Abstract
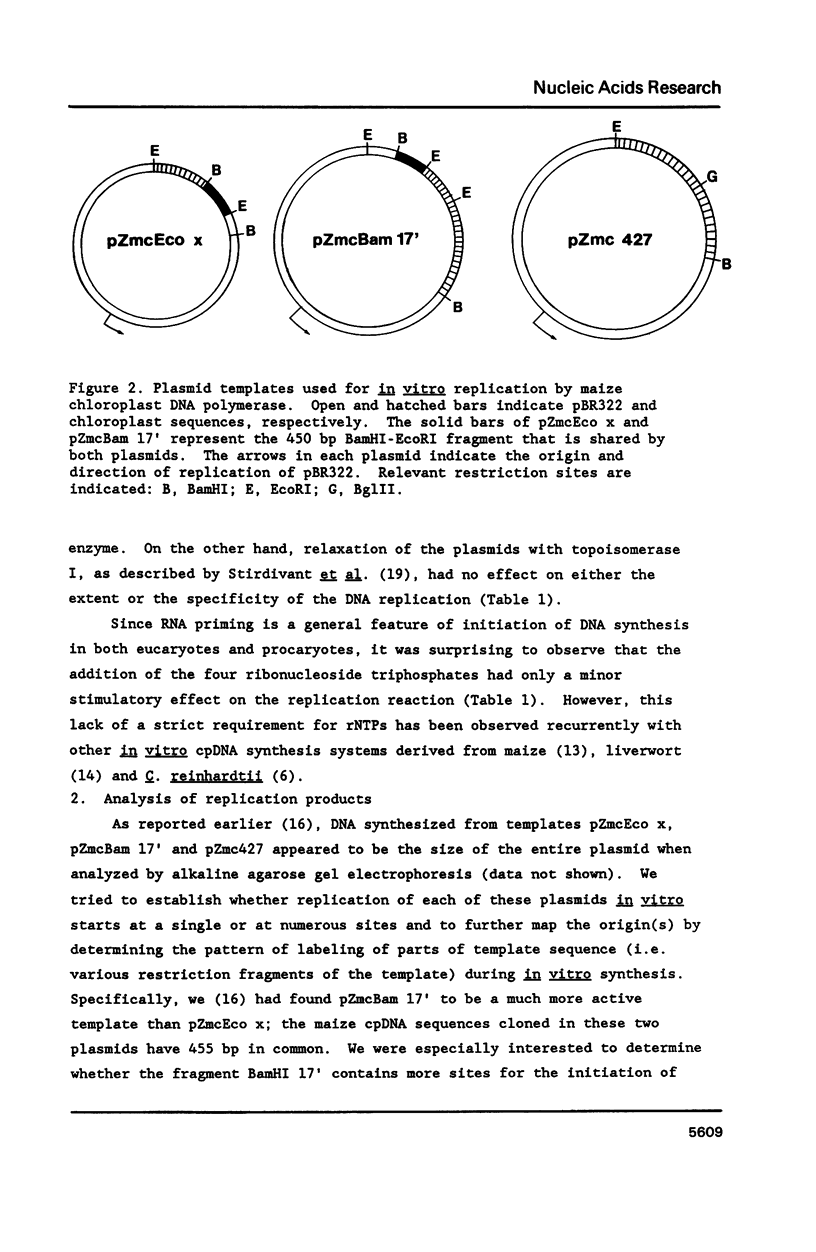
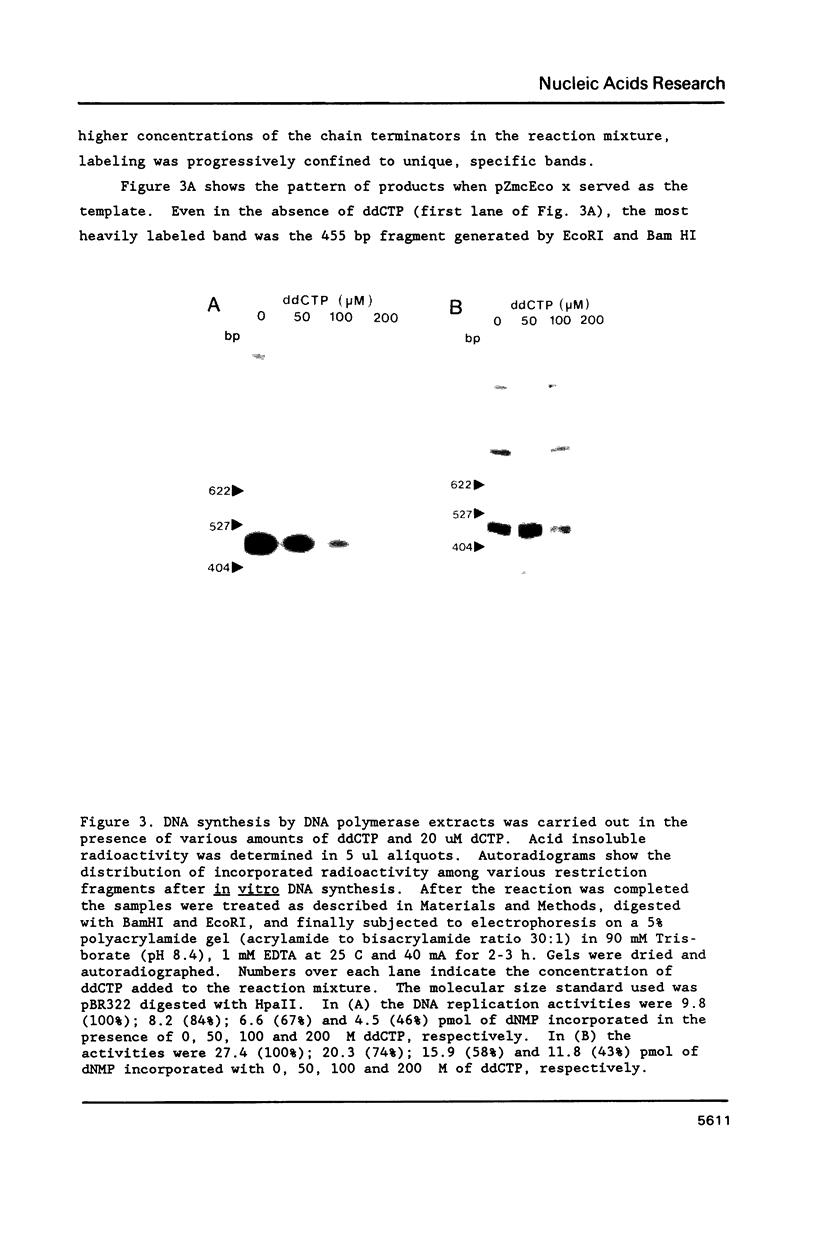
An enzyme system prepared from maize chloroplasts catalyzes the synthesis of DNA from maize chloroplast DNA sequences cloned in bacterial plasmids. Cloned maize chloroplast DNA fragments Bam HI 17' (2470 bp) and Eco RI x (1368 bp) have been shown to be preferred templates for in vitro DNA synthesis catalyzed by pea chloroplast DNA polymerase preparations [Gold et al. (1987) Proc. Natl. Acad. Sci. USA 84, 194-198]. Analysis of replicative intermediates indicates that although the template activity of the recombinant plasmid pZmcBam 17' is substantially greater than that of the pZmcEco x, replication in both cases originates from within a 455 bp region which overlaps the two plasmids. The remaining approximately 1500 basepair portion of maize chloroplast BamHI fragment 17' is not more active because it contains additional origins for replication. The overlapping region shows sequence homology with a portion of the Chlamydomonas reinhardtii chloroplast chromosome that contains a replication origin. Replication is shown to proceed bidirectionally within the 455 bp origin region. Recombinant plasmid pZmc 427, which is also active in the in vitro DNA synthesis assay, promoted localized replication initiation within a 1 kbp Bg1II-Eco RI fragment of the chloroplast DNA insert, a region that includes the 3' terminal part of the psbA gene.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Day A., Ellis T. H. Chloroplast DNA deletions associated with wheat plants regenerated from pollen: possible basis for maternal inheritance of chloroplasts. Cell. 1984 Dec;39(2 Pt 1):359–368. doi: 10.1016/0092-8674(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Gold B., Carrillo N., Tewari K. K., Bogorad L. Nucleotide sequence of a preferred maize chloroplast genome template for in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1987 Jan;84(1):194–198. doi: 10.1073/pnas.84.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardies S. C., Patient R. K., Klein R. D., Ho F., Reznikoff W. S., Wells R. D. Construction and mapping of recombinant plasmids used for the preparation of DNA fragments containing the Escherichia coli lactose operator and promoter. J Biol Chem. 1979 Jun 25;254(12):5527–5534. [PubMed] [Google Scholar]
