Abstract
Although the evidence for the benefit of adding temozolomide (TMZ) to radiotherapy (RT) is limited to glioblastoma patients, there is currently a trend toward treating anaplastic astrocytomas (AAs) with combined RT + TMZ. The aim of the present study was to describe the patterns of care of patients affected by AA and, particularly, to compare the outcome of patients treated exclusively with RT with those treated with RT + TMZ. Data of 295 newly diagnosed AAs treated with postoperative RT ± TMZ in the period from 2002 to 2007 were reviewed. More than 75% of patients underwent a surgical removal. All the patients had postoperative RT; 86.1% of them were treated with 3D-conformal RT (3D-CRT). Sixty-seven percent of the entire group received postoperative chemotherapy with TMZ (n = 198). One-hundred sixty-six patients received both concomitant and sequential TMZ. Prescription of postoperative TMZ increased in the most recent period (2005–2007). One- and 4-year survival rates were 70.2% and 28.6%, respectively. No statistically significant improvement in survival was observed with the addition of TMZ to RT (P = .59). Multivariate analysis showed the statistical significance of age, presence of seizures, Recursive Partitioning Analysis classes I–III, extent of surgical removal, and 3D-CRT. Changes in the care of AA over the past years are documented. Currently there is not evidence to justify the addition of TMZ to postoperative RT for patients with newly diagnosed AA outside a clinical trial. Results of prospective and randomized trials are needed.
Keywords: anaplastic astrocytoma, chemotherapy, radiotherapy, temozolomide, WHO grade III gliomas
The Central Nervous System Study Group of the Italian Association of Radiation Oncology (AIRO) previously reported the results of the Patterns of Care study of 1722 adult astrocytoma patients treated between 1985 and 2001.1 More recently, the group created a multicenter computerized database to collect clinical data regarding high-grade gliomas (HGGs) treated in Italy in the most recent years (2002–2007) in order to call attention to the changes over the time period in which data on temozolomide (TMZ) became available. We recently published the data concerning patients affected with glioblastoma;2 the current study focuses on patients with newly diagnosed anaplastic astrocytoma (AA), treated with radiotherapy in 16 Italian centers.
Postoperative radiotherapy (RT) plus TMZ has become the standard of care for glioblastoma since a large phase III trial showed better survival and longer progression-free survival for postoperative radiochemotherapy compared with RT exclusively.3 On the other hand, the potential benefit of adding TMZ to RT in AA has never been proven because no randomized controlled trial comparing postoperative RT + TMZ versus postoperative RT in WHO grade III gliomas has yet been completed. Nevertheless, many patients with a newly diagnosed AA are currently treated with postoperative TMZ.4–6
In the current study, we retrospectively investigated treatment modalities and outcomes in a large number of AA patients, focusing on the use of TMZ in the postoperative setting.
Methods and Materials
Characteristics of the computerized database to ensure the homogeneity of the data collection were previously illustrated.2
Data on a total of 295 patients diagnosed with WHO grade III astrocytic tumors, treated between January 2002 and June 2007 in 16 Italian centers, were collected. The contribution of each center to the entire data set ranged from 1.3% to 14.5%. The study was approved by the institutional review boards (IRBs) of the participant centers. All enrolled patients signed IRB-approved informed consent forms.
Histopathological reports, operative notes, medical charts regarding RT and chemotherapy, and imaging findings were reviewed.
Only cases with histological specimens analyzed by experienced neuropathologists were included. WHO grade III gliomas with an oligodendroglial component were excluded. Expression of O6-methylguanine-DNA methyltransferase (MGMT) was assessed in only a minority of cases, and consequently it was not possible to evaluate its prognostic and predictive significance in this series. Postoperative imaging was reviewed in order to determine the extent of surgical resection. Gross total resection (GTR) was defined as the absence of residual tumor on postoperative imaging, regardless of the surgeon's assessment of the extent of resection. Type of RT (doses and technique) and chemotherapy approach were recorded.
Clinical and treatment-related factors were correlated with the outcome of the patients by univariate analysis. The Kaplan–Meier method was used to estimate overall survival (OS), calculated from the date of surgery until the time of death or last follow-up examination. Survival curves were compared using the log-rank test. The clinical factors analyzed were gender, patient's age, single vs multifocal lesion, presence or absence of presurgical symptoms (cranial hypertension, seizures, or focal symptoms), preoperative and postoperative Karnofsky Performance Status (KPS), Recursive Partitioning Analysis (RPA) class, extent of surgery (biopsy, subtotal removal, gross total removal), postoperative chemotherapy, delay of RT (45 or less days vs more than 45 days), type of RT (3D-conformal [3D-CRT] vs no 3D-CRT), total dose of RT, and schedule of TMZ (concomitant + sequential TMZ vs other schedules of TMZ). All the factors evaluable for the entire group were assessed using the Cox regression model. Differences among different subgroups of patients were calculated using the chi-square test. All statistical analyses were performed using Statistica for Windows.
Results
Patient Characteristics
Clinical data of the 295 analyzed patients are summarized in Table 1. Age ranged between 18 and 80 years (median, 55 y) with 24 patients younger than 30 years and 33 patients older than 70 years. The female/male ratio was 0.54/1. Almost 95% of patients had a single lesion, with the majority of lesions located in the frontal lobe (n = 74) or in the temporal lobe (n = 51). Only 25 (8.5%) patients had infratentorial lesions. Focal deficits and seizures were the most frequent symptoms at diagnosis (65.7% and 33.2%, respectively). Incidental diagnosis without neurological symptoms occurred in 2 patients involved in a car accident.
Table 1.
