferation was not a significant marker for PFS (P = .53) or OS (P = .14, graphs not shown). In cerebral gliomas high proliferation correlated with shorter OS (Fig. 6B), but not PFS (P = .60, graph not shown). The only subgroup in which high proliferation correlated with shorter PFS was DMBS (Fig. 6C), though it did not correlate with OS (Fig. 6D). Regarding BRAF, tumors with both high proliferation and no BRAF abnormality had shorter PFS and OS compared with tumors with low proliferation and abnormal BRAF (Fig. 6E and F). Unlike p16 deletion, high proliferation did not appear to have any impact on BRAF-abnormal tumors.
Fig. 6.
PFS and OS in pediatric low-grade gliomas by MIB1 proliferation index and BRAF status. (A) There was no difference in the frequency of high proliferation index by tumor site (P = .70). OS was shorter in cerebral tumors with elevated MIB1 (B), as was PFS (but not OS) in DMBS tumors (C and D, respectively). No other comparisons within each subregion were significant. (E) Stratifying all gliomas by MIB1 and any BRAF abnormality (rearrangement or V600E mutation) showed that when MIB1 was high and BRAF was normal, PFS was shorter compared with tumors with low MIB1 and abnormal BRAF (*P = .049). (F) Likewise, gliomas with high MIB1 and normal BRAF had shorter OS compared with tumors with low MIB1 and abnormal BRAF (*P < .0001), as well as compared with tumors with low MIB1 and normal BRAF (*P = .02). Short dotted black line = MIB1 < 10%, BRAF abnormal; long dotted black line = MIB1 < 10%, BRAF normal; solid grey line = MIB1 >10%, BRAF abnormal; solid black line = MIB1 >10%, BRAF normal. DMBS, diencephalon/midbrain/brainstem/spinal cord.
BRAF Rearrangement or V600E Stratified by P16, P53, or MIB1
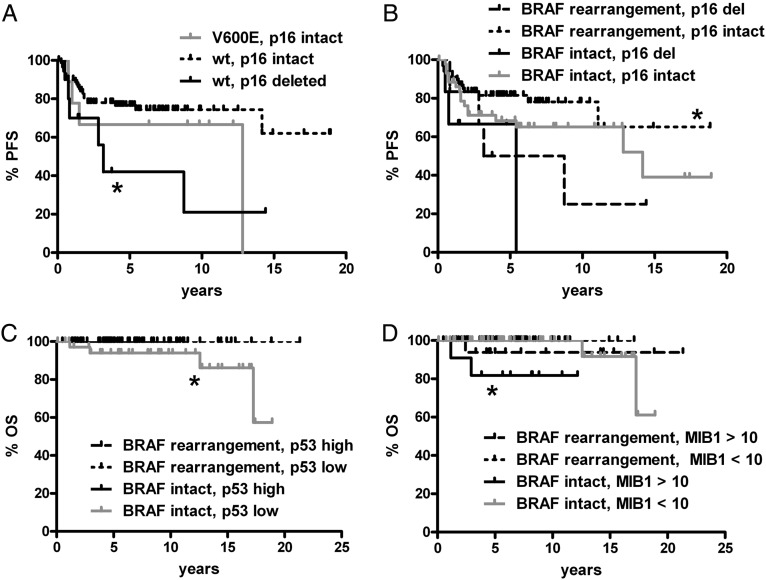
The analyses described above (Figs 4–6) combined gliomas with BRAF rearrangement or V600E into a single group of BRAF-altered tumors, segregated by region. All analyses were also done focusing on either BRAF rearrangement or V600E in all regions pooled together (as splitting by both region and type of BRAF abnormality in the same analysis frequently produced subgroups too small for effective comparisons). Significant associations included shorter PFS in tumors negative for V600E but with p16 deletion, compared with tumors negative for V600E BRAF but with intact p16 (Fig. 7A); shorter PFS in BRAF-rearranged tumors when p16 was deleted versus BRAF-rearranged gliomas with intact p16 (Fig. 7B); shorter OS in gliomas without BRAF rearrangement and with low p53 relative to tumors with BRAF rearrangement and low p53 (Fig. 7C); and shorter OS in tumors without BRAF rearrangement but with high proliferation versus gliomas with a lower proliferation index and either intact or rearranged BRAF (Fig. 7D).