- Karawya E., Swack J. A., Wilson S. H. Improved conditions for activity gel analysis of DNA polymerase catalytic polypeptides. Anal Biochem. 1983 Dec;135(2):318–325. doi: 10.1016/0003-2697(83)90689-9. [DOI] [PubMed] [Google Scholar]
- Koepsel R. R., Khan S. A. Static and initiator protein-enhanced bending of DNA at a replication origin. Science. 1986 Sep 19;233(4770):1316–1318. doi: 10.1126/science.3749879. [DOI] [PubMed] [Google Scholar]
- Koller B., Delius H. Origin of replication in chloroplast DNA of Euglena gracilis located close to the region of variable size. EMBO J. 1982;1(8):995–998. doi: 10.1002/j.1460-2075.1982.tb01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R. D., Tewari K. K. Chloroplast DNA from higher plants replicates by both the Cairns and the rolling circle mechanism. Nature. 1975 Aug 28;256(5520):708–711. doi: 10.1038/256708a0. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. Presence of displacement loops in the covalently closed circular chloroplast deoxyribonucleic acid from higher plants. J Biol Chem. 1975 Nov 25;250(22):8840–8847. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKown R. L., Tewari K. K. Purification and properties of a pea chloroplast DNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2354–2358. doi: 10.1073/pnas.81.8.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pienkos P., Walfield A., Hershberger C. L. Effect of nalidixic acid on Euglena gracilis: induced loss of chloroplast deoxyribonucleic acid. Arch Biochem Biophys. 1974 Dec;165(2):548–553. doi: 10.1016/0003-9861(74)90281-1. [DOI] [PubMed] [Google Scholar]
- Ryder K., Silver S., DeLucia A. L., Fanning E., Tegtmeyer P. An altered DNA conformation in origin region I is a determinant for the binding of SV40 large T antigen. Cell. 1986 Mar 14;44(5):719–725. doi: 10.1016/0092-8674(86)90838-x. [DOI] [PubMed] [Google Scholar]