Patient clinical features and treatment characteristics in the whole group and in the 2 subgroups according to postoperative treatment
| Characteristics | Total | RT Alone | RT + CHT | Chi-square P |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| 295 (100) | 97 (32.9) | 198 (67.1) | ||
| Age | ||||
| ≤50 years | 123 (41.7) | 36 (37.1) | 87 (43.9) | 0.52 |
| 51–60 years | 63 (21.4) | 23 (23.7) | 40 (20.2) | |
| >60 years | 109 (36.9) | 38 (39.2) | 71 (35.9) | |
| Gender | ||||
| Male | 192 (65.1) | 65 (67.0) | 127 (64.1) | 0.62 |
| Female | 103 (34.9) | 32 (33.0) | 71 (35.9) | |
| Number of lesions | ||||
| Single | 280 (94.9) | 92 (94.8) | 188 (94.9) | 0.97 |
| Multiple | 15 (5.1) | 5 (5.2) | 10 (5.1) | |
| Presenting symptoms | ||||
| Focal symptoms | 194 (65.7) | 63 (64.9) | 131 (66.2) | 0.84 |
| Cranial hypertension | 76 (25.8) | 39 (40.2) | 46 (23.2) | 0.15 |
| Seizure | 98 (33.2) | 28 (28.9) | 70 (35.4) | 0.27 |
| None | 2 (0.7) | – | – | – |
| Preoperative KPS | ||||
| 90–100 | 110 (37.3) | 23 (23.7) | 47 (23.7) | 0.14 |
| 80 | 116 (39.3) | 41 (42.3) | 75 (37.9) | |
| ≤70 | 69 (23.4) | 33 (34.0) | 36 (18.2) | |
| Postoperative KPS | ||||
| 90–100 | 161 (54.6) | 41 (42.3) | 120 (60.6) | 0.002 |
| 80 | 60 (20.3) | 20 (20.6) | 40 (20.2) | |
| <70 | 74 (25.1) | 36 (37.1) | 38 (19.2) | |
| RPA class | ||||
| I | 93 (31.6) | 27 (27.8) | 66 (33.3) | 0.19 |
| II | 24 (8.1) | 6 (6.2) | 18 (9.1) | |
| III | 42 (14.2) | 15 (15.5) | 27 (13.6) | |
| IV | 82 (27.8) | 24 (24.7) | 58 (29.3) | |
| V | 54 (18.3) | 25 (25.8) | 29 (14.6) | |
| VI | – | – | – | |
| RPA classes 1–3 vs 4–5 | ||||
| I–III | 159 (53.9) | 48 (49.5) | 111 (56.1) | 0.29 |
| IV–V | 136 (46.1) | 49 (50.5) | 87 (43.9) | |
| RPA classes 1–2 vs 3 vs 4–5 | ||||
| I–II | 117 (39.7) | 33 (23.7) | 84 (42.4) | 0.38 |
| III | 42 (14.2) | 15 (76.3) | 27 (13.6) | |
| IV–V | 136 (46.1) | 49 (50.5) | 87 (43.9) | |
| Surgical procedure | ||||
| Biopsy | 70 (23.7) | 23 (23.7) | 47 (23.7) | 1.00 |
| Exeresis | 225 (76.3) | 74 (76.3) | 151 (76.3) | |
| 3D-CRT | ||||
| Yes | 254 (86.1) | 73 (75.3) | 181 (91.4) | 0.002 |
| No | 41 (13.9) | 24 (24.7) | 17 (8.6) | |
| RT fraction size | ||||
| 1.8 Gy | 19 (6.4) | 4 (4.1) | 15 (7.6) | 0.0007 |
| 2 Gy | 248 (84.1) | 75 (77.3) | 173 (87.4) | |
| 3 Gy | 28 (9.5) | 18 (18.6) | 10 (5.1) | |
| RT total dose for 1.8/2 Gy per fraction | ||||
| <60 Gy | 33 (11.2) | 17 (17.5) | 16 (8.1) | 0.005 |
| 60 Gy | 178 (60.3) | 43 (44.3) | 135 (68.2) | |
| >60 Gy | 56 (19.0) | 19 (19.6) | 37 (18.7) | |
| RT total dose for 3 Gy per fraction | ||||
| ≤39 Gy | 14 (4.7) | 9 (9.3) | 5 (2.5) | 0.05 |
| >39 Gy | 14 (4.7) | 9 (9.3) | 5 (2.5) | |
| Delay of RT | ||||
| ≤45 days | 114 (38.7) | 31 (32.0) | 83 (41.9) | 0.13 |
| >45 days | 124 (42.0) | 45 (46.4) | 79 (39.9) | |
| Unknown | 57 (19.3) | – | – | |
In the majority of patients, a CT scan was followed by an MRI scan before surgery (61.0%), whereas exclusive MRI or CT were performed for 16.3% and 22.7%, respectively. Twenty patients (6.8%) had MR spectroscopy before surgery. Exclusive CT scan was the most frequent postoperative imaging (32.9%); 29.5% and 20.3% of patients in the postoperative setting had both MRI and CT scan or exclusive MRI, respectively. Some patients did not have any postoperative radiological scan before starting the postoperative treatment (17.3%).
Median preoperative KPS was 80, with 15 patients with a score of 50 or less, whereas postsurgical KPS was 90. Patients were stratified according to RPA.7 The most frequent classes were the first one (younger than 50 years and normal mental status) and the fourth one (50 or older years, KPS 70–100, and symptom onset more than 3 months before surgery).
Treatment Characteristics
Surgery
Two hundred twenty-five patients out of 295 (76.3%) underwent a surgical removal. Among patients who had a surgically removed lesion, the extent of resection was based on postoperative imaging. In 67 patients, data regarding extent of removal were not available for lack of postoperative CT or MRI scan (n = 51) or difficulties in interpreting postoperative imaging (n = 16). A quarter of the patients (n = 75) had a gross total removal, as shown by postsurgical imaging. Carmustine wafer implants were not used in any patient.