Fig. 7.
Significant PFS and OS associations in pediatric low-grade gliomas by BRAF V600E or rearrangement and p16, p53, or MIB1. All subregions were pooled together; all comparisons not marked with an asterisk (or not shown) are not significant. (A) Tumors without BRAF V600E (wild type [wt] BRAF) but with p16 deletion had shorter PFS than wt BRAF tumors where p16 was intact (*P = .02 vs V600E, p16 intact). There was only 1 case with concomitant V600E mutation and p16 deletion—a left temporal PXA that progressed after 5.4 years, though the patient is alive 10.3 years after original surgery. (B) Likewise, tumors that had both BRAF rearrangement and intact p16 had better PFS than those with p16 deletion regardless of BRAF status (*P = .049 vs BRAF rearrangement, p16 deleted; *0.03 vs BRAF intact, p16 deleted). (C) OS (*P = .03) was shorter in gliomas with intact BRAF and low p53 expression compared with those with low p53 and BRAF rearrangement. PFS also showed a strong trend in the same direction (P = .06, not shown). (D) Tumors with intact BRAF and high MIB1 proliferation index had shorter OS than tumors with a lower proliferation index and either intact or rearranged BRAF (*P = .007 and .0008, respectively). There was also a trend toward worse OS in BRAF-rearranged tumors when MIB1 was high compared with BRAF-rearranged tumors with low MIB1 (P = .06). Del, deleted.
Rare Tumors with both BRAF Rearrangement and V600E Mutation
In the entire cohort there were 6 tumors with both BRAF rearrangement and V600E mutations (Supplementary Table S1), consistent with prior work showing that the 2 lesions can occur in the same tumor.7 Because such tumors were unusual in this cohort, it is difficult to prove whether their behavior is different from the others. Still, 2 of the 6 were PAs that developed metastatic disease, though both responded well to adjuvant therapy. Aside from a 9-year-old female with a left temporal pleomorphic xanthoastrocytoma, who died of surgical complications, the other 5 are alive and well.
Response to Therapy
Forty cases in the cohort required adjuvant therapy (Table 1 and Supplementary Table S2); 25 were DMBS tumors, 11 were cerebellar tumors, and 4 were cerebral tumors. The clinical/radiographic response of each case was categorized as described in the Methods. Although none of the molecular variables showed significant correlations with response, elevated MIB1 proliferation trended toward worse responses to radiotherapy-containing regimens (Table 3).
Table 3.
Correlation of clinical and molecular factors and response to adjuvant therapy in pediatric low-grade gliomas
| Variable | P value, radiotherapy in regimen (N = 17) | P value, chemotherapy in regimen (N = 11) |
|---|---|---|
| Age <5 years | 0.75 | 0.50 |
| DMBS location | 0.24 | 0.17 |
| Non-PA morphology | 0.64 | 0.62 |
| High p53 | 0.53 | 0.31 |
| P16 deleted | 0.97 | 0.33 |
| MIB1 >10% | 0.081 | 0.50 |
| BRAF rearranged | 0.99 | 1.0 |
| BRAF V600E | 0.68 | 0.18 |
After initial surgery, 40 cases of pediatric low-grade gliomas were exposed to adjuvant chemotherapy and/or radiation, with clinical response judged as either poor, partial with regrowth, or good (see Methods Results, and Supplementary Table S2). By multiple regression, a high MIB1 proliferation index showed a trend toward correlating with worse response to radiotherapy but no correlation with regimens containing chemotherapy. No other variable showed any relation to adjuvant therapeutic responses. Modeling with fewer variables (eg, excluding V600E, p53, and p16) to include more cases showed no major differences in P values (not shown).
