Abstract
Managing a CNS neoplasm during pregnancy presents complex challenges, and population-based studies are lacking. We designed a retrospective cohort study using the Nationwide Inpatient Sample (NIS) to investigate pregnancy outcomes in women with CNS neoplasms. We constructed a logistic regression model for maternal mortality, preterm labor, intrauterine growth restriction (IUGR), and Caesarean delivery, controlling for age, comorbidities, and demographic characteristics. We identified 379 malignant brain tumors, 437 benign brain tumors, and 44 spine tumors among 19 million pregnancy-related admissions from 1988 through 2009. Malignant brain tumors were associated with maternal mortality (odds ratio [OR], 143), preterm labor (OR, 3.4), and IUGR (OR, 2.9). Benign brain tumors were associated with preterm labor (OR, 2.3). A diagnosis of hyperemesis gravidarum was more common in malignant (OR, 2.2) and benign (OR, 2.8) brain tumors. Compared with the general population, Caesarean delivery was more frequent for malignant (OR, 6.4) and benign (OR, 2.8) brain tumors and spine tumors (OR, 3.9). Admission without delivery was more common for malignant (OR, 8.6) and benign (OR, 4.3) brain tumors and spine tumors (OR, 3.8; P < .05 for all outcomes). Thirty-three percent of all hospitalizations involved neurosurgical procedures, but pregnancy complications were not significantly more likely to occur in surgical patients. In conclusion, malignant brain tumors were associated with adverse pregnancy outcomes, and CNS neoplasms were associated with higher rates of Caesarean delivery. Additional research is needed to improve understanding of obstetric risk in these patients and to assist with treatment, counseling, and monitoring during delivery.
Keywords: brain tumors, epidemiology, outcome research, pregnancy, spine tumors
Primary intracranial neoplasms are the fifth leading cause of cancer-related death in women aged 20–39 years.1 Among young adults aged 20–34 years, gliomas are the most common histology (34%), followed by pituitary tumors (27%) and meningiomas (14%).2 Although pregnancy is not considered to be a risk factor for developing a CNS neoplasm and there is no evidence that primary brain tumors occur at greater frequency during pregnancy compared with nonpregnant women of the same age,3,4 physiologic changes during pregnancy may exacerbate tumor-related symptoms or affect the growth of lesions with steroid hormone receptors, such as meningiomas.5,6 Because peritumoral edema can worsen as the result of fluid shifts during the second and third trimesters,1,7 pre-existing lesions may become symptomatic for the first time during pregnancy.8 Although less common than brain tumors, spinal neoplasms may pose risks during the process of labor and delivery, given the frequent need for spinal or epidural anesthesia.9–11
As with other cancers, treatment of CNS tumors during pregnancy depends on the site, biology, and aggressiveness of the tumor and on balancing the complex and interconnected needs of mother and fetus.12 This is particularly true in the case of malignant brain tumors, for which the accepted treatment standard includes radiation therapy, which is usually contraindicated during pregnancy, especially in its early stages. In addition, the stress of surgery and anesthesia during pregnancy has been associated with preterm labor and other complications.13
Although several authors have attempted to clarify pregnancy outcomes in women with CNS tumors, population-based studies addressing this scenario are lacking. Most of the previous work on this topic consists of single case reports or small series.8,13–22 Although neurosurgical procedures performed during pregnancy have not been shown to worsen maternal or neonatal outcomes, prior studies were not adequately powered to draw statistically significant conclusions. Similarly, although some women experienced fetal loss, preterm labor, and other complications related to their CNS neoplasms, it was not possible to quantify their risk for such complications.
We address this clinical question by using a nationally representative administrative dataset to identify hospitalized pregnant women with CNS tumors, thus maximizing sample size and statistical power and illuminating areas of future research to clarify diagnostic and treatment strategies in these patients. We hypothesized that pregnant women with CNS neoplasms, particularly malignant brain tumors, would have an elevated risk of adverse pregnancy outcomes and a higher rate of Caesarean delivery because of more conservative management of their delivery process.
Materials and Methods
Data Source
The Nationwide Inpatient Sample (NIS) of the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality (AHRQ; Rockville, MD), collects administrative and clinical data on US hospital discharges. The 2009 NIS, the most recent available dataset, contains discharge data from 1050 hospitals in 44 states, approximating a 20% stratified sample of all non-federal hospitals.23 We used all datasets from 1988 through 2009, comprising information on 5–8 million hospital discharges per year. Information available from the NIS includes up to 15 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes for each hospitalization, geographic region, hospital characteristics, and payer information. The NIS incorporates all types of hospitals, all payers including the uninsured, and all ages; thus, it is well suited to studying rare conditions in a young patient population.
Study Population
Using the NIS, we designed a retrospective cohort study, defining the population of interest as all pregnancy-related hospitalizations from 1988 through 2009. To identify these hospitalizations, we searched for ICD-9-CM codes related to pregnancy (630–679). To define the study cohorts within this population, we searched for diagnosis codes for all CNS neoplasms, dividing them into 3 cohorts: malignant brain tumors, benign brain tumors, and spine tumors. A full list of relevant ICD-9-CM codes is available from the authors on request.