Radiotherapy
All the patients had postoperative radiation treatment. Most of them were treated with 3D-CRT (86.1%) and with conventional fractionation (90.5%). In 80 cases (27.1%), the treatment plan for CRT was based on coregistered CT and MRI. The median dose for patients treated with conventional fractionation (1.8–2 Gy per fraction) was 60 Gy (range, 54–66 Gy), whereas patients who received a hypofractionated treatment (3 Gy per fraction) had a median dose of 39 Gy (range, 30–45 Gy). The median interval time between surgery and beginning of RT was 47 days (range, 14–91 days).
Postoperative chemotherapy
About two-thirds of the entire group received postoperative chemotherapy with TMZ (n = 198, 67.1%), with 166 of 198 patients (83.8% of patients who had postoperative chemotherapy) having concomitant and sequential TMZ according to the standard schedule.3 A minority of patients had only concomitant (n = 22) or only sequential TMZ (n = 10). No patient received any other chemotherapy agent.
The pretreatment characteristics of patients treated exclusively with RT and patients treated with RT + TMZ were well-balanced between the treatment groups, except for a significant imbalance in terms of postoperative KPS, the use of 3D-CRT, conventional fractionation, and total dose of 60 Gy: all these factors were favorable in the RT + TMZ group.
Changes in postoperative treatment over time
Patients in the present series were stratified according to time of treatment before or after the publication of the phase III trial from the European Organisation for Research and Treatment of Cancer (EORTC)/National Cancer Institute of Canada (NCIC)3 showing the superiority of RT + TMZ treatment for patients with glioblastoma multiforme (GBM) compared with RT exclusively (group A: January 2002–March 2005, n = 139; group B: April 2005–June 2007, n = 156). The use of both chemotherapy (group A: n = 73, 52.5%; group B: n = 125, 80.1%) and 3D-CRT (group A: n = 108, 77.7%; group B: n = 146, 93.6%) significantly increased in the subgroup of patients treated starting from April 2005 (chi-square test P< .0001 for both chemotherapy and use of 3D-CRT).
Survival
Median OS was 20.6 months, with 210 out of 295 patients dead at the time of analysis. Actuarial survival was 70.2% at 1 year and 28.6% at 4 years. The median follow-up, calculated from the end of RT to the last follow-up or death, was 23.1 months.
Univariate Analysis
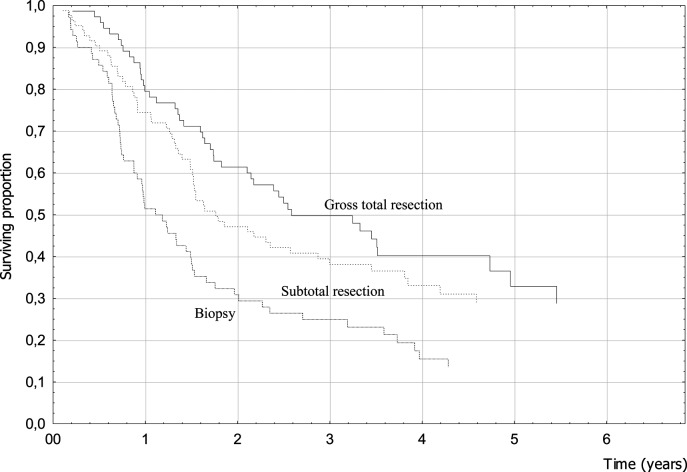
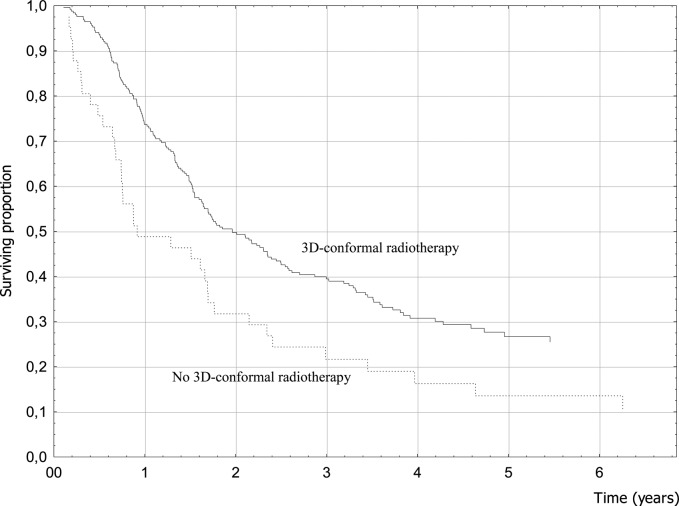
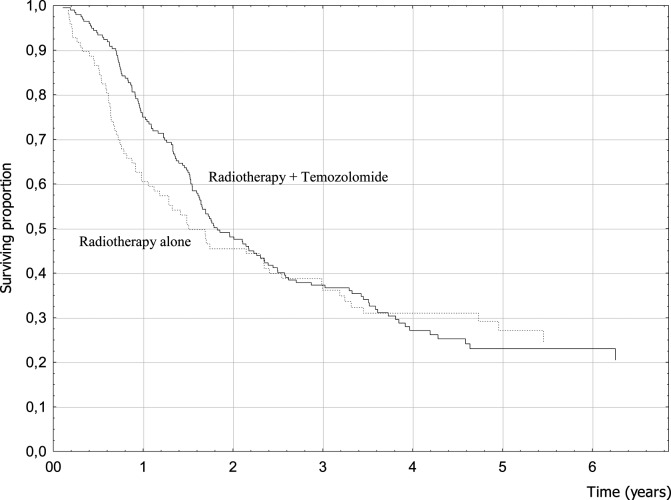
Clinical factors associated with better prognosis were female gender, younger age, seizures as a presenting symptom, absence of focal symptoms at diagnosis, higher preoperative and postoperative KPS, and RPA class (Table 2). Treatment-related factors that resulted in better survival were related to surgery and RT. When the extent of surgery was analyzed, 4-year OS was 32.9% for patients who had undergone tumor removal (40.2% for GTR patients) and only 15.5% for biopsy patients (P = .001; hazard ratio [HR] = 0.59) (Fig. 1). Conformal RT was significantly associated with a better prognosis (P = .01; HR = 0.60) (Fig. 2). Among patients treated with conventional fractionation (n = 178), doses of 60 Gy yielded better results without reaching statistical significance (P = .10). The use of chemotherapy with TMZ was not associated with better survival (P = .59; HR = 0.92) (Fig. 3). When analyzing OS of the subgroup of patients who received a standard schedule of TMZ (concomitant TMZ + sequential TMZ), the difference versus patients who had only RT was still not significant (P = .53).