Multivariate Analyses
Several models of multivariate analyses were tested to determine which factors were the most robust at correlating with PFS (Table 4). Each model varied only by how the BRAF data were treated, focusing on rearrangement only (Table 4, upper left), V600E only (upper right), both rearrangement and V600E grouped together (lower left), or both rearrangement and V600E but analyzed separately (lower right). In all 4 models the most powerful independent adverse prognostic factor by far was midline/DMBS location. In all the models p16 deletion was either an independent adverse prognostic factor or trended in that direction. High MIB1 also trended toward an adverse effect in some models, as did V600E when it was analyzed apart from rearrangement. BRAF rearrangement, on the other hand, was never a significant independent prognostic marker, though its HR consistently suggested reduced risk of progression. Of note, combining V600E and rearrangement into a single variable greatly reduced P value versus just analyzing both types of BRAF abnormality in the same model but as separate variables.
Table 4.
PFS multivariate modeling of clinical, histologic, and molecular factors in pediatric low-grade gliomas
| Variable | HR | 95% CI | P | Variable | HR | 95% CI | P |
|---|---|---|---|---|---|---|---|
| Age <5 y | 1.58 | 0.72–3.44 | .25 | Age <5 y | 1.45 | 0.67–3.28 | .32 |
| Midline location | 3.87 | 1.84–8.11 | .0003 | Midline location | 4.60 | 2.18–9.69 | .0001 |
| Grade I vs II | 1.65 | 0.45–6.00 | .45 | Grade I vs II | 1.54 | 0.45–5.26 | .49 |
| P53 high | 1.53 | 0.66–3.54 | .32 | P53 high | 1.40 | 0.56–3.49 | .47 |
| P16 deleted | 2.88 | 1.03–8.08 | .04 | P16 deleted | 2.50 | 0.91–6.82 | .07 |
| MIB1 >10% | 2.29 | 0.98–5.33 | .05 | MIB1 >10% | 1.87 | 0.77–4.55 | .17 |
| BRAF rearranged | 0.64 | 0.29–1.42 | .27 | BRAF V600E | 2.39 | 0.93–6.15 | .07 |
| Age <5 y | 1.25 | 0.57–2.75 | .57 | Age <5 y | 1.57 | 0.70–3.53 | .27 |
| Midline location | 3.98 | 1.78–8.92 | .0008 | Midline location | 4.54 | 2.08–9.91 | .0001 |
| Grade I vs II | 1.56 | 0.42–5.82 | .51 | Grade I vs II | 1.96 | 0.53–7.21 | .31 |
| P53 high | 1.48 | 0.59–3.73 | .40 | P53 high | 1.60 | 0.63–4.07 | .32 |
| P16 deleted | 2.37 | 0.82–6.80 | .11 | P16 deleted | 3.25 | 1.09–9.65 | .03 |
| MIB1 >10% | 2.01 | 0.81–4.98 | .13 | MIB1 >10% | 2.19 | 0.89–5.42 | .09 |
| Any BRAF abnormality | 0.78 | 0.32–1.91 | .59 | BRAF rearranged | 0.54 | 0.23–1.25 | .15 |
| BRAF V600E | 2.48 | 0.94–6.56 | .07 | ||||
Abbreviations: HR, hazard ratio; CI, confidence interval.
Several models of progression-free survival (PFS) showed that the most consistent powerful marker of shorter PFS was a tumor located in the midline vs either cerebellum or cerebrum. The strongest molecular marker for reduced PFS was homozygous p16 deletion, while high MIB1 similarly trended toward shorter PFS. Interestingly, BRAF rearrangement trended weakly toward longer PFS, yet BRAF V600E strongly trended toward shorter PFS. No variable showed independent prognostic significance for OS in any model. N = 104 (upper left), 96 (upper right), 97 (lower left), and 90 (lower right).
Discussion
The role of constitutive BRAF activation in a large proportion of pediatric low-grade gliomas is of interest both from diagnostic/prognostic and therapeutic perspectives—the former because it may help differentiate indolent tumors from more aggressive tumors, and the latter because BRAF is a “druggable” target for improved adjuvant treatment of residual or recurrent tumors. Trials for anti-BRAF drugs in low-grade pediatric gliomas are in progress, following the favorable results with such agents in melanomas.30,31 From a prognostic perspective it is still unclear whether rearrangement or V600E mutation have differing impacts on outcome or whether other molecular markers can affect the prognostic power of BRAF.
