Abstract
Attentional biases for negative stimuli have been observed in school-age and adolescent children of depressed mothers and may reflect a vulnerability to depression. The direction of these biases and whether they can be identified in early childhood remains unclear. The current study examined attentional biases in 5–7-year-old children of depressed and non-depressed mothers. Following a mood induction, children participated in a dot-probe task assessing biases for sad and happy faces. There was a significant interaction of group and sex: daughters of depressed mothers attended selectively to sad faces, while children of controls and sons of depressed mothers did not exhibit biases. No effects were found for happy stimuli. These findings suggest that attentional biases are discernible in early childhood and may be vulnerability markers for depression. The results also raise the possibility that sex differences in cognitive biases are evident before the emergence of sex differences in the prevalence of depression.
Keywords: Maternal depression, Cognitive vulnerability, Children, Attentional bias, Emotional faces
Introduction
Cognitive theorists have long hypothesized that negatively-biased cognitive schemas, which serve as filters influencing how information is attended to, interpreted, and remembered, are a diathesis for depressive disorders (e.g., Beck 1967; Ingram et al. 1998). Individuals with cognitive diatheses are posited to be at increased risk for developing depressive episodes in the face of life stress. The link between cognitive biases and depressive disorders has been supported by numerous laboratory studies that have reported associations between depression and information-processing biases in attention, judgment, and memory (Joormann 2009; Williams et al. 1988). For example, depression has been linked with sustained attention for negative emotional stimuli (Leyman et al. 2007) and biases in both explicit and implicit memory (Matt et al. 1992; Rinck and Becker 2005). Similar findings have been reported in children and adolescents with high levels of depressive symptoms (Jacobs et al. 2008; Reid et al. 2006). For example, children with elevated levels of depressive symptoms exhibit memory biases for negative information compared to positive information (Bishop et al. 2004). In addition, children and adolescents with depression have been found to display negative biases in their interpretations of the stressfulness of events, and of their personal contributions to the events (Krackow and Rudolph 2008).
Consistent with Beck’s and other cognitive theorists’ work, some of the cognitive factors associated with depression appear to be markers of vulnerability to depression, rather than episode markers for the disorder. A vulnerability is an enduring, trait-like biological or psychological factor that predisposes an individual to a disorder. Thus, a potential vulnerability marker should be present before the development of the disorder, in persons at elevated risk for the disorder, and during remission from the disorder (Ingram and Luxton 2005; Zubin and Spring 1977). There is evidence that self-reported dysfunctional attitudes and a depressotypic inferential style prospectively predict the first-onset of major depressive disorder (MDD) in older adolescents and young adults (Alloy et al. 2006). In addition, laboratory measures of cognitive biases, including selective recall and attention for negative stimuli, have been found to be present in high risk samples and after remission from a depressive episode, particularly when activated by negative mood states (for a review, see Scher et al. 2005).
Most studies using high risk samples to explore potential vulnerability markers have focused on the offspring of parents with the disorder. Parental depression is one of the best established risk factors for depression (Hammen 2009; Weissman et al. 1987). Offspring of parents with a history of recurrent MDD or chronic forms of depression, such as chronic MDD, double depression, and dysthymic disorder, appear to be at particularly high risk for developing depression (Keller et al. 1986; Klein et al. 1988; Warner et al. 1995). Though both paternal and maternal MDD are associated with elevated rates of offspring depression, this link is more consistent for maternal depression, with several large studies either failing to observe an association between paternal and offspring MDD (Brennan et al. 2002) or finding that it was limited to more severe forms of offspring depression (Klein et al. 2005). Indeed, in a meta-analysis of this literature, Connell and Goodman (2002) reported that maternal depression had a significantly larger effect than paternal depression on child internalizing problems and that this difference was particularly strong in younger children.
Children with a history of maternal depression appear to be characterized by some of the same cognitive biases found in depressed individuals. For example, children of mothers with a history of depression have more negative attributional styles and more negative views of the self than do children of parents with no psychopathology (Garber and Robinson 1997; Jaenicke et al. 1987). More recently, Dearing and Gotlib (2009) reported that girls of depressed mothers offer more negative interpretations of ambiguous stimuli than do girls of mothers with no history of depression.
Several recent studies have also examined attentional biases in children of depressed parents. Attentional biases are of interest because they are likely to influence downstream cognitive processes, potentially contributing to the development of evaluation and memory biases. In addition, attentional systems develop early and can be assessed at a younger age than more complex higher-order cognitive processes, hence they may provide a window on the early development of emotional disorders (Pine et al. 2009).
One of the most frequently used approaches to assessing attentional biases is the dot-probe task, in which participants are asked to respond to a probe presented on the left or right side of the screen. Prior to the presentation of the probe, one emotional and one neutral stimulus (e.g., words or faces) are presented simultaneously on the left and right side of the screen and removed before the presentation of the probe; in this way, the probe can be presented in the location where a neutral or emotional stimulus was presented. Response latencies are used to calculate attentional biases towards and away from emotional stimuli (MacLeod et al. 1986). An attentional bias toward emotional stimuli, for instance, would be indicated by shorter response latencies when the emotional stimuli and probe appear on the same side of the screen than when they appear on opposite sides. Attentional biases towards threatening stimuli in individuals with anxiety disorders is a well established finding, but the relation with mood disorders is less clear (Bar-Haim et al. 2007; Mogg and Bradley 2005). While some studies have found attentional biases for negative emotional stimuli in depression (Gotlib et al. 2004a; Mathews et al. 1996; Rinck and Becker 2005), other studies, including several with depressed children and adolescents, have failed to find biases for threatening words or emotional faces (Dalgleish, et al. 2003; MacLeod, et al. 1986; Mogg et al. 2000; Neshat-Doost et al. 2000). The duration and nature of the stimuli and use of mood primes may account for these inconsistent findings.
In anxiety disorders, results from the dot-probe task suggest that attention is captured quickly by negative stimuli, with evidence of biases being reported even when conscious awareness of stimuli is restricted (Mogg and Bradley 2005). Individuals with generalized anxiety disorder (GAD) shift attention towards threatening stimuli faster and more frequently than do those with depression (Mogg et al. 2000). In depression, attentional biases seem to be slower and less automated, perhaps indicative of more conscious processing (Mathews and MacLeod 2005). For this reason, biases in depression might be elicited more easily using longer stimulus presentation durations than are used in anxiety research.