Table 2.
Overall survival analysis: n patients at start, n deaths, OS (%), log-rank test, survival at specific follow-up times (1, 2, 4 y), hazard ratio (HR), and 95% confidence intervals (CI) from univariate regression analysis
| Variable | Pts at Start | n Deaths | Log-rank Testa | Survival (%) at |
HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| 1 y | 2 y | 4 y | |||||
| All | 295 | 210 | – | 70.2 | 47.2 | 28.6 | – |
| Sex | |||||||
| Female | 103 | 66 | 76.4 | 57.7 | 35.2 | 1b | |
| Male | 192 | 144 | 0.02 | 66.9 | 41.8 | 24.9 | 1.41 (1.05–1.89) |
| Age (y) | |||||||
| ≤50 | 123 | 61 | 89.8 | 75.9 | 51.6 | 1b | |
| 51–60 | 63 | 52 | 60.3 | 35.3 | 12.9 | 3.25 (2.23–4.75) | |
| >60 | 109 | 97 | 0.0001 | 54.8 | 22.3 | 11.2 | 3.96 (2.84–5.51) |
| N lesions | |||||||
| Single | 280 | 197 | 71.5 | 48.3 | 29.5 | 1b | |
| Multiple | 15 | 13 | 0.09 | 46.7 | 26.7 | 13.3 | 1.74 (0.99–3.05) |
| Presenting symptoms | |||||||
| Cranial hypertension | |||||||
| Yes | 76 | 57 | 65.5 | 44.7 | 23.4 | 1.23 (0.90–1.66) | |
| No | 219 | 153 | 0.20 | 71.9 | 48.1 | 30.4 | 1b |
| Seizure | |||||||
| Yes | 98 | 54 | 84.4 | 61.5 | 45.5 | 0.50 (0.37–0.69) | |
| No | 197 | 156 | <0.0001 | 63.2 | 40.1 | 19.8 | 1b |
| Focal symptoms | |||||||
| Yes | 194 | 143 | 64.7 | 41.6 | 25.3 | 1.40 (1.04–1.88) | |
| No | 101 | 67 | 0.02 | 80.8 | 58.1 | 34.8 | 1b |
| Preoperative KPS | |||||||
| ≤70 | 69 | 54 | 60.2 | 37.1 | 16.6 | 1b | |
| 80 | 116 | 86 | 68.7 | 44.1 | 25.4 | 0.78 (0.55–1.09) | |
| 90–100 | 110 | 70 | 0.001 | 78.0 | 56.7 | 39.1 | 0.56 (0.39–0.79) |
| Postoperative KPS | |||||||
| ≤70 | 74 | 62 | 54.7 | 30.3 | 12.6 | 1b | |
| 80 | 60 | 44 | 71.7 | 41.7 | 26.9 | 0.64 (0.44–0.95) | |
| 90–100 | 161 | 104 | <0.0001 | 76.7 | 57.0 | 38.0 | 0.46 (0.34–0.64) |
| RPA class | |||||||
| I | 93 | 44 | 90.1 | 79.0 | 56.9 | 1b | |
| II | 24 | 16 | 66.7 | 37.5 | 32.8 | 1.82 (1.12–2.98) | |
| III | 42 | 26 | 80.7 | 55.6 | 26.9 | 2.33 (1.31–4.15) | |
| IV | 82 | 75 | <0.0001 | 57.3 | 26.2 | 9.0 | 4.13 (2.81–6.06) |
| V | 54 | 49 | 49.3 | 22.8 | 9.6 | 4.74 (3.12–7.20) | |
| Surgical procedure | |||||||
| Exeresis | 225 | 151 | 76.2 | 52.3 | 32.9 | 0.59 (0.43–0.79) | |
| Biopsy | 70 | 59 | 0.001 | 51.4 | 30.8 | 15.5 | 1b |
| Extent of surgery | |||||||
| Biopsy | 70 | 59 | 51.4 | 30.8 | 15.5 | 1b | |
| Subtotal removal | 83 | 56 | 74.4 | 47.1 | 33.1 | 0.62 (0.43–0.89) | |
| Gross total removal | 75 | 45 | 0.001 | 79.5 | 61.4 | 40.2 | 0.48 (0.33–0.72) |
| Chemotherapy | |||||||
| Yes | 198 | 142 | 75.0 | 48.1 | 27.1 | 0.92 (0.69–1.23) | |
| No | 97 | 68 | 0.59 | 60.5 | 45.4 | 31.0 | 1b |
| Delay of RT | |||||||
| ≤45 days | 114 | 83 | 69.9 | 46.6 | 24.0 | 1b | |
| >45 days | 124 | 85 | 0.23 | 73.2 | 50.1 | 31.2 | 0.83 (0.61–1.13) |
| 3D-CRT | |||||||
| Yes | 254 | 173 | 0.01 | 73.7 | 49.7 | 30.7 | 0.60 (0.42–0.85) |
| No | 41 | 37 | 48.8 | 31.7 | 16.3 | 1b | |
| RT total dose (Gy) for 1.8/2 Gy fractions | |||||||
| <60 | 33 | 23 | 54.5 | 45.4 | 35.8 | 1.14 (0.72–1.79) | |
| 60 | 178 | 122 | 80.0 | 51.2 | 29.2 | 1b | |
| >60 | 56 | 40 | 0.10 | 64.3 | 50.0 | 30.9 | 1.02 (0.71–1.46) |
| RT total dose (Gy) for 3 Gy fractions | |||||||
| ≤39 | 14 | 12 | 35.7 | 14.3 | 14.3 | – | |
| >39 | 14 | 13 | 0.99 | 42.9 | 21.4 | 7.1 | |
| TMZ schedule | |||||||
| Concomitant + sequential TMZ | 166 | 117 | 73.1 | 48.3 | 28.1 | 1b | |
| Other schedules adjuvantTMZ | 32 | 25 | 0.93 | 84.4 | 46.9 | 21.8 | 1.09 (0.71–1.69) |
aFrom survival analysis, related to possibile difference on OS between different levels of the same parameter.