Outcomes and Covariates
We used ICD-9-CM diagnosis and procedure codes to identify all hospitalizations associated with pregnancy outcomes commonly reported in the obstetric literature: maternal mortality, any delivery, delivery by Caesarean section, preterm labor, intrauterine growth restriction (IUGR), and intrauterine fetal demise (stillbirth). We identified the following relevant associated diagnoses: hyperemesis gravidarum, seizures, hydrocephalus, altered consciousness, cerebral edema, cerebral herniation, and intracranial hemorrhage. Finally, we identified hospitalizations associated with the following neurosurgical procedures: craniotomy, brain biopsy, ventriculostomy, shunt operation, and spine operation.
We selected potential confounders based on known risk factors for pregnancy complications identified by a review of the relevant literature: age, pre-eclampsia, chronic and gestational diabetes, chronic and gestational hypertension, renal disease, multiple gestation, race/ethnicity, and socioeconomic status. Race/ethnicity was defined as white, black, Hispanic, other, or missing. Socioeconomic status was defined as income quartile based on median total family income in the zip code of the patient's primary residence. For Caesarean delivery, we additionally considered year (because of the observed increase in the Caesarean delivery rate for the general population in the United States over the period studied)24 and prior Caesarean delivery (an important determinant of whether a patient is offered the procedure for subsequent deliveries). We also adjusted for potential confounders that could independently affect the likelihood of an invasive procedure being offered: hospital size (small, medium, or large), hospital type (rural, urban nonteaching, or urban teaching), and primary insurance payer (Medicare, Medicaid, privately insured, or self-pay/uninsured).
Statistical Analysis
We used standard statistical tests to compare baseline characteristics among admissions associated with malignant brain tumors, benign brain tumors, and spine tumors: a t test for continuous variables (age), Fisher's test for binary variables, and Pearson's χ2 test for categorical variables. We used multivariable logistic regression to determine odds ratios (ORs) and 95% confidence intervals (CIs) for the association between CNS neoplasms and the outcomes of interest. Final adjusted models included subsets of the covariates described above. We used an automated score selection algorithm to determine the set of covariates that provided the best explanation of the variation in the data, allowing no more than one covariate for every 10 events.25 For rare outcomes (e.g., mortality), we report only univariate analyses.
For all statistical analyses, we used SAS software (version 9.0, SAS Institute). Statistical significance is defined as P < .05; all P values are 2-tailed. Because obtaining accurate national estimates was not a focus of this study, the unweighted NIS dataset was used.
Reporting of Outcomes
All outcomes are reported based on the clinically appropriate at-risk group. The outcome of delivery is reported for the entire pregnant population, whereas mortality is reported both for the entire population and for the subgroup that experienced a delivery during the course of hospitalization. Because preterm labor, IUGR, stillbirth, and Caesarean section are clinically relevant mainly for delivering patients, analyses for these outcomes are reported for deliveries only. Outcomes with zero events are not listed.
For intracranial tumors, we performed analyses on outcomes that might be affected by elective or urgent surgery: maternal mortality, stillbirth and preterm labor (because the stress of surgery might cause adverse effects on the fetus), and Caesarean section (because of the desire for a more controlled delivery process in patients requiring neurosurgery).
The Partners Institutional Review Board approved the use of the NIS for this study. A waiver of informed consent was obtained for use of this publicly available, de-identified database.
Results
Patient Characteristics
We identified 19,750,702 pregnancy-related admissions that occurred from 1988 through 2009. Of these, 379 were associated with malignant brain tumors, including 165 deliveries (44%); 437 were associated with benign brain tumors, including 265 deliveries (61%), and 44 were associated with spine tumors, including 28 deliveries (64%). For comparison, in the general US population, nearly 90% of hospitalizations of pregnant women are associated with delivery of an infant. Both malignant and benign brain tumors were associated with older age (Table 1). Patients with CNS tumors were more likely to be white, less likely to be Hispanic, and more likely to have private health insurance, although the magnitude of these differences was small. Patients with malignant brain tumors were more likely to fall in lower income quartiles, and patients with benign brain tumors were more likely to fall in higher income quartiles. Benign brain tumors were associated with higher rates of gestational diabetes, gestational and chronic hypertension, and multiple gestation. All types of CNS neoplasms during pregnancy were significantly more likely to be managed in large hospitals and/or urban teaching hospitals, compared with the general obstetric population.
Table 1.