In addition, attentional biases in depression seem to be most apparent for mood-congruent stimuli, such as sad emotional faces (Gotlib et al. 2004a; b). Moreover, in studies with depressed and at-risk children and adults, pictorial stimuli appear to be more effective in eliciting attentional biases than verbal stimuli, such as sad words (e.g., Dalgleish et al. 2003; Gibb et al. 2009; Gotlib et al. 2004a; b; Joormann et al. 2007; Neshat-Doost et al. 2000).
Finally, a negative mood state appears to be important for eliciting cognitive and attentional biases in depression. Persons and Miranda (1992) argued that a negative mood state is required to activate cognitive schemas associated with vulnerability to depression. From this perspective, although maladaptive cognitive styles are stable traits in individuals with depression, they exist only in a latent form until they are activated by some type of negative experience, such as a mood induction. Indeed, there is evidence that attentional biases for negative stimuli are linked to mood state (Bradley et al. 1997). In addition, negative mood states seem to exacerbate information-processing biases that are associated with depression. For example, after induction of a negative mood, formerly depressed individuals show more errors than do controls when they are distracted by emotional words (Ingram et al. 1994). In another study, children of depressed mothers showed more negative cognitive biases than did children of non-depressed mothers, but only after having been primed with a negative mood state (Taylor and Ingram 1999).
There is recent evidence suggesting that biases identified in the dot-probe task may be a vulnerability or risk marker for depression. In two studies using longer stimulus durations, pictorial stimuli, and mood primes, Joormann and Gotlib and colleagues reported that attentional biases for sad stimuli were evident after remission from a depressive episode (Joormann and Gotlib 2007), and that 9- to 14-year-old daughters of mothers with a history of recurrent MDD exhibited a bias for sad emotional faces, whereas daughters of non-depressed mothers exhibited a bias for happy emotional faces (Joormann et al. 2007). In addition, a recent dot-probe study without a mood prime found that 8- to 12-year-old children of mothers with a history of depression showed avoidance of sad faces compared to children of mothers with no history of depression (Gibb et al. 2009). These findings raise several important questions. First, although school-age children and younger adolescents at risk for depression appear to exhibit attentional biases to sad faces on the dot-probe task, the direction of these biases (e.g., greater attention versus avoidance) remains unclear. In addition, investigators have not examined whether attentional biases can be identified in even younger offspring of depressed mothers. This is a critical question given that early identification of risk markers for depression would provide increased opportunities for early intervention/prevention (Pine et al 2009). Finally, rates of depression begin to increase more sharply among females than males in early adolescence; it is still unclear, however, exactly what mechanisms underlie this differential risk (Hankin and Abramson 2001; Hyde et al. 2008; Nolen-Hoeksema and Girgus 1994). There is some evidence of sex differences in cognitive styles prior to adolescence. For example, girls appear to recall more emotional memories than boys beginning in childhood, indicating that there may be early differences in the processing of emotional information (Davis 1999). In addition, a longitudinal study of depressive cognitions in children and adolescents found that a number of cognitive diatheses, including perceptions of physical appearance and self-worth, are more stable and trait-like for girls than for boys beginning before the gender difference in depression emerges (Cole et al. 2009). Though girls did not show higher levels of depressive cognitions, the results indicated that these cognitive styles may be more persistent in females which could contribute to gender differences in rates of depression. Examining cognitive biases in males and females at high and low risk for depression prior to adolescence may provide insight into whether these factors precede and, therefore, may contribute to, the sex difference in rates of depression in adolescents and adults.
The current study was designed to examine whether attentional biases can be elicited in young children at risk for developing depressive disorders. We administered a dot-probe task to 5- to 7-year-old male and female offspring of mothers with a history of recurrent or chronic depression and offspring of mothers with no lifetime history of psychopathology. Based on findings from previous research, we used a relatively long stimulus presentation duration, pictorial stimuli, and a negative mood induction. Although attentional biases have been observed without mood primes (e.g., Mathews et al. 1996), findings have been less reliable and even in the opposite direction (Gibb et al. 2009) compared to those studies that include mood primes. We predicted that children of depressed mothers would show attentional biases for sad emotional stimuli. In addition, we examined whether these effects would be moderated by child gender, such that biases for sad faces would be greater among girls than among boys.
Method
Participants
Participants were 118 children with a mean age of 6 years selected from a larger study. The initial sample (n=559) was recruited through a commercial mailing list when they were 3 years old. Children between 3 and 4 years of age who lived with at least one English-speaking biological parent and did not have any significant medical conditions or developmental disabilities were eligible for participation. Only one child per family was included. For the present study, we selected the children of mothers who, in the initial assessment, had a lifetime history of chronic or recurrent MDD or dysthymic disorder as defined by the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (American Psychiatric Association 2000), and the children of mothers who, in the initial assessment, had no lifetime history of axis I diagnoses. Children of mothers with a history of bipolar disorder were not included, and a history of mood disorder not otherwise specified was not sufficient for inclusion in the depression group. Of the children from the initial (age 3) wave of assessments, 331 would have met these criteria, and we included 118 of them in this study. Nineteen of these 118 children were excluded from analyses due to poor performance on the dot probe task, leaving a final sample of 99 children between the ages of 5 and 7 (M= 6.17, SD=0.40). The sample was primarily middle-class, with 25 families falling into social class I, 40 in class II, 29 in class III, and 5 in class IV according to Hollingshead’s Four Factor Index of Social Status (Hollingshead 1975). In terms of race, 85% of the children were Caucasian, 3% were Hispanic, 1% was African American, 2% were Asian American, and 9% were multiracial.
Procedure
Diagnoses of maternal depression were made during the initial wave of assessments. All other assessments occurred approximately 3 years later when the children were approximately 6 years old. Prior to the experimental procedures, children rated their mood. Next, the mood induction procedures were administered. Following the mood prime, children were asked to rate their mood for a second time and were given instructions for the dot-probe task. Then they completed the dot-probe task, as described below.
Parent Assessments
When the children entered the study at age three, their biological mothers were interviewed using the Structured Clinical Interview for DSM-IV non-patient version (First et al. 1996). The SCID is one of the most widely used structured diagnostic interviews and has acceptable levels of interrater reliability and procedural validity (Williams et al. 1992). The SCID has been shown to have fair test-retest reliability (Kappa=0.61–0.69) and good inter-rater reliability (Kappa= 0.80) for diagnoses of major depressive disorder using DSM-IV criteria (Williams et al. 1992; Zanarini et al. 2000). Interviews were conducted by telephone, which generally yields comparable results to face-to-face interviews (Rohde et al. 1997; Sobin et al. 1993). Two Masters-level raters conducted the diagnostic interviews. A second rater derived independent diagnoses based on audiotapes of 30 interviews. The interrater reliability (Kappa) for lifetime depressive disorder was 0.93.