bReference category for univariate regression analysis.
Fig. 1.
Extent of surgery and survival (log-rank test, P = .001).
Fig. 2.
3D-CRT and survival (log-rank test, P = .01).
Fig. 3.
Postoperative chemotherapy with TMZ and survival (log-rank test, P = .93).
Multivariate Analysis
Five factors maintained their significance when analyzed with multivariate analysis (Table 3): younger age (P = .001), epilepsy (P = .04), RPA class (P = .04), surgical removal (P = .001), and 3D-CRT (P = .04). Gender, preoperative and postsurgical KPS, and presence of focal symptoms lost their significance. Chemotherapy with TMZ was confirmed not to be a significant factor.
Table 3.
Multivariate analysis
| Variable | Patients | P-value |
|---|---|---|
| All | ||
| 295 | – | |
| Sex | ||
| Female | 103 | .09 |
| Male | 192 | |
| Age (y) | ||
| ≤50 | 123 | |
| 51–60 | 63 | |
| >60 | 109 | .001 |
| Seizure | ||
| Yes | 98 | |
| No | 197 | .04 |
| Focal symptoms | ||
| Yes | 194 | |
| No | 101 | .38 |
| Preoperative KPS | ||
| ≤70 | 69 | |
| 80 | 116 | |
| 90–100 | 110 | .41 |
| Postoperative KPS | ||
| ≤70 | 74 | |
| 80 | 60 | |
| 90–100 | 161 | .11 |
| RPA class | ||
| I | 93 | |
| II | 24 | |
| III | 42 | |
| IV | 82 | |
| V | 54 | .04 |
| Surgical procedure | ||
| Exeresis | 225 | |
| Biopsy | 70 | .001 |
| 3D-CRT | ||
| Yes | 254 | |
| No | 41 | .04 |
Discussion
AA represents less than 5% of adult brain tumors.4 Only meager data exist that specifically address the optimal management of AA, and the majority of the studies pool together both grade III and IV gliomas (with a strong majority of the patients affected by GBM) or include anaplastic oligodendroglioma or mixed anaplastic oligoastrocytoma. Furthermore, some considerations about the treatment are based on extrapolation from glioblastoma data.
Although no strong evidence justifies the use of postoperative TMZ in patients with newly diagnosed AA, postoperative chemotherapy with TMZ is commonly used for these patients in clinical practice all over the world.4–6 Our data, analyzing the period from 2002 to 2007, strongly confirm that RT is usually associated with TMZ (67% in the whole analyzed period, 80.1% in the period from 2005 to 2007).
Chemotherapy and AA: Existing Randomized Studies
There are few randomized trials reporting results specifically regarding patients with AA (Table 4). Two of them compared RT alone versus RT + chemotherapy (carmustine + dibromodulcitrol8 and procarbazine + carmustine + vincristine [PCV]9). No survival benefit due to the addition of chemotherapy was shown.
Table 4.
Randomized studies that enrolled only WHO grade III gliomas and/or analyzed results for the subgroup of anaplastic astrocytoma
| Author | Treatment Arms | Histotypes | n Patients | Differences in OS | Differences in PFS | Survival Data Regarding OS in AA |
||
|---|---|---|---|---|---|---|---|---|
| Median Survival | Actuarial Survival |
|||||||
| @2y | @4y | |||||||
| Randomized studies that compared RT alone vs RT followed by chemotherapy | ||||||||
| Hildebrand et al8 | RT vs RT + BCNU + DBD | AA | 193 | NS | NS | 23.9 m | 47% | 31% |
| 27.3 m | 52% | 34% | ||||||
| Medical Research Council Brain Tumor Working Party9 | RT vs RT + PCV | HGG | 674 (AA = 113) | NS | na | 13 m | 15% | 6% |
| 15 m | 19% | 5% | ||||||
| Randomized studies that compared RT alone vs exclusive chemotherapy | ||||||||
| Wick et al10 | Adjuvant RT + PCV or TMZ at disease progression vs adjuvant PCV or TMZ + RT at disease progression | AA, AO, AOA | 318 (AA = 144) | NS | NS | na | na | |
Abbreviations: RT, radiotherapy; BCNU, carmustine; PCV, procarbazine, lomustine, and vincristine; DBD, dibromodulcitol; DFMO, α-difluoromethylornithine; TMZ, temozolomide; HGG, high-grade glioma; AA, anaplastic astrocytoma; AO, anaplastic oligodendroglioma; AOA, mixed anaplastic oligoastrocytoma; OS, overall survival; PFS, progression-free survival; NS, not significant; na, not available.