Characteristics of pregnancy-related hospitalizations in patients with CNS tumors and in the general obstetric population, US, 1988–2009
| Characteristic | MBT (N= 379) | BBT (N= 437) | ST (N= 44) | Control (N= 19,750,702) | P |
|---|---|---|---|---|---|
| Age | |||||
| Mean (SD) | 29.0 (6.3) | 30.3 (5.9) | 27.1 (5.3) | 27.1 (6.1) | † |
| Age ≥35, % | 18.7 | 25.9 | a | 12.9 | † |
| Race, % | |||||
| White | 45.1 | 46.2 | 59.1 | 39.1 | .001 |
| Black | 9.5 | 11.2 | a | 10.3 | |
| Hispanic | 11.6 | 10.1 | a | 14.6 | |
| Other | 5.0 | 8.0 | a | 5.9 | |
| Missing | 28.8 | 24.5 | 22.7 | 30.2 | |
| Income quartile, % | |||||
| 1 (least wealthy) | 28.8 | 16.0 | 25.0 | 23.9 | † |
| 2 | 17.7 | 18.8 | a | 22.6 | |
| 3 | 21.1 | 26.3 | 22.7 | 21.0 | |
| 4 (most wealthy) | 29.8 | 37.3 | 29.6 | 30.0 | |
| Missing | 2.6 | a | a | 2.6 | |
| Insurance payor, % | |||||
| Medicare | 3.7 | 2.3 | 0 | 0.5 | † |
| Medicaid | 32.8 | 28.2 | 31.8 | 37.2 | |
| Private | 56.1 | 65.1 | 59.1 | 53.9 | |
| Self-pay/Other | 7.4 | 4.4 | a | 8.5 | |
| Comorbidities, % | |||||
| Gestational DM | 4.5 | 3.4 | a | 3.7 | .016 |
| Chronic DM | a | 2.8 | 0 | 1.1 | .811 |
| Gestational HTN | a | 3.7 | a | 2.3 | .003 |
| Chronic HTN | 2.6 | 5.5 | a | 1.8 | .030 |
| Pre-eclampsia | a | 5.7 | a | 3.4 | † |
| Renal disease | a | a | a | 0.3 | .060 |
| Multiple gestation | a | 3.0 | 0 | 1.5 | .048 |
| Hospital setting, % | |||||
| Rural | 5.3 | 6.6 | a | 11.6 | † |
| Urban non-teaching | 29.6 | 34.2 | 31.8 | 46.6 | |
| Urban teaching | 65.2 | 59.2 | 61.4 | 41.8 | |
| Hospital size, % | |||||
| Small | 6.9 | 8.5 | a | 11.5 | † |
| Medium | 21.1 | 24.1 | 27.3 | 28.2 | |
| Large | 72.0 | 67.4 | 65.9 | 60.3 | |
Abbreviations: MBT, malignant brain tumors; BBT, benign brain tumors; ST, spine tumors.
aPer HCUP policy, exact number and frequency cannot be reported when cell size <10.
†P < .0001.
Relevant associated symptoms and examination findings noted during hospitalizations of pregnant women with brain tumors included seizures (24% for malignant and 9.4% for benign) and hydrocephalus (7.7% for malignant and 4.6% for benign). A small minority of brain tumor–related hospitalizations were accompanied by diagnoses of cerebral herniation or edema, intracranial hemorrhage, and altered consciousness.
Malignant Brain Tumors
Malignant brain tumors were strongly associated with in-hospital maternal mortality, both in the delivering and the nondelivering population (Table 2). Malignant brain tumors were also associated with hospitalization not resulting in delivery, and a diagnosis of hyperemesis gravidarum was more likely. In the delivering population, serious complications, including preterm labor, IUGR, and stillbirth, were more common in the malignant brain tumor cohort. Finally, malignant brain tumors were strongly associated with delivery by Caesarean section.
Table 2.
Odds ratio (OR) and 95% confidence interval (CI) for outcomes of interest by cohort and at-risk group
| MBT | Outcome | % Cohort | % Control | OR | P |
|---|---|---|---|---|---|
| All pregnancies | Mortality | <10 | 0.02 | 143 (71–288) | † |
| Non-delivery | 56.5 | 13.1 | 8.6 (7.0–10.5)a | † | |
| Hyperemesis | <10 | 1.1 | 2.2 (1.1–4.3) | .001 | |
| Deliveries | Mortality | <10 | 0.01 | 326 (134–796) | † |
| Preterm labor | 19.4 | 6.9 | 3.4 (2.3–5.1)b | † | |
| IUGR | <10 | 1.5 | 2.9 (1.4–6.2) | .01 | |
| Stillbirth | <10 | 0.48 | 3.9 (1.2–12) | .05 | |
| Caesarean | 64.9 | 25.7 | 6.4 (4.5–9.0)c | † | |
| BBT | Outcome | % Cohort | % Control | OR | P |
| All pregnancies | Mortality | <10 | 0.02 | 15 (2.1–108) | .06 |
| Non-delivery | 39.4 | 13.1 | 4.3 (3.5–5.2)a | † | |
| Hyperemesis | 3.0 | 1.1 | 2.8 (1.6–4.9) | .001 | |
| Deliveries | Preterm labor | 15.9 | 6.9 | 2.3 (1.6–3.3)d | † |
| IUGR | <10 | 1.5 | 1.8 (0.8–3.8) | .13 | |
| Stillbirth | <10 | 0.48 | 1.6 (0.4–6.4) | .36 | |
| Caesarean | 49.4 | 25.7 | 2.8 (2.1–3.6)e | † | |
| ST | Outcome | % Cohort | % Control | OR | P |
| All pregnancies | Non-delivery | 36.4 | 13.1 | 3.8 (2.0–7.0)f | † |
| Deliveries | Preterm labor | <10 | 6.9 | 2.3 (0.8–6.5) | .12 |
| IUGR | <10 | 1.5 | 5.0 (1.2–21.1) | .07 | |
| Caesarean | 57.1 | 25.7 | 3.9 (1.8–8.2) | † |
Abbreviations: MBT, malignant brain tumors; BBT, benign brain tumors; ST, spine tumors; IUGR, intrauterine growth restriction.