The strong trend toward improved PFS in BRAF-rearranged low-grade gliomas (P = .06, not shown), plus its preponderance in PAs relative to most other tumors (Table 2), could be taken at first glance to be evidence that BRAF rearrangement is indicative of a PA even if tumor morphology does not quite match. However, this trend might not have been because BRAF rearrangement was more common in PAs per se, but rather because BRAF rearrangement is more common in the cerebellum, where tumors are usually more resectable. Multivariate analysis suggested as much (Table 4), and non-PA tumors with BRAF rearrangement are not rare, either in this cohort or in another recently studied cohort.7 On the other hand, even though neither PFS nor OS was significantly different by BRAF rearrangement when adjusting for tumor location (Fig. 2), it is noteworthy that in the high-risk midline tumors, the only deaths were from tumors lacking rearrangement (Fig. 2F). Still, caution is suggested before equating BRAF rearrangement with PA, especially in locations where non-PA low-grade gliomas are more frequent.
Prior work showed that BRAF rearrangement was an independently favorable prognostic factor for a cohort of 70 incompletely resected low-grade pediatric gliomas of the optic pathway, brainstem, and spinal cord.7 In the current cohort there were no non-NF1 optic gliomas and only 20 cases of midbrain, brainstem, and spinal cord tumors, though this cohort had more diencephalic, cerebral, and cerebellar tumors (Table 2). The closest comparison to that prior work is the DMBS subset in this study, where rearrangement did not significantly stratify PFS but did, as mentioned above, show fewer deaths compared with BRAF-intact midline tumors (Fig. 2E and F). Thus, these results do not preclude a favorable effect of rearrangement on PFS in certain contexts but that such an effect may be outweighed by other variables like midline location and p16 status (Table 4).
The p16 protein is located on the CDKN2A locus on 9p21 and is a major checkpoint in the cell cycle. Loss of p16 is well known to correlate with increased WHO glioma grade32 and has been seen in BRAF-driven pediatric low-grade gliomas that behaved more aggressively.13,16,19 Our data support those studies but further suggest that p16 deletion is an adverse marker independent of BRAF status (Figs 4 and 7, Table 4). Interestingly, nuclear atypia also tended to be higher in p16-deleted gliomas (see Results). Likewise, elevated MIB1 generally corresponds to higher glioma grade and was recently reported to connote a higher risk of recurrence in partially resected PAs,26 though its general significance in PAs is unclear.26,33,34 The current data suggest that very high MIB1 indices do trend toward shorter PFS and possibly a higher risk of resistance to radiotherapy but may not specifically modify the prognosis of BRAF-driven gliomas (Figs 6 and 7, Tables 3 and 4).
Regarding p53, its increased expression is more characteristic of grade II astrocytomas rather than grade I PAs,35 which might account for the shorter PFS seen in DMBS tumors with high p53 expression (Fig. 5B). However, the data suggest that high p53 does not modify BRAF-driven outcomes and probably does not add as much clinical value as p16 and MIB1.
Univariate analysis did not show consistently worse PFS in BRAF V600E tumors (Fig. 3), but V600E trended toward worse PFS on multivariate analysis, with an HR opposite to that of BRAF rearrangement. While the latter did not reach significance on multivariate analysis, the more favorable HR associated with rearrangements was consistent with prior work.7,34 These results seem counterintuitive, considering that both rearrangement and V600E produce constitutive BRAF activity and that V600E can induce PA growth but eventual senescence in vivo.16,18
One hypothesis is that although both types of BRAF alterations have the same basic activity, other molecular lesions may preferentially be found in BRAF V600E or BRAF-rearranged tumors and thus drive the tumors toward different outcomes. In our cohort there was no difference in p53 expression, p16 deletion, or MIB1 proliferation between BRAF-rearranged and BRAF V600E gliomas (not shown), so none of these variables are likely to account for the divergent HRs. BRAF V600E mutation may therefore be indicative of a tumor that is at higher risk of worse behavior than a grade I PA. Along those lines, a recent study showed that 10% of malignant pediatric astrocytomas had the BRAF V600E mutation,31 which is a far higher percentage than those that had BRAF rearrangement. Clearly, more cases with dual V600E and rearrangement testing are needed to firmly establish whether there is a true difference in behavior. Until then, caution is recommended when comparing tumors with rearrangement or V600E or when combining them in outcome-based studies like clinical trials.