A recent phase III randomized trial10 compared RT alone or chemotherapy alone with TMZ or PCV; when patients treated with postoperative RT experienced disease progression, they were randomly assigned to receive either TMZ or PCV; relapsed patients treated with first-line chemotherapy were treated with RT. The primary endpoint was time to progression after both treatment modalities had failed, which did not significantly differ between the treatment arms. Upfront RT yielded a better response rate and longer time to progression than did initial chemotherapy, but the difference did not reach statistical significance. Although the interpretation of the results is complicated by its unusual design,4 the study showed equivalence between the 2 treatment arms in terms of OS and progression-free survival (PFS).
RT + TMZ and AA: Existing Series
Some phase II trials assessed the efficacy of neoadjuvant TMZ (alone11,12 or plus carmustine13) in patients with high-grade gliomas (Table 5). The best results in patients affected by AA were reported by Gilbert et al.,11 with a response rate of 39% and a median survival of 23.5 months. Brada et al.12 selected patients who had undergone biopsy alone in order to evaluate the objective response without the confounding effects of surgery. The overall response rate (partial response + minimal response) in AA was only 14%. Median survival was 14 months. Brandes et al.14 retrospectively assessed outcome of postoperative chemotherapy in patients with AA treated with RT, comparing postoperative PCV with sequential TMZ (no concomitant TMZ was prescribed). There was no cohort of patients treated with RT exclusively. There were no significant differences between outcomes in terms of OS and PFS between the 2 different chemotherapy regimens. It should be noted that the subgroup of patients treated with RT + TMZ and analyzed in this series was characterized by favorable prognostic factors (50% of patients were younger than 40 years old; median KPS at diagnosis was 90; only 13% of patients had undergone a biopsy). This should explain the optimal results in terms of survival outcome. Combs et al.15 published a retrospective analysis of 20 patients treated with RT + TMZ. The schedule used (TMZ at 50 mg/m2 each day throughout radiation treatment) was based on clinical experience previously reported by the same authors. No sequential TMZ was administered. The outcome of the combined approach was compared with a 1:2 matched-pair analysis of historical controls treated with RT exclusively (n = 40). The addition of such a schedule of concomitant TMZ did not have a significant impact on PFS or OS.
Table 5.
Studies that addressed the use of postoperative radiotherapy + temozolomide in anaplastic astrocytoma
| Author | Study | Treatment arms | Histotypes | n Patients | Differences in OS | Differences in PFS | Survival Data Regarding OS in AA |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Median Survival | Actuarial survival |
|||||||||
| @1y | @2y | @4y | ||||||||
| Gilbert et al11 | Ph II | Neoadj TMZ + RT | HGG | n = 57 (AA n = 18) | – | – | 23.5 m | na | 50% | na |
| Brada et al12 | Ph II | Neoadj TMZ + RT | HGG | n = 162 (AA = 37) | – | – | 14 m | 56% | na | na |
| Chang et al13 | Ph II | Neoadj TMZ + BCNU ± RT | WHO grade III gliomas | n = 41 (AA n = 33) | – | – | na | na | ||
| Brandes et al14 | Retrosp | RT + sTMZ vs RT + PCV | AA | n = 109 (RT + TMZ n = 60) | NS | NS | na | na | 75% | na |
| na | 83% | na | ||||||||
| Combs et al15 | Retrosp | RT vs RT + cTMZ | AA + AOA (AA n = 54) | n = 60 (RT + TMZ n = 20) | NS | NS | na | na | ||
| Current study | Retrosp | RT vs RT + cTMZ + sTMZ | AA | n = 295 (RT + cTMZ + sTMZ n = 166) | NS | na | 18.1 m | 60.5% | 45.4% | 31.0% |
| 21.4 m | 75.0% | 48.1% | 27.1% | |||||||
Abbreviations: OS, overall survival; PFS, progression-free survival; Ph II, phase II study; Retrosp, retrospective study; Neoadj, neoadjuvant; TMZ, temozolomide; RT, radiotherapy; HGG, high-grade glioma; AA, anaplastic astrocytoma; BCNU, carmustine; sTMZ, sequential temozolomide; PCV, procarbazine + lomustine + vincristine; cTMZ, concomitant temozolomide; AOA, mixed anaplastic oligoastrocytoma; NS, not significant; na, not available.
Prognostic Factors
Multivariate analysis showed statistical significance for age, presence of seizures, RPA classes I–III, surgical removal, and use of 3D-CRT.
Age was reported to be a significant prognostic factor for HGGs1,7 and also in several studies addressing the prognostic factors for only AA.14,16–19 The presence of epilepsy has been validated as a prognostic factor in brain tumors, both in low-grade gliomas20,21 and in WHO grade III gliomas.22 The Radiation Therapy Oncology Group RPA has recently been validated in 2 series: Park et al.23 showed that this classification retained its relative prognostic significance in patients with WHO grade III glioma (AA, n = 56), and Paravati et al.24 showed that the RPA classes can successfully predict survival of patients with HGG (AA n = 45) treated with intensity-modulated RT and postoperative TMZ. Extent of resection is among the major determinants of survival of patients with HGG.1,7,14,25,26 The use of 3D-CRT showed a significant prognostic value also in our previous study including GBM,2 although it should be noted that the prognostic significance of 3D-CRT could be due to a selection bias, since it is likely that patients amenable to CRT had focal tumor and smaller lesions without an extensive infiltrative component.