Outcomes are not reported for subgroups with zero events. ORs are univariate unless otherwise noted.
aAdjusted for age, pre-eclampsia, chronic and gestational diabetes, chronic and gestational hypertension, renal disease, multiple gestation, race, and socioeconomic status.
bAdjusted for pre-eclampsia, multiple gestation, and race.
cAdjusted for age, year, previous Caesarean delivery, pre-eclampsia, chronic and gestational diabetes, chronic and gestational hypertension, multiple gestation, and insurance payor.
dAdjusted for pre-eclampsia, chronic diabetes, multiple gestation, and race.
eAdjusted for age, year, previous Caesarean delivery, pre-eclampsia, chronic and gestational diabetes, chronic and gestational hypertension, multiple gestation, renal disease, insurance payor, socioeconomic status, and hospital type.
fAdjusted for chronic diabetes and race.
†P < .0001
Neurosurgical procedures were performed in 48% of hospitalizations involving malignant brain tumors (Table 3). Adverse outcomes were not significantly associated with neurosurgical procedures, although significantly more Caesarean sections were performed in women who required neurosurgery during their hospitalizations (Table 4).
Table 3.
Neurosurgical procedures performed during hospitalizations of pregnant woman with intracranial neoplasms, US, 1988–2009
| Procedure | Malignant, N (%) | Benign, N (%) |
|---|---|---|
| Craniotomy | 119 (31.4) | 70 (16.0) |
| Biopsy | 34 (9.0) | a |
| Ventriculostomy | 16 (4.2) | a |
| Shunt operation | 11 (2.9) | a |
| All | 180 (47.5) | 84 (19.2) |
aAccording to HCUP policy, exact number and frequency cannot be reported when cell size <10.
Table 4.
Odds ratio (OR) and 95% confidence interval (CI) for outcomes of interest in hospitalizations associated with intracranial neoplasms, organized by cohort and surgery status
| Cohort | Outcome | Surgery (% w/outcome) | No surgery (% w/outcome) | OR (95% CI) | P |
|---|---|---|---|---|---|
| MBT | Mortality | <10 | <10 | 1.7 (0.4–6.8) | .48 |
| Preterm labor | 32.3 | 16.4 | 2.4 (1.0–5.9) | .07 | |
| Stillbirth | <10 | <10 | 2.2 (0.2–25) | .47 | |
| Caesarean | 83.9 | 60.4 | 3.3 (1.2–9.2)a | .02 | |
| BBT | Mortality | <10 | 0 | 15 (0.6–368) | .17 |
| Preterm labor | <10 | 15.6 | 1.2 (0.4–3.7) | .76 | |
| Stillbirth | <10 | <10 | 12 (0.7–191) | .16 | |
| Caesarean | 77.3 | 46.9 | 3.9 (1.4–11)b | .01 |
Abbreviations: MBT, malignant brain tumors; BBT, benign brain tumors.
Outcomes are not reported for subgroups with zero events. ORs are univariate unless otherwise noted.
aAdjusted for gestational diabetes and socioeconomic status.
bAdjusted for age, hypertension, race, and socioeconomic status.
†P < .0001
Benign Brain Tumors
Benign brain tumors were not associated with maternal mortality, and in fact, there were no deaths in the delivering population (Table 2). However, similar to malignant brain tumors, benign brain tumors were associated with hospitalizations not resulting in delivery and with a diagnosis of hyperemesis gravidarum. In the delivering population, only preterm labor was more likely to occur. Like malignant brain tumors, benign brain tumors were strongly associated with Caesarean delivery.
Neurosurgical procedures were performed in 19% of hospitalizations involving benign brain tumors (Table 3). As with malignant brain tumors, patients having neurosurgery were not significantly more likely to have adverse pregnancy outcomes, compared with the general population, although they had significantly more Caesarean deliveries (Table 4).
Spine Tumors
There were no mortalities in the spine tumor cohort, and preterm labor, IUGR, and stillbirth were not more likely to occur in the small spine tumor cohort than in the general obstetric population. However, spine tumors, similar to brain tumors, were strongly associated with Caesarean delivery (Table 2). Neurosurgical procedures were performed during 41% of spine tumor–related admissions.