Except for our prior work focused on key molecular and cell cycle variables, including BRAF rearrangement, p53, p16, and MIB1, in the outcome of pediatric PAs,8 to our knowledge this is the first study to weigh the prognostic and predictive significance of both BRAF rearrangement and BRAF V600E, plus the aforementioned cell cycle markers, in a large cohort of PA and non-PA pediatric low-grade gliomas. Some limitations include the retrospective nature of this study, the predominance of PAs versus other tumor subtypes, and the fact that substratification by region, then by BRAF status (especially V600E), then by p53/MIB1/p16 was often difficult from a statistical perspective. And other than knowing that the vast majority of midline tumors had subtotal resections while most cerebellar and cerebral cases had gross total resections, the intraoperative impression of the degree of resection was not available in most cases. This precludes separating out the individual contributions of resection extent and tumor location, though it is likely that the midline location is an adverse factor due in large part to the difficulty in achieving total tumor resection. Furthermore, specific information on doses and durations of radiotherapy and chemotherapy were often not retrievable in this cohort, thus limiting that aspect of the analysis.
Nevertheless, our data suggest that p16 deletion independently shortens PFS in these gliomas, including in BRAF-altered tumors; that tumors with very high MIB1 proliferation indices may be at higher risk of adjuvant radiotherapy resistance and/or worse outcome, though none of these markers is a strong predictor of response; that the behavior of tumors with BRAF rearrangement might be different from those with V600E mutations; and that despite all of these molecular biomarker discoveries, tumor location (and by extension, tumor resectability) remains by far the most important prognostic variable.
Supplementary Material
Supplementary material is available at Neuro-Oncology Journal online (http://neuro-oncology.oxfordjournals.org/).
Conflict of interest statement. None declared.
Funding
Callie Rohr American Brain Tumor Association Basic Research Fellowship (to C.H.), National Institutes of Health (K08 CA155764-01A1 to C.H.); University of Kentucky College of Medicine Physician Scientist Program Award (to C.H.). I.P. was supported by an NIH R01NS37704 and a grant from the Pediatric Low-Grade Glioma Initiative.
Supplementary Material
References
- 1.Bar EE, Lin A, Tihan T, Burger PC, Eberhart CG. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67:878–887. doi: 10.1097/NEN.0b013e3181845622. doi:10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 2.Pfister S. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–1749. doi: 10.1172/JCI33656. doi:10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones DT, Kocialkowski S, Liu L, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. doi:10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forshew T, Tatevossian RG, Lawson AR, et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218(2):172–181. doi: 10.1002/path.2558. doi:10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 5.Jones DT, Kocialkowski S, Liu L, Pearson DM, Ichimura K, Collins VP. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene. 2009;28(20):2119–2123. doi: 10.1038/onc.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cin H, Meyer C, Herr R, et al. Oncogenic FAM131B-BRAF fusion resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway activation in pilocytic astrocytoma. Acta Neuropathol. 2011;121(6):763–774. doi: 10.1007/s00401-011-0817-z. doi:10.1007/s00401-011-0817-z. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins C, Walker E, Mohamed N, et al. BRAF-KIAA1549 fusion predicts better clinical outcome in pediatric low-grade astrocytoma. Clin Cancer Res. 2011;17(14):4790–4798. doi: 10.1158/1078-0432.CCR-11-0034. doi:10.1158/1078-0432.CCR-11-0034. [DOI] [PubMed] [Google Scholar]