Mothers completed the Diagnostic Inventory for Depression (DID; Zimmerman et al. 2004) when their children returned for a second assessment at age 6. The DID is a self-report measure designed to assess DSM-IV criteria for MDD. The DID has a sensitivity of 90.1% and a specificity of 77% with respect to SCID diagnoses of MDD (Zimmerman et al. 2004). The DID is also highly correlated with other measures of depression, including the Beck Depression Inventory (r=0.83) and the Hamilton Rating Scale for Depression (r=0.73), and it is more highly correlated with measures of depressive symptoms than other classes of symptoms. The DID also has high internal consistency (Cronbach’s α=0.91) and test-retest reliability (r=0.91) (Zimmerman et al. 2004). DID scores were used to rule out the development of a recent MDD episode in mothers with no history of axis I disorders at the time of the SCID. Thus, mothers selected for the control group had no lifetime history of axis I disorders on the SCID at the initial assessment and did not meet criteria for a MDD episode on the DID in the follow-up assessment. Offspring of mothers with a history of chronic or recurrent depression were included in the study regardless of DID scores.
Child Assessments
Child Depression
Parents were interviewed using the Pre-school Age Psychiatric Assessment (PAPA; Egger et al. 1999) at both assessments. The PAPA uses a structured format and an interviewer-based approach. Most symptoms are assessed for the 3 months prior to the interview to maximize recall. Good test-retest reliability (Kappa=0.72) has been reported for diagnoses of depression using independent interviews (Egger et al. 2006). Interviews were conducted by advanced graduate students in clinical psychology and Masters-level research staff who were trained on administration by a member of the PAPA development team. At both assessments, a second rater independently rated audiotapes of a subset of PAPA interviews (N’s=21 and 35, respectively). No children in the inter-rater reliability sub-samples met full DSM-IV criteria for MDD at either the initial or follow-up assessment. However, the ICCs for the dimensional depressive symptom scale were 0.85 and 0.95, respectively.
In addition, the Children’s Depression Inventory (CDI; Kovacs 1992) was administered to the children during the second (age 6) wave of assessments. The CDI is a self-report measure that assesses depressive symptoms in youth and has been demonstrated to have good internal consistency (Cronbach’s α=0.86) and test-retest reliability (r=0.82) with a 2 week interval between administrations (Kovacs 1992; Finch et al. 1987). The CDI has also been shown to have a sensitivity of 81% and a specificity of 84% using a cut-off score of 17 when compared to diagnoses of major depression in youth aged 12–18 years (Craighead et al. 1995). Although it is generally used in children older than 7 years, Ialongo et al. (2001) have reported evidence for the validity of the CDI in children as young as 5 years. Because of the young age of the children, the items were read aloud by a researcher, and two items related to school work were excluded from the questionnaire to ensure that all of the items were developmentally appropriate.
Peabody Picture Vocabulary Test
The Peabody Picture Vocabulary Test-III (PPVT; Dunn and Dunn 1997) was administered during the follow-up (age 6) assessment. The PPVT is a widely used measure of receptive vocabulary. It is highly reliable, with an internal consistency of 0.95 and a test-retest reliability of 0.92. Its concurrent validity has been demonstrated through high correlations with a number of other measures of verbal ability (Dunn and Dunn 1997). Scores were included in the analyses to reduce the influence of variability associated with differences in verbal comprehension and cognitive ability within groups.
Mood Induction
Prior to beginning the dot-probe task, participants viewed a short clip from the film My Dog Skip (Russell 2000), which depicts a young boy’s tearful reaction to his injured pet dog, and were asked to think about how they would feel if they were the little boy. According to a meta-analysis of mood induction procedures, films or stories are most effective in inducing mood states, particularly if they are paired with explicit instructions to enter the desired mood state (Westermann et al. 1996). Participants were asked to rate their mood as very happy, happy, OK, sad, or very sad, using a chart showing the word and facial expression for each emotion. Ratings were taken prior to and after the induction. During practice trials and between blocks of the dot-probe task, the researcher gave corrective feedback to ensure that the child was properly completing the task but refrained from providing positive reinforcement in order to maintain the effect of the mood induction.
Dot-Probe Task
Emotional face pictures from the NimStim stimulus set developed by the MacArthur Foundation Research Network on Early Experience and Brain Development1 were used for the task. Six male and six female actors’ faces, each with happy, sad, and neutral expressions, were chosen from the set. Each emotional face was paired with the neutral expression of the same actor. A face pair for each actor was presented eight times for a total of 96 trials, which were divided into two blocks. Order of actors and the emotional face shown in each trial were randomly presented, with happy and sad pairs appearing with equal probability. A grey fixation cross appeared in the middle of the screen for 1,000 ms at the start of each trial and was replaced by a face pair that was presented for 1,500 ms. A dot then appeared in the location of one of the pictures and 1 Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham at tott0006@tc. umn.edu for more information concerning the stimulus set. remained until the participant responded by pressing one of the mouse buttons. The emotional face was randomly presented on the left or right side of the screen with the neutral face in the other position, and the dot appeared with equal probabilities on the left and right side. The task was presented on a 13-inch Dell color monitor using Presentation software (Neurobehavioral Systems 2006) to present the stimuli and record responses. The size of each picture presented was approximately 8 cm×9.5 cm with a space of 4.5 cm between the two pictures. Participants held a mouse in both hands with one thumb on each of the buttons. They were informed that they should press the mouse button that corresponded with the side of the screen on which the dot was presented. To demonstrate, the researcher pointed to each side of the screen and to the corresponding button. Twenty-four practice trials were presented prior to the start of the actual task: 12 consisting of just the dot probe, and 12 with a face pair followed by the dot probe. During the practice trials, the researcher monitored responses and gave feedback in order to ensure that the participants understood the task.
Attentional bias scores were calculated for each emotion using the following equation (MacLeod and Mathews 1988; Mogg et al. 1995):
In this equation, R and L refer to the right and left sides of the screen, while e refers to the emotional face and p to the probe. This calculation was made separately for sad and happy trials. The equation subtracts the mean latency when the probe and emotional face are on the same side (congruent trials) from the mean latency when the probe and emotional face are on opposite sides (incongruent trials) for both the left and right sides, and then takes the average of the two scores. If participants attend to the emotional face, latencies will be shorter for congruent compared to incongruent trials, and attentional bias scores will be positive. If participants avoid emotional faces, latencies will be longer for congruent compared to incongruent trials, and bias scores will be negative.