Discussion
In the present study, we investigated pregnancy outcomes in more than 800 pregnancy-related hospitalizations in women with brain and spine tumors. To date, there are no large population-based studies of obstetric outcomes in patients with CNS neoplasms. Evidence-based guidelines are not available, making it difficult for physicians to counsel patients and to monitor the pregnancy and delivery process. In contrast, guidelines exist for the management of other cancers during pregnancy. Because of the increasing mean age at which US women give birth, cancer during pregnancy is becoming more common. Improving outcomes for breast and cervical cancer and long clinical experience with chemotherapy have informed this discussion for well over a decade.26–30 However, the incidence of CNS tumors is much lower than many other cancers in this age group; a recent population-based assessment estimated the incidence of maternal malignant brain tumors at 3.6 per 1 million live births.4
Several studies have attempted to collect cases of CNS neoplasms and other lesions amenable to neurosurgical intervention during pregnancy. Although most are reports of single cases, there are 2 retrospective series comprising a total of 36 pregnant women with intracranial neoplasms.13,14 Johnson et al found significantly more gliomas in those diagnosed prior to pregnancy and more meningiomas diagnosed during pregnancy. Cohen-Gadol et al published an institutional series including 14 patients with intracranial neoplasms, 9 of whom underwent a neurosurgical procedure while pregnant. No fetal or maternal complications were directly related to these procedures, and all mothers who underwent surgery at or near term subsequently delivered healthy infants.13 Neither study had a large enough sample size to achieve statistical significance.
Given these limitations, a large registry or administrative database, such as the NIS, is probably the only feasible way to conduct an adequately powered study of this clinical question. Because the NIS includes all payers and all types of hospitals, it enables sampling of young, otherwise healthy patients, such as pregnant women, who would not be represented in other US databases, such as the Medicare and VA registries. Our approach allowed us to collect the largest possible number of pregnant women with CNS tumors, an effort that could take years or decades if subjects were recruited prospectively. Our study also addresses the issue of referral bias, which in prior institutional series may have excluded patients presenting in very poor clinical condition. Encompassing a large sample of hospitalized pregnant women in the United States, we demonstrated that patients with CNS neoplasms during pregnancy are far more likely to be cared for at a tertiary referral center. However, we also were able to capture those treated at smaller hospitals. Thus, our estimates for adverse outcomes, whether surgery was performed, are more likely to be realistic national estimates.
We found that serious pregnancy complications, including preterm labor, IUGR, and mortality, were more likely to occur in patients with malignant brain tumors. This may be related to maternal compromise caused by the underlying malignancy. For example, seizures, hydrocephalus, and aspiration pneumonia are common sequelae of malignant brain tumors and can all have deleterious effects on a pregnancy. Although we found a markedly increased risk of in-hospital mortality among pregnant women with malignant brain tumors, the baseline maternal mortality rate in the United States is very low (most recently estimated at 13 deaths per 100,000 live births);31 thus, the absolute risk of mortality is still quite low.
For benign brain tumors, preterm labor was the only complication that occurred at a significantly higher frequency. There are many risk factors for preterm labor, including older age and a greater frequency of chronic comorbid illnesses. However, the difference persisted even when adjusting for these factors. For both cohorts, early planned induction or Caesarean delivery was likely performed in some cases as soon as fetal lung maturity was documented, to commence definitive treatment as soon as possible.
Similar to other adults with brain tumors, pregnant women commonly presented with seizures and hydrocephalus. Moreover, we found an increased frequency of hyperemesis gravidarum in patients with both malignant and benign brain tumors, suggesting that nausea and vomiting may be misattributed to the pregnancy itself in women with elevated intracranial pressure due to CNS pathology. This highlights the importance of a thorough examination for suspicious symptoms during pregnancy.
Hospitalizations of patients with brain tumors differed in several ways from the general population. They were much less likely to deliver while in the hospital, perhaps implying that they delivered their infants during a subsequent hospitalization or that they experienced more antenatal hospitalizations. They were more likely to have a Caesarean delivery, probably because of conservative management of the delivery process.