This is a retrospective review and, as such, is subject to all the usual limitations. As in other studies addressing AA,8,15,24 histological diagnosis was confirmed by only local neuropathologists; the lack of a pathological review is the most critical limitation of this study. Central pathological review of grade III tumors remains crucial, considering that disagreement in pathological diagnosis for WHO grade III gliomas is not uncommon.16 The high average age of the current series (55 y) could be an indirect sign of this possible inaccuracy since it could suggest that some cases had an undergraded WHO grade IV glioma. This study may also criticized for other pitfalls. First, the lack of biological markers is a consistent drawback of our survey, considering the predictive and prognostic importance of such data.19 Data regarding the genetic profile of the tumor might help in predicting chemosensitivity, allowing better tailoring of treatment strategies. Thus, it could be hypothesized that we would be able to understand which patients could benefit by the addition of TMZ. The best-known pathway involved in resistance to TMZ relies on MGMT, a DNA repair protein that inhibits the cross-linking of double-stranded DNA due to TMZ. The methylation of the MGMT promoter results in lack of MGMT expression and, therefore, in a greater cytotoxicity following treatment with TMZ. This finding was confirmed by Hegi et al27 in patients enrolled in the EORTC/NCIC phase III trial, which demonstrated a survival benefit associated with TMZ among glioblastoma patients with a methylated MGMT promoter region. It should be noted that some authors pointed out that MGMT promoter methylation status alone does not suffice to provide information about the sensitivity of grade III gliomas to alkylating agents,28,29 as MGMT protein expression is also regulated by other independent pathways, such as MGMT mRNA expression.30 Furthermore, the lower expression of the MGMT protein observed in grade III gliomas compared with WHO grades II and IV gliomas31 suggests that other relevant molecular factors in tumor chemosensitivity should be assessed—for example, a role of DNA mismatching repair gene alterations in the resistance to TMZ could also be advocated.32 The absence of data on genetic features and especially on MGMT makes it difficult to know whether the treatment groups were well-balanced. Second, we did not record any data regarding supportive care. This is an important point for the risk of significant interactions between antiepileptic drugs and TMZ;33,34 furthermore, the use of steroids concomitantly with radiation and TMZ may result in severe reductions in CD4 count that could have a negative impact on prognosis.35
Nevertheless, examining 295 biopsy-proven pure AA patients, the present series differs from the majority of other AA studies that also included GBM9,11,12 or mixed gliomas with an oligodendroglial component10,13,15 and gives a comprehensive analysis of the current management of this disease. To our knowledge, this is the largest series regarding the use of TMZ + RT in AA, and it is the only existing study reporting the outcome of both concomitant and sequential TMZ in the postoperative be setting, including a control group with patients treated with RT alone.
Based on the results of the few existing studies to date regarding the use of TMZ, as well as on the results of the current study, we can draw the conclusion that although RT + TMZ improves overall survival in patients with GBM, this therapeutic strategy does not significantly improve outcome in AAs. Only the findings of the prematurely closed Radiation Therapy Oncology Group 9813 trial and the results of the ongoing EORTC-26053 trial addressing the potential benefit of concomitant and/or sequential TMZ in AA will either support or refute these results.
Acknowledgments
The authors are very grateful to Tracy Scudieri, who reviewed the English version of the manuscript. Her help was invaluable.
Conflict of interest statement. None declared.
References
- 1.Magrini SM, Ricardi U, Santoni R, et al. Patterns of practice and survival in a retrospective analysis of 1722 adult astrocytoma patients treated between 1985 and 2001 in 12 Italian radiation oncology centers. Int J Radiat Oncol Biol Phys. 2006;65:788–799. doi: 10.1016/j.ijrobp.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 2.Scoccianti S, Magrini SM, Ricardi U, et al. Patterns of care and survival in a retrospective analysis of 1059 patients with glioblastoma multiforme treated between 2002 and 2007: a multicenter study by the Central Nervous System Study Group of Airo (Italian Association of Radiation Oncology) Neurosurgery. 2010;67:446–458. doi: 10.1227/01.NEU.0000371990.86656.E8. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, Van Den Bent MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;52:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.DeAngelis LM. Anaplastic glioma: how to prognosticate outcome and choose a treatment strategy. J Clin Oncol. 2009;27:5861–5862. doi: 10.1200/JCO.2009.24.5985. [DOI] [PubMed] [Google Scholar]
- 5.Omar A, Mason WP. Temozolomide: the evidence for its therapeutic efficacy in malignant astrocytoma. Core Evid. 2009;4:93–111. doi: 10.2147/ce.s6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dresemann G. Temozolomide in malignant glioma. Oncotargets Ther. 2010;3:139–146. doi: 10.2147/ott.s5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 8.Hildebrand J, Gorlia T, Kros JM, et al. Adjuvant dibromodulcitol and BCNU chemotherapy in anaplastic astrocytoma: results of a randomised European Organisation for Research and Treatment of Cancer phase III study (EORTC study 26882) Eur J Cancer. 2008;44:1210–1216. doi: 10.1016/j.ejca.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Medical Research Council Brain tumor Working Party. Randomized trial of procarbazine, lomustine and vincristine in the adjuvant treatment of high-grade astrocytoma. J Clin Oncol. 2001;19:509–518. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- 10.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert MR, Friedman HS, Kuttesch JF, et al. A phase II study of temozolomide in patients with newly diagnosed supratentorial malignant glioma before radiation therapy. Neuro Oncol. 2002;4:261–267. doi: 10.1093/neuonc/4.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brada M, Ashley S, Dowe A, et al. Neoadjuvant phase II multicentre study of new agents in patients with malignant glioma after minimal surgery. Report of a cohort of 187 patients treated with temozolomide. Ann Oncol. 2005;16:942–949. doi: 10.1093/annonc/mdi183. [DOI] [PubMed] [Google Scholar]