Results
Maternal Characteristics
Means and standard deviations of maternal age and DID scores and percentages of married parents and mothers who completed four-year college degrees are presented in Table 1. Two-way ANOVAs with depression group and child’s sex as the independent variables showed that the groups did not differ in maternal age, Fs(1, 95)<1, p>0.05. There was a significant main effect of depression group on DID scores, F(1, 89)=21.84, p<0.01, though only one of the mothers with a history of depression met symptom criteria for current MDD on the DID (data were missing for six of the mothers with a history of depression). Chi-square tests confirmed that the groups did not significantly differ with regard to the proportion of married parents, χ2(3)= 1.63, p>0.05, or the number of mothers who completed college degrees, χ2(3)=2.24, p>0.05.
Table 1.
Means and Standard Deviations of Maternal Age and DID Scores and Distribution of Marital Status and Education by Maternal Depression and Child Gender
| Characteristic | At-risk females
|
Control females
|
At-risk males
|
Control males
|
||||
|---|---|---|---|---|---|---|---|---|
| N=15 | N=27 | N=21 | N=36 | |||||
| M | SD | M | SD | M | SD | M | SD | |
| Maternal age | 38.07 | 5.12 | 39.19 | 3.62 | 38.29 | 4.93 | 38.67 | 4.72 |
| Maternal DID score | 8.07 | 7.04 | 3.59 | 3.37 | 8.31 | 5.70 | 2.81 | 4.20 |
| % | % | % | % | |||||
| Married | 80.0 | 92.6 | 90.5 | 88.9 | ||||
| College degree | 73.3 | 63.0 | 52.4 | 69.4 | ||||
Of the mothers in the chronic/recurrent depression groups, 66.7% met criteria for lifetime MDD and 52.8% met criteria for lifetime dysthymic disorder. Mothers with a history of MDD experienced a mean of 3.25 (SD=3.80) episodes with a mean duration of 34.17 (SD=33.70) months for the longest episode. The average age of onset was 23.42 (SD=8.19) for MDD and 22.63 (SD=9.88) for dysthymic disorder. Of the mothers in the depressed group, 52.8% also had a lifetime history of a specific anxiety disorder and 27.8% had a history of substance use disorders. Mothers of male and female children did not differ significantly with respect to rates of MDD or dysthymic disorder, number of MDD episodes, duration of the longest episode, age of onset of dysthymia or MDD, or comorbidity with anxiety or substance use disorders.
Child Characteristics
Demographic characteristics, CDI scores, and PPVT scores for each group are presented in Table 2. The groups did not differ on age, Fs(1, 95)<1, p>0.05, laterality, Fs(1, 95)<1, p>0.05, or ethnicity, χ2(3)=0.91, p>0.05. PPVT scores in this sample are slightly higher than the population mean of 100. Mean CDI scores are similar to those in other community samples (Twenge and Nolen-Hoeksema 2002). Two male children of mothers with a history of depression and one male child of a mother with no history of axis I disorders had raw scores on the CDI at or above the 85th percentile for children 7–12 years old. None of the children met full DSM-IV criteria for MDD or dysthymic disorder on the PAPA at age 3 or age 6. Two-way ANOVAs with depression group and child’s sex as the independent variables revealed significant main effects of sex on both PPVT scores, F(1, 95)=4.18, p<0.05, and CDI scores, F(1,95)=9.55, p<0.01, with girls exhibiting higher PPVT and lower CDI scores than boys. However, none of the group or group X sex effects on PPVT or CDI were significant.
Table 2.
Means and Standard Deviations/Frequencies of Children’s Characteristics and Dot-Probe Variables as a Function of Maternal Depression and Child Gender
| Characteristic | At-risk females
|
Control females
|
At-risk males
|
Control males
|
||||
|---|---|---|---|---|---|---|---|---|
| N=15 | N=27 | N=21 | N=36 | |||||
| % | % | % | % | |||||
| Caucasian | 80.0 | 88.9 | 81.0 | 86.1 | ||||
| Right-handed | 86.7 | 85.2 | 85.7 | 94.4 | ||||
| M | SD | M | SD | M | SD | M | SD | |
| Age | 6.15 | 0.40 | 6.10 | 0.33 | 6.21 | 0.50 | 6.19 | 0.39 |
| PPVT score | 110.80 | 10.28 | 111.67 | 9.71 | 103.95 | 11.03 | 109.83 | 9.57 |
| CDI score | 5.07 | 3.15 | 6.30 | 3.51 | 9.00 | 4.97 | 7.94 | 4.71 |
| Mood induction | −1.33 | 1.40 | −1.26 | 1.51 | −1.10 | 1.00 | −0.97 | 1.42 |
| Accuracy | 0.85 | 0.11 | 0.82 | 0.11 | 0.89 | 0.08 | 0.86 | 0.11 |
| Sad attention bias | 39.74 | 67.15 | −18.62 | 63.15 | −10.02 | 55.66 | −10.43 | 80.84 |
| Happy attention bias | 13.26 | 66.70 | 0.69 | 79.01 | 0.27 | 57.13 | −1.06 | 63.28 |
Dot-Probe Task
Trials with correct responses and latencies between 100 ms and 1,300 ms were included in the analyses. The upper and lower bounds chosen for response latencies are similar to those used in previous dot-probe studies with children (Gibb et al. 2009; Joormann et al. 2007). Participants with less than 60% useable trials were excluded from analyses, which resulted in the loss of 19 subjects or 16% of the original sample (7 male controls, 7 female controls, 5 at-risk females and no at-risk males). A chi-square test showed that the groups did not differ significantly with regard to number of excluded subjects, χ2(3)=5.71, p> 0.05. Means and standard deviations of mood induction effects, accuracy rates, and attentional bias scores for each group are shown in Table 2. The negative values for mood induction effect indicated that, on average, participants in each group reported feeling somewhat sadder following the mood induction clip. A two-way repeated measures ANOVA yielded no significant main or interaction effects of sex and depression group on mood or mood change before and after the clip, Fs(1, 95)<1, p>0.05. Sad attentional bias scores were not significantly correlated with baseline mood ratings (r=−0.01, p>0.05) or change in mood following the induction, (r=0.05, p>0.05). There were also no significant correlations between happy attentional bias scores and baseline mood (r=−0.001, p> 0.05) or change in mood (r=−0.06, p>0.05). After excluding children with fewer than 60% useable trials, the remaining children produced correct responses within an acceptable range of latencies on about 85% of trials. There were no significant effects of sex, group, or their interaction on accuracy. In addition, accuracy was not significantly correlated with age (r=0.13), PPVT (r=0.02), or CDI scores (r=0.06) in this sample. Nevertheless, we included PPVT scores, CDI scores, age in months, and overall accuracy as covariates in the analyses of attentional bias scores in order to reduce the influence of nuisance variables and increase power when testing the effects of group and gender (Miller and Chapman 2001).