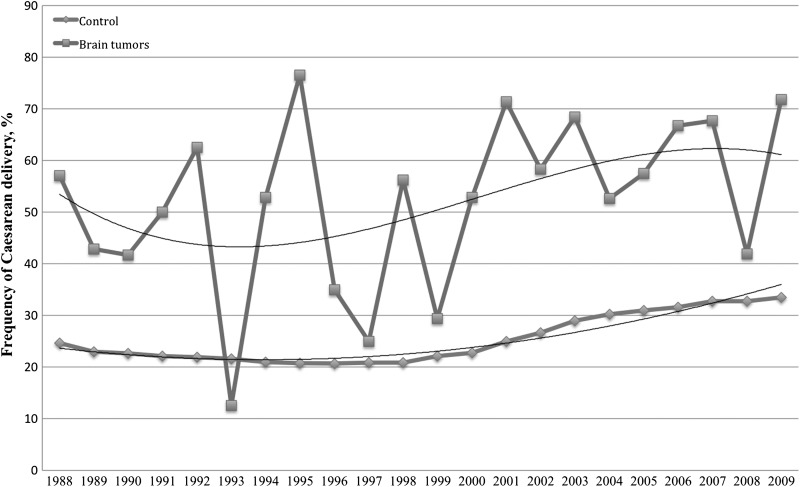
Both malignant and benign brain tumors were strongly associated with Caesarean delivery even when controlling for potential confounders, probably because of conservative management of the delivery process in these patients. Pregnancies in patients with intracranial lesions may be perceived as higher risk and, therefore, may be managed by Caesarean delivery. For example, some obstetricians discourage pushing during the second stage of delivery, because this can dramatically increase intracranial pressure and theoretically exacerbate cerebral edema or precipitate an intratumoral hemorrhage.1 As mentioned, some patients may undergo an early scheduled Caesarean delivery or labor induction, which confers a higher rate of Caesarean delivery if labor fails to progress. Finally, we must use caution in interpreting an increased OR for an outcome that is common even in the general population experiencing normal pregnancy and delivery (Figure 1). However, because our results were fairly consistent between the univariate and multivariate analyses, it seems unlikely that including more or different covariates in the model would have appreciably changed the results.
Fig. 1.
Trend in US Caesarean delivery rate for brain tumors and the general population, 1988–2009.
In patients with brain tumors who underwent a neurosurgical procedure, adverse outcomes were not more likely to occur, although these patients experienced significantly more Caesarean deliveries, likely as part of a definitive treatment plan. This confirms the findings of smaller observational series, which have not demonstrated increased maternal or fetal risks with neurosurgical procedures. Thus, there seems to be no compelling reason to delay indicated surgery because of concerns that it would pose additional risks to the pregnancy.
Similar to brain tumors, spine tumors were associated with hospitalization not resulting in a delivery and with a higher rate of Caesarean delivery. No significant associations between spine tumors and pregnancy complications were observed, although small sample size warrants caution in interpretation of these findings as negative.
There are limitations to our approach. In any large database, clinical detail is lacking. Of importance, because ICD-9-CM diagnosis codes generally do not include information on histology, low-grade gliomas are classified as malignant brain tumors, even though their natural history and prognosis are very different from those of glioblastomas or CNS metastatic disease. Similarly, pituitary tumors and craniopharyngiomas, although their pathology and clinical behavior are very different, share the same ICD-9-CM code.
Many patients, especially those with asymptomatic, small benign lesions, may be treated entirely in the outpatient setting, with definitive treatment deferred until after delivery.1,7 In addition, patients with long-term controlled CNS neoplastic disease, such as low-grade astrocytoma, may have a relatively uneventful pregnancy and delivery course. Our study of hospitalizations is inherently biased toward the larger and more symptomatic tumors encountered in the inpatient setting, and thus, our estimates may represent an upper limit of risk. In addition, because there is no way to link maternal and neonatal hospitalizations in the NIS, we were unable to obtain information on neonatal outcomes, an area that should be addressed by future research.
We chose to adjust for race and ethnicity in our statistical analysis, because African-American race has been reported as an independent risk factor for adverse pregnancy outcomes, possibly as a result of the higher baseline rate of chronic hypertension and other medical comorbidities in African-Americans.32 However, because not every state reports data on race, approximately 30% of such data are missing. To address this, we created a separate “missing” category and adjusted for it in the statistical analysis.
In the United States, where the proportion of births taking place at home is less than 1%,33 nearly all pregnancies result in at least one hospital admission (for delivery). Therefore, we are confident that we captured a representative sample of women during their pregnancies. However, because the unit of analysis in the NIS is a hospitalization rather than an individual patient, we could not account for multiple admissions related to a single pregnancy. Because the probability of delivery twice during a calendar year is quite low (0.25%),34,35 hospitalizations associated with a definitive delivery likely reflect individual patients, and we feel that these estimates are the most accurate.
In summary, we found an increased frequency of adverse pregnancy outcomes among pregnant women with brain tumors, particularly malignant brain tumors; these risks were not increased for spine tumors. Physicians who care for patients with CNS neoplasms should be aware of the potential for enhanced obstetric risk. Because of the challenges of studying pregnancy outcomes in a rare disease, well-designed prospective studies are needed to address the aforementioned limitations and to provide detailed patient-level information. Our hope is that, by illuminating areas of enhanced risk during pregnancy, we can contribute to the understanding and management of pregnancy in patients with CNS disease. By collecting the largest possible sample of pregnant women with both malignant and benign CNS tumors, we hope to move beyond the individualization of care and expert opinion and lay the groundwork for future research that will help better define obstetric risk in this patient population.
Funding
This work was supported by the John and Cyndy Leahy Fellowship Fund. The authors were independent of the funders in all aspects of study design, data analysis, and writing of the manuscript.
Supplementary Material
Acknowledgments
We thank Dr. Robert Martuza and the MGH Neurosurgical Service for generous financial support of relevant coursework at the Harvard School of Public Health. A preliminary version of this work was presented as a poster at the annual Society for Neuro-Oncology meeting in Orange County, CA, November 2011.