- 13.Chang SM, Prados MD, Yung WKA, et al. Phase II study of neoadjuvant 1,3-bis (2-chloroethyl)-1-nitrosourea and temozolomide for newly diagnosed anaplastic glioma. A North American Brain Tumor Consortium trial. Cancer. 2004;100:1712–1716. doi: 10.1002/cncr.20157. [DOI] [PubMed] [Google Scholar]
- 14.Brandes AA, Nicolardi L, Tosoni A, et al. Survival following adjuvant PCV or temozolomide for anaplastic astrocytoma. Neuro Oncol. 2006;8:253–260. doi: 10.1215/15228517-2006-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Combs SE, Nagy M, Edler L, et al. Comparative evaluation of radiochemotherapy with temozolomide versus standard-of-care postoperative radiation alone in patients with WHO grade III astrocytic tumors. Radiother Oncol. 2008;88:177–182. doi: 10.1016/j.radonc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Stupp R, Reni M, Gatta G, Mazza E, Vecht C. Anaplastic astrocytoma in adults. Crit Rev Oncol Hematol. 2007;63:72–80. doi: 10.1016/j.critrevonc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Tortosa A, Vinolas N, Villa S, et al. Prognostic implication of clinical, radiologic and pathologic features in patients with anaplastic gliomas. Cancer. 2003;97:1063–1071. doi: 10.1002/cncr.11120. [DOI] [PubMed] [Google Scholar]
- 18.Perry A, Jenkins RB, O'Fallon JR, et al. Clinicopathological study of 85 similarly treated patients with anaplastic astrocytic tumors. An analysis of DNA content, cellular proliferation and p53 expressions. Cancer. 1996;86:672–683. doi: 10.1002/(sici)1097-0142(19990815)86:4<672::aid-cncr17>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 19.Compostella A, Tosoni A, Blatt V, Franceschi E, Brandes AA. Prognostic factors for anaplastic astrocytomas. J Neurooncol. 2007;81:295–303. doi: 10.1007/s11060-006-9232-z. [DOI] [PubMed] [Google Scholar]
- 20.Leighton C, Fisher B, Bauman G, et al. Supratentorial low-grade glioma in adults: An analysis of prognostic factors and the timing of radiation. J Clin Oncol. 1997;15:1294–1301. doi: 10.1200/JCO.1997.15.4.1294. [DOI] [PubMed] [Google Scholar]
- 21.van Veelen MLC, Avezaat CJJ, Kros JM, et al. Supratentorial low-grade astrocytoma: Prognostic factors, dedifferentiation, and the issue of early versus late surgery. J Neurol Neurosurg Psych. 1998;64:581–587. doi: 10.1136/jnnp.64.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krzyszkowski T, Czepko R, Betlej M. Prognostic value of epileptic seizures in patients with cerebral gliomas. Ann Acad Med Stetin. 2004;50:35–40. [PubMed] [Google Scholar]
- 23.Park CK, Lee SH, Han JH, et al. Recursive partitioning analysis of prognostic factors in WHO grade III glioma patients treated with radiotherapy or radiotherapy plus chemotherapy. BMC Cancer. 2009;9:450–456. doi: 10.1186/1471-2407-9-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paravati AJ, Heron DE, Landsittel D, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma and anaplastic astrocytoma: validation of Radiation Therapy Oncology Group–Recursive Partitioning Analysis in the IMRT and temozolomide era. J Neurooncol. 2011;104:339–349. doi: 10.1007/s11060-010-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen ZR, Suki D, Shi W, et al. Surgical resection of anaplastic astrocytoma: prognostic factors and outcome. Neuro-Oncol. 2002;4:367. [Google Scholar]
- 26.Mc Girt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110:156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 27.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 28.Brell M, Tortosa A, Verger E, et al. Prognostic significance of O6-methylguanine-DNA methyltransferase determined by promoter hypermethylation and immunohistochemical expression in anaplastic gliomas. Clin Cancer Res. 2005;11:5167–5174. doi: 10.1158/1078-0432.CCR-05-0230. [DOI] [PubMed] [Google Scholar]
- 29.Siker ML, Chakravarti A, Mehta MP. Should concomitant and adjuvant treatment with temozolomide be used as standard therapy in patients with anaplastic glioma? Crit Rev Oncol Hematol. 2006;60:99–111. doi: 10.1016/j.critrevonc.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Kreth S, Thon N, Eigenbrod S, et al. O6-methylguanine-DNA methyltransferase (MGMT) mRNA expression predicts outcome in malignant glioma independent of MGMT promoter methylation. Plos One. 2011;6:e17156. doi: 10.1371/journal.pone.0017156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capper D, Mittelbronn M, Meyermann R, Schittenhelm J. Pitfalls in the assessment of MGMT expression and in its correlation with survival in diffuse astrocytomas: proposal of a feasible immunohistochemical approach. Acta Neuropathol. 2008;115:249–259. doi: 10.1007/s00401-007-0310-x. [DOI] [PubMed] [Google Scholar]
- 32.Felsberg J, Thon N, Eigenbrod S, et al. Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int J Cancer. 2011;129:659–670. doi: 10.1002/ijc.26083. [DOI] [PubMed] [Google Scholar]
- 33.Kargiotis O, Markoula S, Kyritsis AP. Epilepsy in cancer patient. Cancer Chemother Pharmacol. 2011;67:489–501. doi: 10.1007/s00280-011-1569-0. [DOI] [PubMed] [Google Scholar]
- 34.Yap KY, Chui WK, Chan A. Drug interactions between chemotherapeutic regimens and antiepileptics. Clin Ther. 2008;30:1385–1407. doi: 10.1016/j.clinthera.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Grossman SA, Ye X, Lesser GJ, et al. Iatrogenic Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]