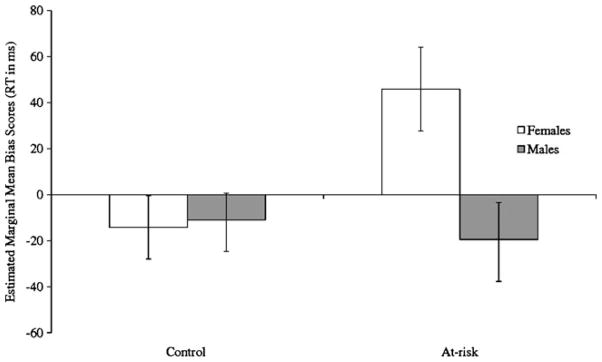
A two-way ANCOVA conducted to examine the effect of maternal depression and sex of the child on attentional biases for happy emotional faces yielded no significant main or interaction effects, Fs(1, 91)<1, p>0.5. A second two-way ANCOVA was conducted to examine the effect of maternal depression and sex of the child on attentional biases for sad emotional faces. The main effects of sex, F(1, 91)=3.83, p=0.054, and maternal psychopathology, F(1, 91)=2.97, p=0.09, approached significance. These two effects were qualified, however, by a significant interaction of sex and maternal psychopathology, F(1, 91)= 5.37, p<0.05 (see Fig. 1). To examine the nature of this interaction, we conducted tests of simple main effects to examine the difference between depression groups within each sex. Whereas at-risk and control males did not differ significantly from each other, F(1, 95)=0.00, p>0.05, at-risk females had higher bias scores than did control females, F(1, 95)=6.82, p=0.01. In addition, we also conducted tests of the simple main effect of sex within each depression group. There was no significant difference between males and females in the control group, F(1, 95)=0.22, p>0.05; in contrast, females had higher scores than did males in the maternal depression group, F(1, 95)=4.50, p<0.05. Lastly, to determine which of the groups showed attentional biases we conducted one-sample t-tests comparing sad bias scores for each group to zero. Daughters of depressed mothers exhibited an attentional bias towards sad faces, t(14)=2.29, p<0.05, while daughters of controls showed no biases, t(26)=−1.53, p>0.05. In addition, neither sons of depressed mothers, t(20)= −0.83, p>0.05, nor sons of controls, t(35)=−0.78, p>0.05, exhibited significant biases for sad faces. None of the groups exhibited significant biases for happy faces, all ts<1, p>0.05. Thus, daughters of mothers with a history of chronic or recurrent depression selectively attended to sad emotional faces, whereas daughters of non-depressed mothers and sons in both groups showed a pattern of avoidance or no attentional biases for sad faces.
Fig. 1.
Estimated marginal means and standard errors of sad attentional bias scores for males and females in each group
Discussion
Although findings from dot-probe studies of attentional biases in depressed youth and adults are mixed, there is a growing literature indicating that depression is associated with selective attention for sad emotional stimuli when extended stimulus durations, pictorial stimuli, and mood primes are used (Gotlib et al. 2004a; b; Joormann and Gotlib 2007; Joormann et al. 2007). The current results are consistent with a previous study reporting that the 9- to 14-year-old daughters of depressed mothers exhibited an attentional bias towards sad emotional stimuli on the dot-probe task (Joormann et al. 2007). Our findings cannot be attributed to levels of depressive symptoms in the children or to recent exposure to maternal depressive symptoms. Levels of depression in the children were low and did not differ between groups, and although mothers with a history of depression reported higher levels of current symptoms than never mentally ill mothers, this difference was small and not clinically significant. Thus, the present findings provide further evidence that young girls at increased risk for depression selectively attend to negative stimuli.
However, our findings contrast with results presented by Gibb et al. (2009) who, while also reporting evidence of attentional biases in the children of mothers with a history of depression, found that these at-risk youth showed a pattern of avoidance of sad stimuli. Although Gibb et al. (2009) also used longer stimulus durations and emotional face stimuli, they did not use a sad mood induction, and suggested that mood priming may shift the direction of an attentional bias from avoidance to selective attending. As our study did not include a non-primed condition, we cannot test this hypothesis.
Our study extends Joormann et al.’s (2007) and Gibb et al.’s (2009) studies by indicating that the link between attentional biases and risk for depression may be evident as early as 5–7 years of age. These findings are important because early identification of information-processing biases associated with depression offers a larger window for prevention or early intervention. This may be particularly important given evidence suggesting that some interventions are more effective in younger children than in older youth and adults, perhaps owing to neuroplasticity being greater earlier in development (Pine et al. 2009). This may be particularly relevant to interventions targeting cognitive biases, which appear to stabilize and become more trait-like in late childhood and early adolescence (Cole et al. 2008; Hankin 2008).
Previous studies using dot-probe tasks in youth have focused on middle-to-late childhood and adolescence. Our data indicate that children as young as 5 years of age are capable of performing dot-probe tasks, although accuracy rates were much lower than was the case in studies of later childhood and early adolescence (e.g., Joormann et al. 2007). Indeed, 16% of the sample had valid data on less than 60% of the trials. The greater error variance in the performance of younger children could lead to smaller effect sizes and require larger samples than studies using older youth and adults. Nevertheless, it is important to note that the offspring of mothers with and without a history of depression did not differ in accuracy, and the greater error variance cannot account for the differences in attentional biases.
Unlike Joormann et al. (2007), we did not find attentional biases for happy stimuli in children of mothers with no history of psychopathology. Indeed, the daughters of depressed mothers showed the greatest tendency for a bias for happy faces, although this did not approach significance and the magnitude of the effect was exceedingly small (the interaction effect for happy bias scores had an effect size of partial η2=0.001). It is possible that biases for happy stimuli are less evident in younger children or that the negative mood induction may have a more salient effect on younger children, altering biases towards happy stimuli that may be apparent otherwise. However, it also worth noting that fewer studies have found differences between depressed and healthy groups on biases for happy than sad stimuli (Gibb et al. 2009; Gotlib et al. 2004a; b).