Conflict of interest statement. None declared.
References
- 1.Stevenson CB, Thompson RC. The clinical management of intracranial neoplasms in pregnancy. Clin Obstet Gynecol. 2005;48(1):24–37. doi: 10.1097/01.grf.0000153209.70749.d0. doi:10.1097/01.grf.0000153209.70749.d0. [DOI] [PubMed] [Google Scholar]
- 2.CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2007. February 2011.
- 3.Roelvink NC, Kamphorst W, van Alphen HA, Rao BR. Pregnancy-related primary brain and spinal tumors. Arch Neurol. 1987;44(2):209–215. doi: 10.1001/archneur.1987.00520140069020. doi:10.1001/archneur.1987.00520140069020. [DOI] [PubMed] [Google Scholar]
- 4.Haas JF, Janisch W, Staneczek W. Newly diagnosed primary intracranial neoplasms in pregnant women: a population-based assessment. J Neurol Neurosurg Psychiatry. 1986;49(8):874–880. doi: 10.1136/jnnp.49.8.874. doi:10.1136/jnnp.49.8.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowppli-Bony A, Bouvier G, Rue M, et al. Brain tumors and hormonal factors: review of the epidemiological literature. Cancer Causes Control. 2011;22(5):697–714. doi: 10.1007/s10552-011-9742-7. doi:10.1007/s10552-011-9742-7. [DOI] [PubMed] [Google Scholar]
- 6.Hatch EE, Linet MS, Zhang J, et al. Reproductive and hormonal factors and risk of brain tumors in adult females. Int J Cancer. 2005;114(5):797–805. doi: 10.1002/ijc.20776. doi:10.1002/ijc.20776. [DOI] [PubMed] [Google Scholar]
- 7.Karnad DR, Guntupalli KK. Neurologic disorders in pregnancy. Crit Care Med. 2005;33(suppl 10):S362–S371. doi: 10.1097/01.ccm.0000182790.35728.f7. doi:10.1097/01.CCM.0000182790.35728.F7. [DOI] [PubMed] [Google Scholar]
- 8.Su TM, Lan CM, Yang LC, Lee TC, Wang KW, Hung KS. Brain tumor presenting with fatal herniation following delivery under epidural anesthesia. Anesthesiology. 2002;96(2):508–509. doi: 10.1097/00000542-200202000-00041. doi:10.1097/00000542-200202000-00041. [DOI] [PubMed] [Google Scholar]
- 9.Han IH, Kuh SU, Kim JH, et al. Clinical approach and surgical strategy for spinal diseases in pregnant women: a report of ten cases. Spine (Phila Pa 1976) 2008;33(17):E614–E619. doi: 10.1097/BRS.0b013e31817c6c7d. doi:10.1097/BRS.0b013e31817c6c7d. [DOI] [PubMed] [Google Scholar]
- 10.Tsutsumi S, Nonaka Y, Abe Y, Yasumoto Y, Ito M. Spinal angiolipoma in a pregnant woman presenting with acute epidural hemorrhage. J Clin Neurosci. 2011;18(6):849–851. doi: 10.1016/j.jocn.2010.09.017. doi:10.1016/j.jocn.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Akhaddar A, Oukabli M, En-Nouali H, Boucetta M. Acute postpartum paraplegia caused by spinal extradural capillary hemangioma. International Journal of Gynaecology and Obstetrics: the Official Organ of the International Federation of Gynaecology and Obstetrics. 2010;108(1):75–76. doi: 10.1016/j.ijgo.2009.08.012. doi:10.1016/j.ijgo.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Esmaeilzadeh M, Dictus C, Kayvanpour E, et al. One life ends, another begins: Management of a brain-dead pregnant mother-A systematic review. BMC Med. 2010;8:74. doi: 10.1186/1741-7015-8-74. doi:10.1186/1741-7015-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen-Gadol AA, Friedman JA, Friedman JD, Tubbs RS, Munis JR, Meyer FB. Neurosurgical management of intracranial lesions in the pregnant patient: a 36-year institutional experience and review of the literature. J Neurosurg. 2009;111(6):1150–1157. doi: 10.3171/2009.3.JNS081160. doi:10.3171/2009.3.JNS081160. [DOI] [PubMed] [Google Scholar]
- 14.Johnson N, Sermer M, Lausman A, Maxwell C. Obstetric outcomes of women with intracranial neoplasms. Int J Gynaecol Obstet. 2009;105(1):56–59. doi: 10.1016/j.ijgo.2008.11.037. doi:10.1016/j.ijgo.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 15.Erdogan B, Sen O, Aydin MV, Bagis T, Bavbek M. Cerebellar hemangioblastoma in pregnancy. A case report. J Reprod Med. 2002;47(10):864–866. [PubMed] [Google Scholar]
- 16.Hayashi S, Takeda N, Komura E. Symptomatic cerebellar hemorrhage from recurrent hemangioblastoma during delivery. Case report. Neurol Med Chir (Tokyo) 2010;50(12):1105–1107. doi: 10.2176/nmc.50.1105. doi:10.2176/nmc.50.1105. [DOI] [PubMed] [Google Scholar]