- Schreier P. H., Cortese R. A fast and simple method for sequencing DNA cloned in the single-stranded bacteriophage M13. J Mol Biol. 1979 Mar 25;129(1):169–172. doi: 10.1016/0022-2836(79)90068-8. [DOI] [PubMed] [Google Scholar]
- Snyder M., Buchman A. R., Davis R. W. Bent DNA at a yeast autonomously replicating sequence. Nature. 1986 Nov 6;324(6092):87–89. doi: 10.1038/324087a0. [DOI] [PubMed] [Google Scholar]
- Stirdivant S. M., Crossland L. D., Bogorad L. DNA supercoiling affects in vitro transcription of two maize chloroplast genes differently. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4886–4890. doi: 10.1073/pnas.82.15.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K. K. Purification and properties of chloroplast DNA polymerase. Methods Enzymol. 1986;118:186–201. doi: 10.1016/0076-6879(86)18073-6. [DOI] [PubMed] [Google Scholar]
- Waddell J., Wang X. M., Wu M. Electron microscopic localization of the chloroplast DNA replicative origins in Chlamydomonas reinhardii. Nucleic Acids Res. 1984 May 11;12(9):3843–3856. doi: 10.1093/nar/12.9.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. M., Chang C. H., Waddell J., Wu M. Cloning and delimiting one chloroplast DNA replicative origin of Chlamydomonas. Nucleic Acids Res. 1984 May 11;12(9):3857–3872. doi: 10.1093/nar/12.9.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Wu M., Lou J. K., Chang D. Y., Chang C. H., Nie Z. Q. Structure and function of a chloroplast DNA replication origin of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6761–6765. doi: 10.1073/pnas.83.18.6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn K., Blattner F. R. Sequence-induced DNA curvature at the bacteriophage lambda origin of replication. Nature. 1985 Oct 3;317(6036):451–453. doi: 10.1038/317451a0. [DOI] [PubMed] [Google Scholar]
- Zimmermann W., Weissbach A. Deoxyribonucleic acid synthesis in isolated chloroplasts and chloroplast extracts of maize. Biochemistry. 1982 Jul 6;21(14):3334–3343. doi: 10.1021/bi00257a014. [DOI] [PubMed] [Google Scholar]