The origins of attentional biases in MDD are still unclear. Beck (1967) and subsequent cognitive theorists (e.g., Ingram 2003) have hypothesized that cognitive biases develop in response to early stress and adversity. Alternatively, attentional biases may reflect genetic influences (e.g., Beevers et al. 2009; Gibb et al. 2009), develop in association with early-emerging individual differences in emotional reactivity and regulation (i.e., temperament) (e.g., Mauer and Borkenau 2007), or be shaped by interactions between temperament and the early environment (Pine et al. 2009).
In the present study, attentional biases for sad emotional stimuli were observed only in girls, but not in boys, at risk for depression. This is of particular interest because one of the two previous studies finding attentional biases in children of depressed mothers looked only at female offspring (Joormann et al. 2007). In contrast, Gibb et al. (2009) did not find that sex moderated the effect of maternal depression on sad attentional biases in offspring. In light of the present findings, future studies should continue to examine sex differences in cognitive vulnerabilities prior to adolescence; such findings could have significant implications for understanding the higher rate of depression in females that emerges in adolescence. For example, theorists have proposed that risk factors, such as cognitive vulnerabilities, may already be more common among girls than boys prior to adolescence. These vulnerabilities are posited to interact with the life stressors associated with adolescence to produce the marked increase in depression in adolescent girls (Hankin and Abramson 2001; Hyde et al. 2008; Nolen-Hoeksema and Girgus 1994). Our finding of sex differences in attentional biases among offspring of depressed mothers is consistent with this hypothesis.
We can only speculate regarding the source of the sex difference in attentional biases in children of depressed mothers. There is evidence of sex-specific genetic influences in the transmission of MDD (Kendler et al. 2001). Alternatively, depressed parents may differ in their treatment of sons and daughters, or daughters may be more susceptible than boys to modeling their mother’s depressive cognitive biases (Goodman 2007). Further work is needed to elucidate the causes of sex differences in cognitive biases in children, and evaluate whether attentional biases in childhood interact with stressors in adolescence to produce the increased rate of depression in post-pubertal females.
We should note a number of limitations to the current study. First, the size of some of the groups was relatively small when broken down by sex, particularly daughters of mothers with chronic or recurrent depression. Importantly, however, despite the smaller sample size, it was this group of participants who differed significantly from the other three groups in their attentional bias to sad faces. Second, because children were included in the chronic or recurrent depression group even if their mothers had met criteria for another axis I disorder, it is possible that the attentional biases found in daughters of depressed mothers may be associated with maternal psychopathology in general, rather than with maternal depression specifically. Specifically, over half the mothers with a history of depression also had a history of anxiety disorders. As anxiety has also been shown to be associated with attentional biases, it is possible that factors related to risk for anxiety disorders may account for the findings. However, in two studies using procedures similar to the current study, Gotlib et al. (2004a; b) compared adults with MDD to groups with social phobia and GAD, and found that the attention bias to sad faces was specific to depression.
Third, because the effects of maternal depression on offspring are greater than the effects of paternal depression, particularly for younger children (Brennan et al. 2002; Connell and Goodman 2002; Klein et al. 2005), the current study focused only on maternal depression. However, as fathers’ depression is also linked to depression in offspring, future research is needed to better understand the association between paternal depression and attentional biases in children.
Lastly, the assessment of maternal depression was limited in that SCID diagnoses were made approximately 3 years prior to the attentional bias assessment. Though DID scores were used to rule out current MDD in the control group, it is possible that some mothers in this group developed a first onset of depression after the first assessment but recovered prior to the second assessment. However, if this were to affect the results, it would most likely have reduced the effect of maternal diagnosis on offspring attentional biases and attenuated our findings. In addition, for mothers with a history of depression, we do not have information regarding the course of depression in the 3 years preceding the current study. It is possible that the recurrence, duration, and/or severity of depression during these years may have influenced children’s attention towards emotional information.
In summary, the current study suggests that attentional biases towards negative emotional stimuli may be a vulnerability marker for depression. Moreover, we were able to identify attentional biases as early as ages 5–7, suggesting that an attentional bias toward sad stimuli is evident long before the risk period for depression. Finally, attentional biases were limited to young girls at risk for depression, raising the possibility that females may exhibit higher levels of cognitive vulnerability than males many years before the emergence of sex differences in the prevalence of depression. Future research is needed to determine whether findings are similar for other cognitive biases linked to risk for depression. In addition, longitudinal research is needed to evaluate whether attentional biases in early childhood are related to the later development of depressive disorders.
Acknowledgments
This work was supported by the following grants: National Institute of Mental Health grant RO1 MH069942 (DNK) and GCRC Grant M01-RR10710 to Stony Brook University from the National Center for Research Resources.
Contributor Information
Autumn J. Kujawa, Email: autumn.kujawa@stonybrook.edu, Department of Psychology, Stony Brook University, Stony Brook, NY 11794-2500, USA
Dana Torpey, Department of Psychology, Stony Brook University, Stony Brook, NY 11794-2500, USA.
Jiyon Kim, Department of Psychology, Stony Brook University, Stony Brook, NY 11794-2500, USA.
Greg Hajcak, Department of Psychology, Stony Brook University, Stony Brook, NY 11794-2500, USA.
Suzanne Rose, Department of Psychology, Stony Brook University, Stony Brook, NY 11794-2500, USA.