- 8.Horbinski C, Hamilton RL, Nikiforov Y, Pollack IF. Association of molecular alterations, including BRAF, with biology and outcome in pilocytic astrocytomas. Acta Neuropathol. 2010;119(5):641–649. doi: 10.1007/s00401-009-0634-9. doi:10.1007/s00401-009-0634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawson AR, Tatevossian RG, Phipps KP, et al. RAF gene fusions are specific to pilocytic astrocytoma in a broad paediatric brain tumour cohort. Acta Neuropathol. 2010;120(2):271–273. doi: 10.1007/s00401-010-0693-y. doi:10.1007/s00401-010-0693-y. [DOI] [PubMed] [Google Scholar]
- 10.Sievert AJ, Jackson EM, Gai X, et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single-nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 2009;19(3):449–458. doi: 10.1111/j.1750-3639.2008.00225.x. doi:10.1111/j.1750-3639.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H, Okamoto Y, Yokoo H, et al. Gene expression profiling and subgroup identification of oligodendrogliomas. Oncogene. 2004;23(35):6012–6022. doi: 10.1038/sj.onc.1207781. doi:10.1038/sj.onc.1207781. [DOI] [PubMed] [Google Scholar]
- 12.Hasselblatt M, Riesmeier B, Lechtape B, et al. BRAF-KIAA1549 fusion transcripts are less frequent in pilocytic astrocytomas diagnosed in adults. Neuropathol Appl Neurobiol. 2011;37(7):803–806. doi: 10.1111/j.1365-2990.2011.01193.x. doi:10.1111/j.1365-2990.2011.01193.x. [DOI] [PubMed] [Google Scholar]
- 13.Schiffman JD, Hodgson JG, VandenBerg SR, et al. Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant astrocytomas. Cancer Res. 2010;70(2):512–519. doi: 10.1158/0008-5472.CAN-09-1851. doi:10.1158/0008-5472.CAN-09-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. doi: 10.1007/s00401-011-0802-6. doi:10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 15.Dougherty MJ, Santi M, Brose MS, et al. Activating mutations in BRAF characterize a spectrum of pediatric low-grade gliomas. Neuro Oncol. 2010;12(7):621–630. doi: 10.1093/neuonc/noq007. doi:10.1093/neuonc/noq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raabe EH, Lim KS, Kim JM, et al. BRAF activation induces transformation and then senescence in human neural stem cells: A pilocytic astrocytoma model. Clin Cancer Res. 2011;17(11):3590–3599. doi: 10.1158/1078-0432.CCR-10-3349. doi:10.1158/1078-0432.CCR-10-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob K, Quang-Khuong DA, Jones DT, et al. Genetic aberrations leading to MAPK pathway activation mediate oncogene-induced senescence in sporadic pilocytic astrocytomas. Clin Cancer Res. 2011;17(14):4650–4660. doi: 10.1158/1078-0432.CCR-11-0127. doi:10.1158/1078-0432.CCR-11-0127. [DOI] [PubMed] [Google Scholar]
- 18.Gronych J, Korshunov A, Bageritz J, et al. An activated mutant BRAF kinase domain is sufficient to induce pilocytic astrocytoma in mice. J Clin Invest. 2011;121(4):1344–1348. doi: 10.1172/JCI44656. doi:10.1172/JCI44656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez EF, Scheithauer BW, Giannini C, et al. PI3K/AKT pathway alterations are associated with clinically aggressive and histologically anaplastic subsets of pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):407–420. doi: 10.1007/s00401-010-0784-9. doi:10.1007/s00401-010-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollack IF, Campbell JW, Hamilton RL, Martinez AJ, Bozik ME. Proliferation index as a predictor of prognosis in malignant gliomas of childhood. Cancer. 1997;79(4):849–856. doi:10.1002/(SICI)1097-0142(19970215)79:4<849::AID-CNCR23>3.0.CO;2-Z. [PubMed] [Google Scholar]