- 17.Mackenzie AP, Levine G, Garry D, Figueroa R. Glioblastoma multiforme in pregnancy. J Matern Fetal Neonatal Med. 2005;17(1):81–83. doi: 10.1080/14767050400028709. doi:10.1080/14767050400028709. [DOI] [PubMed] [Google Scholar]
- 18.Magne N, Marcie S, Pignol JP, Casagrande F, Lagrange JL. Radiotherapy for a solitary brain metastasis during pregnancy: a method for reducing fetal dose. Br J Radiol. 2001;74(883):638–641. doi: 10.1259/bjr.74.883.740638. [DOI] [PubMed] [Google Scholar]
- 19.Razak AR, Nasser Q, Morris P, Alcutt D, Grogan L. Medulloblastoma in two successive pregnancies. J Neurooncol. 2005;73(1):89–90. doi: 10.1007/s11060-004-4209-2. doi:10.1007/s11060-004-4209-2. [DOI] [PubMed] [Google Scholar]
- 20.Semple PL, Denny L, Coughlan M, Soeters R, Van Wijk L. The role of neurosurgery in the treatment of cerebral metastases from choriocarcinoma: a report of two cases. Int J Gynecol Cancer. 2004;14(1):157–161. doi: 10.1111/j.1048-891x.2004.14147.x. doi:10.1111/j.1048-891x.2004.14147.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith IF, Skelton V. An unusual intracranial tumour presenting in pregnancy. Int J Obstet Anesth. 2007;16(1):82–85. doi: 10.1016/j.ijoa.2006.04.016. doi:10.1016/j.ijoa.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Varveris H, Mazonakis M, Damilakis J, et al. Peripheral primitive neuroectodermal tumour during pregnancy. Br J Radiol. 2002;75(894):543–547. doi: 10.1259/bjr.75.894.750543. [DOI] [PubMed] [Google Scholar]
- 23.HCUP Databases. Healthcare Cost and Utilization Project (HCUP) http://www.hcup-us.ahrq.gov/nisoverview.jsp .
- 24.Declercq E, Menacker F, Macdorman M. Maternal risk profiles and the primary cesarean rate in the United States, 1991–2002. Am J Public Health. 2006;96(5):867–872. doi: 10.2105/AJPH.2004.052381. doi:10.2105/AJPH.2004.052381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. doi:10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 26.Amant F, Deckers S, Van Calsteren K, et al. Breast cancer in pregnancy: recommendations of an international consensus meeting. Eur J Cancer. 2010;46(18):3158–3168. doi: 10.1016/j.ejca.2010.09.010. doi:10.1016/j.ejca.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Azim HA, Jr, Del Mastro L, Scarfone G, Peccatori FA. Treatment of breast cancer during pregnancy: regimen selection, pregnancy monitoring and more. Breast. 2011;20(1):1–6. doi: 10.1016/j.breast.2010.10.008. doi:10.1016/j.breast.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Amant F, Brepoels L, Halaska MJ, Gziri MM, Calsteren KV. Gynaecologic cancer complicating pregnancy: an overview. Best Pract Res Clin Obstet Gynaecol. 2010;24(1):61–79. doi: 10.1016/j.bpobgyn.2009.08.001. doi:10.1016/j.bpobgyn.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284. doi: 10.1136/bmj.a1284. doi:10.1136/bmj.a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367(9509):489–498. doi: 10.1016/S0140-6736(06)68181-6. doi:10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 31.Main EK. Maternal mortality: new strategies for measurement and prevention. Curr Opin Obstet Gynecol. 2010;22(6):511–516. doi: 10.1097/GCO.0b013e3283404e89. doi:10.1097/GCO.0b013e3283404e89. [DOI] [PubMed] [Google Scholar]
- 32.Samadi AR, Mayberry RM, Reed JW. Preeclampsia associated with chronic hypertension among African-American and White women. Ethn Dis. 2001;11(2):192–200. Spring-Summer. [PubMed] [Google Scholar]
- 33.Malloy MH. Infant outcomes of certified nurse midwife attended home births: United States 2000 to 2004. J Perinatol. 2010;30(9):622–627. doi: 10.1038/jp.2010.12. doi:10.1038/jp.2010.12. [DOI] [PubMed] [Google Scholar]
- 34.Declercq E, Barger M, Cabral HJ, et al. Maternal outcomes associated with planned primary cesarean births compared with planned vaginal births. Obstet Gynecol. 2007;109(3):669–677. doi: 10.1097/01.AOG.0000255668.20639.40. doi:10.1097/01.AOG.0000255668.20639.40. [DOI] [PubMed] [Google Scholar]
- 35.Kuklina EV, Whiteman MK, Hillis SD, et al. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J. 2008;12(4):469–477. doi: 10.1007/s10995-007-0256-6. doi:10.1007/s10995-007-0256-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.