Ian H. Gotlib, Department of Psychology, Stanford University, Stanford, CA, USA
Daniel N. Klein, Department of Psychology, Stony Brook University, Stony Brook, NY 11794-2500, USA
References
- Alloy L, Abramson L, Whitehouse W, Hogan M, Panzarella C, Rose D. Prospective incidence of first onsets and recurrences of depression in individuals at high and low cognitive risk for depression. Journal of Abnormal Psychology. 2006;115(1):145–156. doi: 10.1037/0021-843X.115.1.145. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kraneburg MJ, van Ijzendoom MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression. New York: Harper and Row; 1967. [Google Scholar]
- Beevers C, Wells T, Ellis A, McGeary J. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. Journal of Abnormal Psychology. 2009;118(3):670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ, Dalgleish T, Yule W. Memory for emotional stories in high and low depressed children. Memory. 2004;12(2):214–230. doi: 10.1080/09658210244000667. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Lee SC. Attentional biases for negative information in induced and naturally occurring dysphoria. Behaviour Research and Therapy. 1997;35(10):911–927. doi: 10.1016/s0005-7967(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Hammen C, Katz AR, Le Brocque RM. Maternal depression, paternal psychopathology, and adolescent diagnostic outcomes. Journal of Consulting and Clinical Psychology. 2002;70:1075–1085. doi: 10.1037//0022-006x.70.5.1075. [DOI] [PubMed] [Google Scholar]
- Cole DA, Ciesla JA, Dallaire DH, Jacquez FM, Pineda AQ, LaGrange B, et al. Emergence of attributional style and its relation to depressive symptoms. Journal of Abnormal Psychology. 2008;117:16–31. doi: 10.1037/0021-843X.117.1.16. [DOI] [PubMed] [Google Scholar]
- Cole D, Jacquez FM, Truss AE, Pineda AQ, Weitlauf AS, Tilghman-Osborne CE, et al. Gender differences in the longitudinal structure of cognitive diatheses for depression in children and adolescents. Journal of Clinical Psychology. 2009;65(12):1312–1326. doi: 10.1002/jclp.20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell AM, Goodman SH. The association between psychopathology in fathers versus mothers and children’s internalizing and externalizing behavior problems: a meta-analysis. Psychological Bulletin. 2002;128:746–773. doi: 10.1037/0033-2909.128.5.746. [DOI] [PubMed] [Google Scholar]
- Craighead WE, Curry JF, Ilardi SS. Relationship of children’s depression inventory factors to major depression among adolescents. Psychological Assessment. 1995;7(2):171–176. [Google Scholar]
- Dalgleish T, Taghavi R, Neshat-Doost H, Moradi A, Canterbury R, Yule W. Patterns of processing bias for emotional information across clinical disorders: a comparison of attention, memory, and prospective cognition in children and adolescents with depression, generalized anxiety, and posttraumatic stress disorder. Journal of Clinical Child and Adolescent Psychology. 2003;32(1):10–21. doi: 10.1207/S15374424JCCP3201_02. [DOI] [PubMed] [Google Scholar]
- Davis PJ. Gender differences in autobiographical memory for childhood emotional experiences. Journal of Personality and Social Psychology. 1999;76(3):498–510. doi: 10.1037//0022-3514.76.3.498. [DOI] [PubMed] [Google Scholar]
- Dearing KF, Gotlib IH. Interpretation of ambiguous information in girls at risk for depression. Journal of Abnormal Child Psychology. 2009;37(1):79–91. doi: 10.1007/s10802-008-9259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody picture vocabulary test-III. Circle Pines: American Guidance Service; 1997. [Google Scholar]
- Egger HK, Ascher BH, Angold A. Unpublished Interview Schedule. Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center; 1999. The preschool age psychiatric assessment: Version 1.1. [Google Scholar]
- Egger H, Erkanli A, Keeler G, Potts E, Walter B, Angold A. Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA) Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(5):538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- Finch A, Saylor C, Edwards G, McIntosh J. Children’s depression inventory: reliability over repeated administrations. Journal of Clinical Child Psychology. 1987;16(4):339–341. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The structured clinical interview for DSM-IV axis I disorders—non-patient editions. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Garber J, Robinson NS. Cognitive vulnerability in children at risk for depression. Cognition and Emotion. 1997;11(5):619–635. [Google Scholar]
- Gibb BE, Benas JS, Grassia M, McGeary J. Children’s attentional biases and 5-HTTLPR genotype: potential mechanisms linking mother and child depression. Journal of Clinical Child and Adolescent Psychology. 2009;38(3):415–426. doi: 10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. Depression in mothers. Annual Review of Clinical Psychology. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Kasch KL, Traill S, Arnow BA, Johnson SL, Joormann J. Coherence and specificity of information-processing biases in depression and social phobia. Journal of Abnormal Psychology. 2004a;113(3):386–398. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004b;113(1):127–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Hammen CL. Children of depressed parents. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2. New York: Guilford; 2009. pp. 275–297. [Google Scholar]
- Hankin BL. Stability of cognitive vulnerabilities to depression: a short-term prospective multiwave study. Journal of Abnormal Psychology. 2008;117:324–333. doi: 10.1037/0021-843X.117.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: an elaborated cognitive vulnerability–transactional stress theory. Psychological Bulletin. 2001;127(6):773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven: Yale University; 1975. Unpublished manuscript. [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological Review. 2008;115(2):291–313. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Ialongo NS, Edelsohn G, Kellam SG. A further look at the prognostic power of young children’s reports of depressed mood. Child Development. 2001;72(3):736–747. doi: 10.1111/1467-8624.00312. [DOI] [PubMed] [Google Scholar]
- Ingram R. Origins of cognitive vulnerability to depression. Cognitive Therapy and Research. 2003;27(1):77–88. [Google Scholar]
- Ingram RE, Luxton DD. Vulnerability-stress models. In: Hankin BL, Abela JRZ, editors. Developmental psychopathology. Thousand Oaks: Sage; 2005. pp. 32–46. [Google Scholar]
- Ingram RE, Bernet CZ, McLaughlin SC. Attentional allocation processes in individuals at risk for depression. Cognitive Therapy and Research. 1994;18(4):317–332. [Google Scholar]
- Ingram RE, Miranda J, Segal ZV. Cognitive vulnerability to depression. New York: Guilford; 1998. [Google Scholar]
- Jacobs RH, Reinecke MA, Gollan JK, Kane P. Empirical evidence of cognitive vulnerability for depression among children and adolescents: a cognitive science and developmental perspective. Clinical Psychology Review. 2008;28:759–782. doi: 10.1016/j.cpr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke C, Hammen C, Zupan B, Hiroto D. Cognitive vulnerability in children at risk for depression. Journal of Abnormal Child Psychology. 1987;15(4):559–572. doi: 10.1007/BF00917241. [DOI] [PubMed] [Google Scholar]