- 21.Pollack IF, Finkelstein SD, Burnham J, et al. Age and TP53 mutation frequency in childhood malignant gliomas: results in a multi-institutional cohort. Cancer Res. 2001;61(20):7404–7407. [PubMed] [Google Scholar]
- 22.Pollack IF, Finkelstein SD, Woods J, et al. Expression of p53 and prognosis in children with malignant gliomas. N Engl J Med. 2002;346(6):420–427. doi: 10.1056/NEJMoa012224. doi:10.1056/NEJMoa012224. [DOI] [PubMed] [Google Scholar]
- 23.Pollack IF, Hamilton RL, Burnham J, et al. Impact of proliferation index on outcome in childhood malignant gliomas: results in a multi-institutional cohort. Neurosurgery. 2002;50(6):1238–1244. doi: 10.1097/00006123-200206000-00011. discussion 1244–1235. [DOI] [PubMed] [Google Scholar]
- 24.Pollack IF, Hamilton RL, Finkelstein SD, et al. The relationship between TP53 mutations and overexpression of p53 and prognosis in malignant gliomas of childhood. Cancer Res. 1997;57(2):304–309. [PubMed] [Google Scholar]
- 25.Margraf LR, Gargan L, Butt Y, Raghunathan N, Bowers DC. Proliferative and metabolic markers in incompletely excised pediatric pilocytic astrocytomas—an assessment of 3 new variables in predicting clinical outcome. Neuro Oncol. 2011;13(7):767–774. doi: 10.1093/neuonc/nor041. doi:10.1093/neuonc/nor041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horbinski C, Hamilton RL, Lovell C, Burnham J, Pollack IF. Impact of morphology, MIB-1, p53 and MGMT on outcome in pilocytic astrocytomas. Brain Pathol. 2009;20(3):581–588. doi: 10.1111/j.1750-3639.2009.00336.x. doi:10.1111/j.1750-3639.2009.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollack IF, Hamilton RL, Sobol RW, et al. O6-methylguanine-DNA methyltransferase expression strongly correlates with outcome in childhood malignant gliomas: results from the CCG-945 Cohort. J Clin Oncol. 2006;24(21):3431–3437. doi: 10.1200/JCO.2006.05.7265. doi:10.1200/JCO.2006.05.7265. [DOI] [PubMed] [Google Scholar]
- 28.Ciampi R, Knauf JA, Kerler R, et al. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest. 2005;115(1):94–101. doi: 10.1172/JCI23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94(6):2092–2098. doi: 10.1210/jc.2009-0247. doi:10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 30.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. doi:10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolaides T, Li H, Solomon D, et al. Targeted therapy for BRAFV600E malignant astrocytoma. Clin Cancer Res. 2011;17(24):7595–7604. doi: 10.1158/1078-0432.CCR-11-1456. doi:10.1158/1078-0432.CCR-11-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasheed A, Herndon JE, Stenzel TT, et al. Molecular markers of prognosis in astrocytic tumors. Cancer. 2002;94(10):2688–2697. doi: 10.1002/cncr.10544. doi:10.1002/cncr.10544. [DOI] [PubMed] [Google Scholar]
- 33.Giannini C, Scheithauer BW, Burger PC, et al. Cellular proliferation in pilocytic and diffuse astrocytomas. J Neuropathol Exp Neurol. 1999;58(1):46–53. doi: 10.1097/00005072-199901000-00006. doi:10.1097/00005072-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Tihan T, Ersen A, Qaddoumi I, et al. Pathologic Characteristics of pediatric intracranial pilocytic astrocytomas and their impact on outcome in 3 countries: a multi-institutional study. Am J Surg Pathol. 2011;36(1):43–55. doi: 10.1097/PAS.0b013e3182329480. [DOI] [PubMed] [Google Scholar]
- 35.Cheng Y, Pang JC, Ng HK, et al. Pilocytic astrocytomas do not show most of the genetic changes commonly seen in diffuse astrocytomas. Histopathology. 2000;37(5):437–444. doi: 10.1046/j.1365-2559.2000.01005.x. doi:10.1046/j.1365-2559.2000.01005.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.