- Joormann J. Cognitive aspects of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2. New York: Guilford; 2009. pp. 298–321. [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. Journal of Abnormal Psychology. 2007;116(1):80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116(1):135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Keller MB, Beardslee WR, Dorer DJ, Lavori PW. Impact of severity and chronicity of parental affective illness on adaptive functioning and psychopathology in children. Archives of General Psychiatry. 1986;43(10):930–937. doi: 10.1001/archpsyc.1986.01800100020004. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychological Medicine. 2001;31:605–616. doi: 10.1017/s0033291701003907. [DOI] [PubMed] [Google Scholar]
- Klein DN, Clark DC, Dansky L, Margolis ET. Dysthymia in the offspring of parents with primary unipolar affective disorder. Journal of Abnormal Psychology. 1988;97(3):265–274. doi: 10.1037//0021-843x.97.3.265. [DOI] [PubMed] [Google Scholar]
- Klein DN, Lewinsohn PM, Rohde P, Seeley JR, Olino TM. Psychopathology in the adolescent and young adult offspring of a community sample of mothers and fathers with major depression. Psychological Medicine. 2005;35(3):353–365. doi: 10.1017/s0033291704003587. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children’s depression inventory. Toronto: Multi-Health Systems; 1992. [Google Scholar]
- Krackow E, Rudolph KD. Life stress and the accuracy of cognitive appraisals in depressed youth. Journal of Clinical Child and Adolescent Psychology. 2008;37(2):376–385. doi: 10.1080/15374410801955797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyman L, De Raedt R, Schacht R, Koster EHW. Attentional biases for angry faces in unipolar depression. Psychological Medicine. 2007;37(3):393–402. doi: 10.1017/S003329170600910X. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A. Anxiety and the allocation of attention to threat. The Quarterly Journal of Experimental Psychology A: Human Experimental Psychology. 1988;40(4):653–670. doi: 10.1080/14640748808402292. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95(1):15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1(1):167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mathews A, Ridgeway V, Williamson DA. Evidence for attention to threatening stimuli in depression. Behaviour Research and Therapy. 1996;34(9):695–705. doi: 10.1016/0005-7967(96)00046-0. [DOI] [PubMed] [Google Scholar]
- Matt GE, Vázquez C, Campbell WK. Mood-congruent recall of affectively toned stimuli: a meta-analytic review. Clinical Psychology Review. 1992;12(2):227–255. [Google Scholar]
- Mauer N, Borkenau P. Temperament and early information processing: temperament-related attentional bias in emotional Stroop tasks. Personality and Individual Differences. 2007;43:1063–1073. [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Attentional bias in generalized anxiety disorder versus depressive disorder. Cognitive Therapy and Research. 2005;29(1):29–45. [Google Scholar]
- Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: The role of awareness. The British Journal of Clinical Psychology. 1995;34(1):17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. Journal of Abnormal Psychology. 2000;109(4):695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- Neshat-Doost HT, Moradi AR, Taghavi MR, Yule W, Dalgleish T. Lack of attentional bias for emotional information in clinically depressed children and adolescents on the dot-probe task. Journal of Child Psychology and Psychiatry. 2000;41(3):363–368. [PubMed] [Google Scholar]
- Neurobehavioral Systems. Presentation Software (Version 10.1) 2006. [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychological Bulletin. 1994;115(3):424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- Persons JB, Miranda J. Cognitive theories of vulnerability to depression: reconciling negative evidence. Cognitive Therapy and Research. 1992;16(4):485–502. [Google Scholar]
- Pine DS, Helfinstein SM, Bar-Haim Y, Nelson E, Fox NA. Challenges in developing novel treatments for childhood disorders: lessons from research on anxiety. Neuropsychophar-macology. 2009;34:213–228. doi: 10.1038/npp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SC, Salmon K, Lovibond PF. Cognitive biases in childhood anxiety, depression, and aggression: are they pervasive or specific? Cognitive Therapy and Research. 2006;30(5):531–549. [Google Scholar]
- Rinck M, Becker ES. A comparison of attentional biases and memory biases in women with social phobia and major depression. Journal of Abnormal Psychology. 2005;114(1):62–74. doi: 10.1037/0021-843X.114.1.62. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Comparability of telephone and face-to-face interviews in assessing axis I and II disorders. The American Journal of Psychiatry. 1997;154(11):1593–1598. doi: 10.1176/ajp.154.11.1593. [DOI] [PubMed] [Google Scholar]
- Russell J. My dog skip [Motion picture] United States: Alcon Entertainment; 2000. [Google Scholar]
- Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clinical Psychology Review. 2005;25(4):487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Sobin C, Weissman MM, Goldstein RB, Adams P. Diagnostic interviewing for family studies: comparing telephone and face-to-face methods for the diagnosis of lifetime psychiatric disorders. Psychiatric Genetics. 1993;3(4):227–233. [Google Scholar]
- Taylor L, Ingram RE. Cognitive reactivity and depressotypic information processing in children of depressed mothers. Journal of Abnormal Psychology. 1999;108(2):202–210. doi: 10.1037//0021-843x.108.2.202. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Nolen-Hoeksema S. Age, gender, race, socioeconomic status, and birth cohort differences on the children’s depression inventory: a meta-analysis. Journal of Abnormal Psychology. 2002;111(4):578–588. doi: 10.1037//0021-843x.111.4.578. [DOI] [PubMed] [Google Scholar]
- Warner V, Mufson L, Weissman MM. Offspring at high and low risk for depression and anxiety: mechanisms of psychiatric disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(6):786–797. doi: 10.1097/00004583-199506000-00020. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Gammon GD, John K, Merikangas KR. Children of depressed parents: increased psychopathology and early onset of major depression. Archives of General Psychiatry. 1987;44(10):847–853. doi: 10.1001/archpsyc.1987.01800220009002. [DOI] [PubMed] [Google Scholar]
- Westermann R, Spies K, Stahl G, Hesse FW. Relative effectiveness and validity of mood induction procedures: a meta-analysis. European Journal of Social Psychology. 1996;26(4):557–580. [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorders. Oxford England: Wiley; 1988. [Google Scholar]
- Williams JB, Gibbon M, First MB, Spitzer RL. The structured clinical interview for DSM-III-R (SCID): II. Multisite test-retest reliability. Archives of General Psychiatry. 1992;49(8):630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Skodol AE, Bender D, Dolan R, Sanislow C, Schaefer E, et al. The collaborative longitudinal personality disorders study: reliability of axis I and II diagnoses. Journal of Personality Disorders. 2000;14(4):291–299. doi: 10.1521/pedi.2000.14.4.291. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Sheeran T, Young D. The diagnostic inventory for depression: a self-report scale to diagnose DSM-IV major depressive disorder. Journal of Clinical Psychology. 2004;60(1):87–110. doi: 10.1002/jclp.10207. [DOI] [PubMed] [Google Scholar]
- Zubin J, Spring B. Vulnerability: a new view of schizophrenia. Journal of Abnormal Psychology. 1977;86(2):103–126. doi: 10.1037//0021-843x.86.2.103. [DOI] [PubMed] [Google Scholar]